- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Novel Imaging Technique for The On-site Assessment of Renal Biopsy Specimens
(*contributed equally to this work) Published: Vol 12, Iss 18, Sep 20, 2022 DOI: 10.21769/BioProtoc.4517 Views: 1725
Reviewed by: Alessandro DidonnaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Fast and Efficient Decellularization Method for Tissue Slices
Maria Narciso [...] Isaac Almendros
Nov 20, 2022 2674 Views

In-Gel Activity Assay of Mammalian Mitochondrial and Cytosolic Aconitases, Surrogate Markers of Compartment-Specific Oxidative Stress and Iron Status
Wing-Hang Tong and Tracey A. Rouault
Dec 5, 2024 2202 Views

Assessment of SREBP Activation Using a Microsomal Vesicle Budding Assay
Mingfeng Xia [...] Shunxing Rong
Dec 20, 2024 1546 Views
Abstract
When performing renal biopsy, it is necessary to identify the cortex, where glomeruli are exclusively distributed, to ensure the quality of the specimen for histological diagnosis. However, conventional methods using a stereomicroscope or magnifying lens often fail to clarify the quality of the specimen. We have established a fluorescent-based imaging technique for the on-site assessment of renal biopsy specimens. The fluorescent images can be easily obtained by adding an optical filter to the microscope and with a short incubation of an activatable fluorescent probe. This novel imaging technique can be applied to renal biopsy specimens for distinguishing the renal cortex.
Keywords: Activatable fluorescent probeBackground
Renal biopsy is one of the most important procedures for the assessment of kidney diseases. Upon performing renal biopsy, it is necessary to obtain a sufficient number of glomeruli, which are exclusively distributed in the renal cortex. A stereomicroscope or magnifying lens are usually used for this purpose; however, it is often difficult to clearly identify the cortex. Therefore, novel methods for the on-site assessment of renal biopsy specimens need to be established.
Gamma-glutamyl hydroxymethyl rhodamine green (gGlu-HMRG) is a recently developed activatable fluorescent probe (Urano et al., 2011). This probe is characterized by immediate fluorescence emission upon enzymatic catalysis by gamma-glutamyl transpeptidase (GGTP). gGlu-HMRG was originally developed for the detection of several types of cancer that express high levels of GGTP (Mitsunaga et al., 2013), and has not been applied to renal biopsy specimens.
Our recent work has focused on investigating the feasibility of gGlu-HMRG for the on-site assessment of renal biopsy specimens (Iyama et al., 2020). Renal cortex, in which most of the glomeruli are contained, showed rapid induction of fluorescence upon the incubation of gGlu-HMRG and could be clearly distinguished from renal medulla. We herein present a protocol for the induction of fluorescence by gGlu-HMRG with some modifications for better clarity of the images.
Materials and Reagents
Pipettes (M&S Instruments, catalog numbers: F144059M, F144058, and F144055M)
Pipette tips (Violamo, catalog numbers: V-1000, V-200, and V-10)
Eppendorf centrifuge tubes, 1.5 mL
6 cm dishes (AS ONE, catalog number: 2-8590-02)
Phosphate buffer saline (PBS) (FUJIFILM, catalog number: 166-23555)
Dimethyl sulfoxide (FUJIFILM, catalog number: 041-29351)
Normal saline (Otsuka Pharmaceutical Factory, catalog number: 035081517)
ProteoGREENTM-gGlu (GORYO Chemical, catalog number: GC801). Dissolve in dimethyl sulfoxide at 1 mM and store at -20 °C before use (unnecessary to filter)
Equipment
Stereomicroscope (BioTools, catalog number: BS-3048BT)
Fluorescent unit (BioTools, catalog number: BT-ExSM)
Band pass filter (FUJIFILM, catalog number: BPB-45). This filter should be set at the light source of the fluorescent unit
Sharp cut filter (FUJIFILM, catalog number: SC-52). This filter should be set at the lens side of the fluorescent unit (Figure 1)

Figure 1. Setup of the fluorescent unit for the imaging. Band pass filter and sharp cut filter should be set as illustrated.
Procedure
Preparation of the fluorescent probe
Thaw ProteoGREENTM-gGlu stock solution on ice (protect from light).
Dilute ProteoGREENTM-gGlu stock solution into PBS to prepare fluorescent solution (concentration of 50 μM).
Handling of renal biopsy specimen
Perform renal biopsy (Donovan et al., 1991).
Briefly rinse biopsy specimen with 10 mL of normal saline in 6 cm dish. Protect the specimen from drying.
Apply 100 μL of the fluorescent solution to the biopsy specimen. Incubate for 3 min under dark conditions at room temperature.
Illuminate and observe the specimen. Excitation wavelength of 450 nm passes through the band pass filter; the fluorescent solution is excited and emits fluorescence. Sharp cut filter eliminates redundant wavelengths except for fluorescence.
Capture images of the renal biopsy specimen at 2.0× magnification ratio. The corticomedullary junction of the specimen can be easily identified (Figure 2).
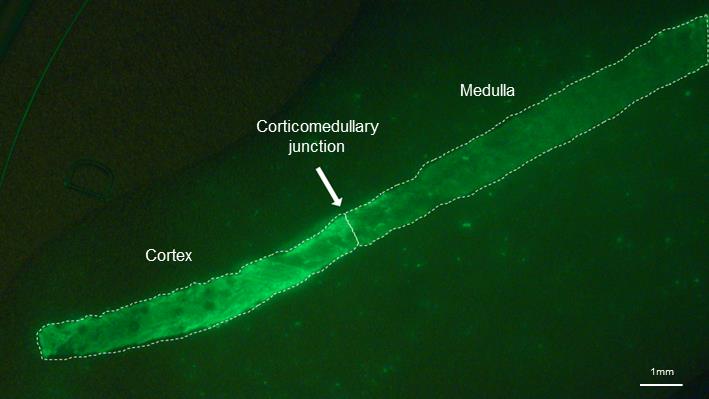
Figure 2. Fluorescent image of the renal biopsy specimen incubated with gGlu-HMRG. Cortex emits stronger fluorescence than the medulla. Scale bar = 1 mm.
Notes
Prepare fluorescent solution each time just before use.
Fluorescent solution should be applied to the specimen before fixation.
Renal carcinoma deriving from the renal proximal tubular epithelial cells might influence the fluorescence.
Acknowledgments
This protocol is based on “A novel method for assessing the renal biopsy specimens using an activatable fluorescent probe,” published in Scientific Reports (Iyama et al., 2020).
Competing interests
The authors declare that they have no financial or non-financial conflicts of interest.
Ethics
This study was conducted in accordance with the Declaration of Helsinki and approved by the ethical committee of Tottori University Hospital (approval number: 18A135). Informed consent was obtained from all subjects.
References
- Iyama, T., Takata, T., Yamada, K., Mae, Y., Taniguchi, S., Ida, A., Ogawa, M., Yamamoto, M., Hamada, S., Fukuda, S., et al. (2020). A novel method for assessing the renal biopsy specimens using an activatable fluorescent probe. Sci Rep 10(1): 12094.
- Mitsunaga, M., Kosaka, N., Choyke, P. L., Young, M. R., Dextras, C. R., Saud, S. M., Colburn, N. H., Sakabe, M., Nagano, T., Asanuma, D., et al. (2013). Fluorescence endoscopic detection of murine colitis-associated colon cancer by topically applied enzymatically rapid-activatable probe. Gut 62(8): 1179-1186.
- Urano, Y., Sakabe, M., Kosaka, N., Ogawa, M., Mitsunaga, M., Asanuma, D., Kamiya, M., Young, M. R., Nagano, T., Choyke, P. L., et al. (2011). Rapid cancer detection by topically spraying a gamma-glutamyltranspeptidase-activated fluorescent probe. Sci Transl Med 3(110): 110ra119.
- Donovan, K. L., Thomas, D. M., Wheeler, D. C., Macdougall, I. C. and Williams, J. D. (1991). Experience with a new method for percutaneous renal biopsy. Nephrol Dial Transplant 6(10): 731-733.
Article Information
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Takata, T., Isomoto, H., Iyama, T. and Yamada, K. (2022). A Novel Imaging Technique for The On-site Assessment of Renal Biopsy Specimens. Bio-protocol 12(18): e4517. DOI: 10.21769/BioProtoc.4517.
Category
Biophysics > Bioengineering > Medical biomaterials
Molecular Biology > Protein > Activity
Biochemistry > Other compound > Peptide
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









