- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Novel Non-invasive Qualitative Assay Using Urinary Fluorescence Imaging to Assess Kidney Disease
(*contributed equally to this work) Published: Vol 13, Iss 9, May 5, 2023 DOI: 10.21769/BioProtoc.4670 Views: 899
Reviewed by: Komuraiah MyakalaAnonymous reviewer(s)
Abstract
In patients with chronic kidney disease, it is necessary to identify the etiology of glomerular disease. Renal biopsy is the gold standard for assessing the underlying pathology; however, it has the risk of potential complications. We have established a urinary fluorescence imaging technique to assess enzymatic activity using an activatable fluorescent probe targeting two enzymes: gamma-glutamyl transpeptidase and dipeptidyl-peptidase. The urinary fluorescence images can be easily obtained by adding an optical filter to the microscope with short incubation of the fluorescent probes. Urinary fluorescence imaging could help to assess underlying etiologies of kidney diseases and is a potential non-invasive qualitative assessment technique for kidney diseases in patients with diabetes.
Key features
• Non-invasive assessment of kidney disease.
• Urinary fluorescent imaging with enzyme-activatable fluorescent probes.
• Enables differentiation of diabetic kidney disease and glomerulonephritis.
Keywords: Activatable fluorescent probeBackground
Chronic kidney disease (CKD) is the major public health burden worldwide. Three major etiologies of CKD leading to end-stage renal disease include diabetic nephropathy, glomerulonephritis, and nephrosclerosis. Although these etiologies can be identified by clinical presentation and laboratory tests in typical cases, some cases require histological assessment to clearly differentiate these diseases, especially in patients with diabetes mellitus. Renal biopsy is the most important procedure to assess the underlying pathologies in CKD. However, it has potential complications such as bleeding, blood transfusion, and loss of renal function. Therefore, a convenient and noninvasive method is warranted for the assessment of kidney disease. Several urinary biomarkers have been suggested to be useful in assessing kidney diseases (Joachim et al., 2021; Takata et al., 2023); however, no biomarkers have been proved to be directly linked to the diagnosis of diabetic kidney disease. Recent advancements in the field of light-emitting substances have opened new approaches for the diagnosis of kidney disease (Huang et al., 2017; Yan et al., 2019). An enzyme-activatable fluorescent probe was recently developed for photodynamic diagnosis (Urano et al., 2011). This technique is based on the fluorescent emittance from hydroxymethyl rhodamine green (HMRG) upon enzymatic reaction with target aminopeptidases, such as gamma-glutamyl transpeptidase (GGTP) and dipeptidyl-peptidase (DPP). While this technique was originally developed for detecting cancer, it can be applied to renal biopsy specimens (Iyama et al., 2020; Takata et al., 2022). Our recent work has focused on investigating the feasibility of the activatable fluorescent probe for noninvasive assessment of the underlying kidney disease (Yamada et al., 2022). Urinary fluorescence was significantly stronger in patients with diabetic nephropathy than those with glomerulonephritis upon DPP-HMRG incubation. Besides, it was stronger in patients with nephrosclerosis compared to those with glomerulonephritis after GGTP-HMRG. Underlying kidney disease can be differentiated by urinary fluorescence imaging in combination with GGTP-HMRG and DPP-HMRG. This study has some limitations regarding the applicability to clinical settings: the results require further confirmation with larger studies, and a quantitative threshold needs to be determined. However, since urinary fluorescence imaging can be easily and quickly obtained, this technique would help to detect kidney diseases, particularly during health checks. We herein present a protocol for the induction of fluorescence by activatable fluorescent probe with some modifications for better clarity of the images.
Materials and Reagents
Pipettes (M&S Instruments, catalog numbers: F144059M, F144058, and F144055M) and pipette tips (VIOLAMO, catalog numbers: V-1000, V-200, and V-10)
1.5 mL Eppendorf centrifuge tubes
Phosphate buffer saline (PBS) (FUJIFILM, catalog number: 166-23555)
Dimethyl sulfoxide (FUJIFILM, catalog number: 041-29351)
EP-HMRG (GORYO Chemical, catalog number: GC811), which targets DPP. Dissolve in dimethyl sulfoxide at 1 mM and store at -20 °C before use (not necessary to filter)
ProteoGREENTM-gGlu (GORYO Chemical, catalog number: GC801), which targets GGTP. Dissolve in dimethyl sulfoxide at 1 mM and store at -20 °C before use (not necessary to filter)
Aqua-auto Kainos CRE-III plus (KAINOS, catalog number: TKA7500), for creatinine measurement
Equipment
Stereomicroscope (BioTools, catalog number: BS-3048BT)
Fluorescent unit (BioTools, catalog number: BT-ExSM)
Band pass filter (FUJIFILM, catalog number: BPB-45). This filter should be set at the light source of the fluorescent unit
Sharp cut filter (FUJIFILM, catalog number: SC-52). This filter should be set at the lens side of the fluorescent unit
Labnet C-1200 mini centrifuge (Marshall Scientific, product code: C-1200)
Procedure
Preparation of the fluorescent probe
Thaw EP-HMRG/ProteoGREENTM-gGlu stock solution at room temperature (protect from light).
Dilute EP-HMRG/ProteoGREENTM-gGlu stock solution into PBS to prepare working solution at a final concentration of 10 μM.
Scheme representing how the probe works is showed in Figure 1.
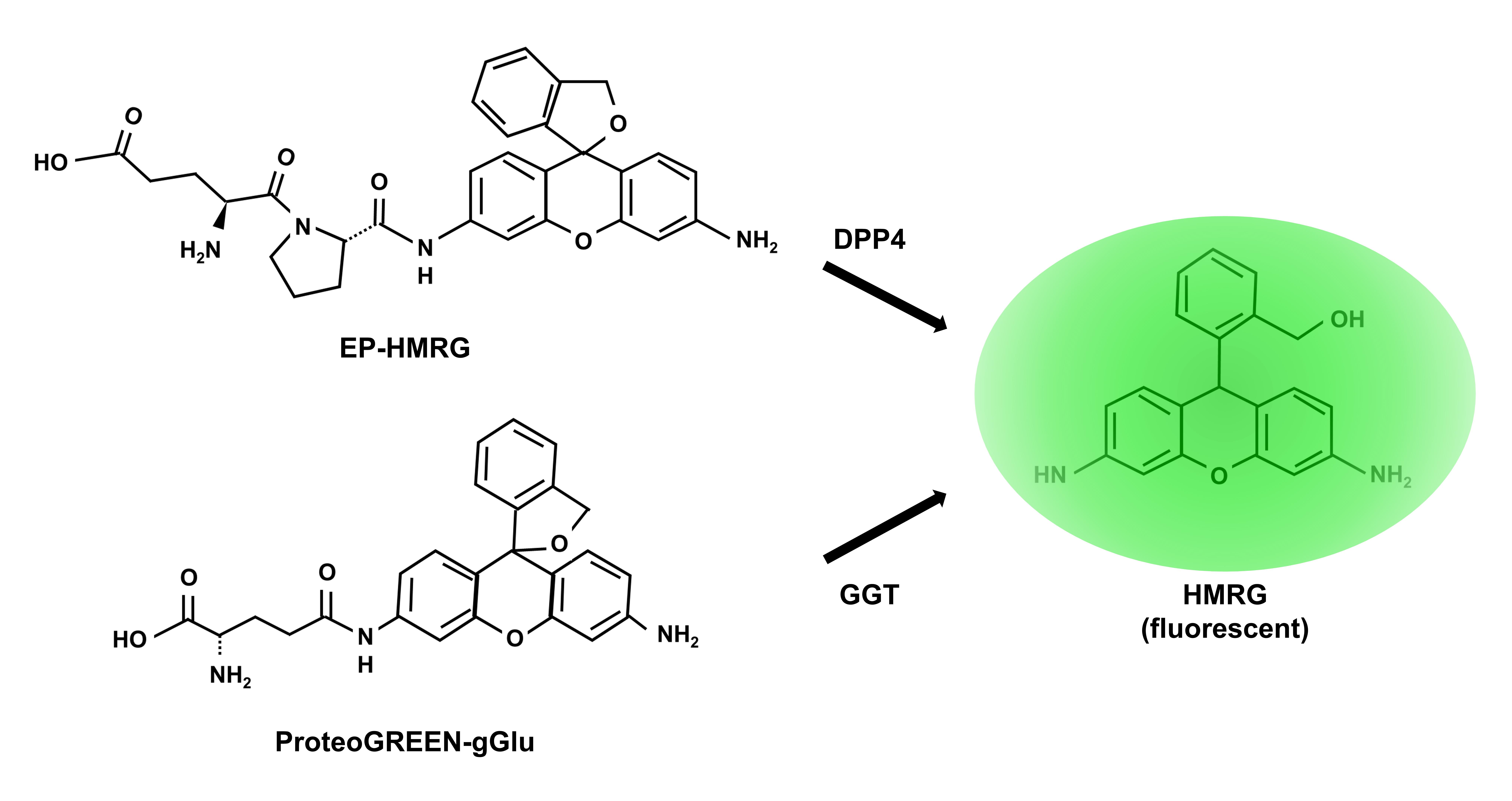
Figure 1. Scheme of the reaction of fluorescent probes. Figure adapted from Yamada et al. (2022).
Fluorescence imaging of the urine
Collect a urine sample from human subjects (1 mL is enough).
Briefly (a few seconds) spin down to remove sediment and transfer supernatant to another tube for testing.
Dilute urine sample in PBS to a creatinine concentration of 1 μg/μL.
Mix 10 μL of diluted urine sample and 10 μL of EP-HMRG/ProteoGREENTM-gGlu stock solution by pipetting to prepare fluorescent working solution. The final concentration of creatinine should be 0.5 μg/μL and fluorescent probes should be 5 μM (20 μL is enough for fluorescent imaging).
Incubate for one minute under dark conditions at room temperature.
Illuminate and observe the fluorescence. An excitation wavelength of 450 nm can pass through the band pass filter; then, the fluorescent working solution is excited and emits fluorescence. The sharp cut filter eliminates redundant wavelengths except for fluorescence (~520 nm).
Capture images of the samples at 2.0× magnification.
Scheme of the fluorescent imaging is showed in Figure 2.
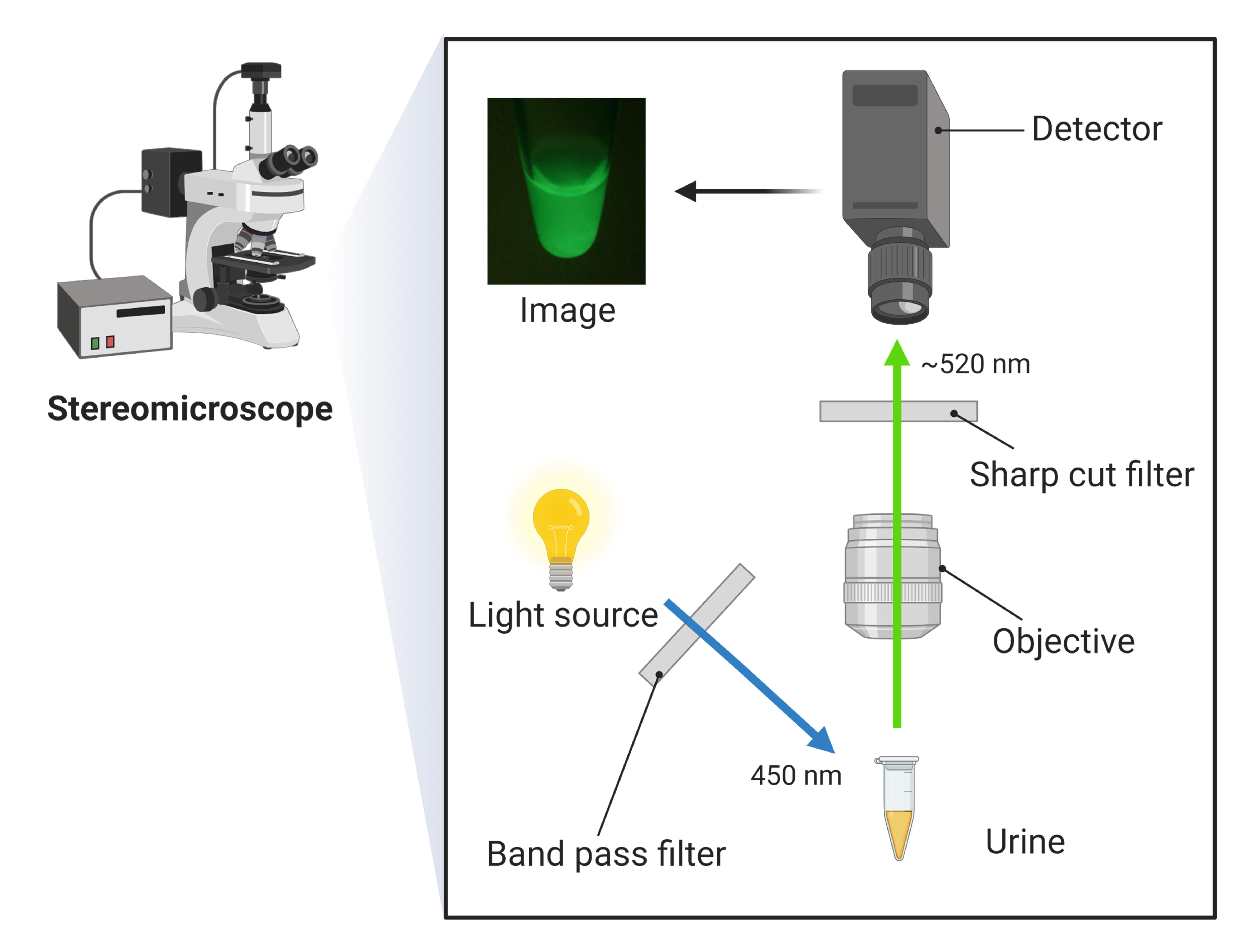
Figure 2. Scheme of the fluorescent unit for the imaging
Notes
Prepare fluorescent working solution each time just before usage.
Acknowledgments
Protocol was based on “Fluorescence imaging using enzyme-activatable probes for detecting diabetic kidney disease and glomerular diseases”, in International Journal of Molecular Sciences (Yamada et al., 2022).
Competing interests
This research was founded by JSPS KAKENHI, grant number JP22K16242 (T.I.).
Ethics
This study was conducted in accordance with the Declaration of Helsinki and approved by the ethical committee of Tottori University Hospital (approval number: 18A135 and 20A001). Informed consent was obtained from all subjects.
References
- Joachim, H. and Shilpak, M. G. (2021). The promise of tubule biomarkers in kidney disease: A review. Am J Kidney Dis 78(5): 719-727.
- Takata, T. and Isomoto, H. (2023). The versatile role of uromodulin in renal homeostasis and its relevance in chronic kidney disease. Intern Med doi: 10.2169/internalmedicine.1342-22.
- Yan, F., Tian, X., Luan, Z., Feng, L., Ma, X. and James, T. D. (2019). NAG-targeting fluorescence based probe for precision diagnosis of kidney injury. Chem Commun (Camb) 55(13): 1955-1958.
- Huang, J., and Gretz, N. (2017). Light-emitting agents for noninvasive assessment of kidney function. ChemistryOpen 6(4); 456-471.
- Urano, Y., Sakabe, M., Kosaka, N., Ogawa, M., Mitsunaga, M., Asanuma, D., Kamiya, M., Young, M. R., Nagano, T., Choyke, P. L. and Kobayashi, H. (2011). Rapid cancer detection by topically spraying a gamma-glutamyltranspeptidase-activated fluorescent probe. Sci Transl Med 3(110): 110ra119.
- Iyama, T., Takata, T., Yamada, K., Mae, Y., Taniguchi, S., Ida, A., Ogawa, M., Yamamoto, M., Hamada, S., Fukuda, S., Kanda, T., Sugihara, T., Isomoto, H. and Urano, Y. (2020). A novel method for assessing the renal biopsy specimens using an activatable fluorescent probe. Sci Rep 10(1): 12094.
- Takata, T., Isomoto, H., Iyama, T. and Yamada, K. (2022). A novel imaging technique for the on-site assessment of renal biopsy specimens. Bio Protoc 12: e4517.
- Yamada, K., Takata, T., Iyama, T., Hamada, S., Mae, Y., Sugihara, T. and Isomoto, H. (2022). Fluorescence imaging using enzyme-activatable probes for detecting diabetic kidney disease and glomerular diseases. Int J Mol Sci 23: 8150.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Takata, T., Iyama, T., Yamada, K. and Isomoto, H. (2023). A Novel Non-invasive Qualitative Assay Using Urinary Fluorescence Imaging to Assess Kidney Disease. Bio-protocol 13(9): e4670. DOI: 10.21769/BioProtoc.4670.
Category
Medicine
Medicine
Biological Sciences > Biological techniques
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Tips for asking effective questions
+ Description
Write a detailed description. Include all information that will help others answer your question including experimental processes, conditions, and relevant images.
Share
Bluesky
X
Copy link