Protocols for the CRISPR/Cas Technology
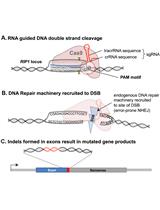
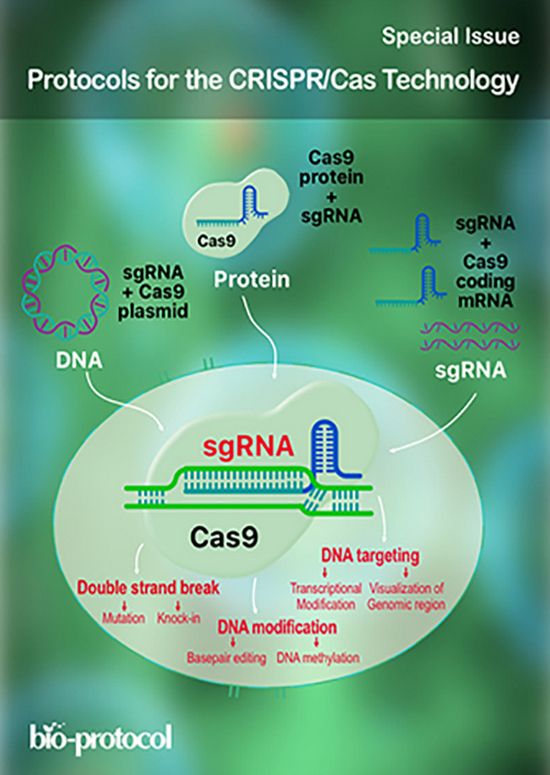
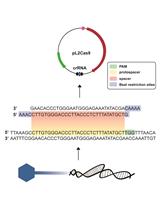
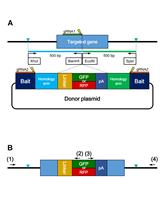
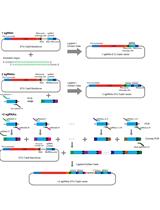
CRISPR/Cas-based technologies witness a growing number of applications in life sciences. These technologies are based on the CRISPR/Cas (Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated proteins) adaptive immune system in prokaryotes, which functions by storing small DNA fragments derived from nucleic acid invaders as spacers in genomic CRISPR loci. CRISPR loci are transcribed and processed into CRISPR RNA (crRNA) guides that are utilized by Cas effector proteins, such as Cas9 or Cas12a, to target and degrade invading nucleic acids in a sequence-specific manner. The high specificity of these ribonucleoprotein complexes and their potential to introduce double strand breaks in target DNA have been the foundation for many technical innovations (such as targeted genome engineering, gene silencing, and visualization of genomic regions) that enable us to address important biological questions.
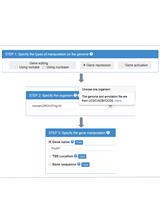
Our CRISPR/Cas special issue presents a comprehensive collection of detailed and peer reviewed protocols focused on CRISPR/Cas applications (Section 1), different approaches for guide RNA design (Section 2), delivery mechanisms of Cas effector proteins and/or guide RNAs into cells and organisms (Section 3), as well as protocols that allow further investigation of the biological role and mechanisms of CRISPR/Cas systems (Section 4). Bio-protocols’ uniquely interactive platform supports communication between scientists – through feedback, Q&A, and protocol updates sections – and will allow you to set up CRISPR/Cas based technologies for your research. Bio-protocol is a living platform and our CRISPR/Cas special issue will grow with the CRISPR/Cas field, giving you access to the latest developments.
Erik Sontheimer, Professor
RNA Therapeutics Institute, University of Massachusetts Medical School
Asma Hatoum-Aslan, Assistant Professor
Department of Biological Sciences, The University of Alabama






















































.jpg)




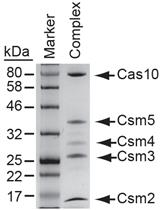



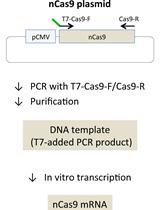



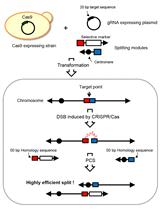





.jpg)