- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Triple-challenge Mouse Model of Allergic Airway Disease, Primary Influenza Infection, and Secondary Bacterial Infection
Published: Vol 10, Iss 8, Apr 20, 2020 DOI: 10.21769/BioProtoc.3583 Views: 4531
Reviewed by: Kristin L. ShinglerAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

TCRβ Clonotype Analysis of EBV and CMV-specific Human CD8+ T Cells
Nening M. Nanlohy [...] Debbie van Baarle
Oct 5, 2015 8729 Views

Multicolor Stimulated Emission Depletion (STED) Microscopy to Generate High-resolution Images of Respiratory Syncytial Virus Particles and Infected Cells
Masfique Mehedi [...] Ursula J. Buchholz
Sep 5, 2017 10119 Views

TetR Regulated in vivo Repression Technology to Identify Conditional Gene Silencing in Genetically Engineerable Bacteria Using Vibrio cholerae Murine Infections as Model System
Franz G. Zingl [...] Stefan Schild
Oct 5, 2020 3744 Views
Abstract
Asthma is a global problem that affects millions of individuals. An increased risk of respiratory viral and bacterial infections is one of the complications of asthma. We recently reported that mice with ovalbumin-induced allergic airway disease (AAD) are protected against influenza-Streptococcus pneumoniae co-infection. Here, we describe in detail a protocol on how to induce AAD and influenza-S. pneumoniae co-infection in mice and to evaluate the specific roles of asthma on immunity to viral and bacterial pathogens in the hope of translating findings to benefit asthmatic individuals.
Keywords: AsthmaBackground
The global prevalence of individuals with asthma is increasing, with 300 million presently suffering and an additional 100 million new incidences predicted by 2025 (Nunes et al., 2017). Because of the altered immune system, asthmatic individuals are believed to have an increased risk of susceptibility to influenza infection. Seasonal and pandemic influenza infection can result in concurrent bacterial infection which can lead to airway respiratory distress syndrome, a potentially life-threatening condition (Gilca et al., 2011). Whether or not asthmatics are more susceptible and have reduced protective anti-influenza immune responses, is controversial. During the recent 2009 H1N1 pandemic, asthmatics were more likely to be hospitalized due to influenza. However, clinical data also suggest that asthmatics were less likely to die or require ICU admission than those without asthma. The majority of deaths during influenza pandemics are not caused by the viral infection per se, but instead, are due to complications from secondary bacterial infections (Morens et al., 2008; MacIntyre et al., 2018). We previously reported that mice with AAD are resistant to a single infection with influenza virus or S. pneumoniae (Furuya et al., 2015; Sanfilippo et al., 2015). However, there was no murine model that addressed the influence of allergic airway disease (AAD) on immunity to influenza virus and bacterial co-infection. Thus, we have developed a triple challenge model in mice to test the hypothesis that asthmatics are protected from severe influenza because they are less likely to develop severe secondary bacterial pneumonia (Roberts et al., 2019).
Materials and Reagents
- 1.5 ml conical tubes (Fisher Scientific, catalog number: 05-408-129 ), autoclaved
- 50 ml tubes (Corning, catalog number: 352070 )
- 1 ml TB syringes with 25 G needle (BD, catalog number: 309626 )
- 1.2 ml cryovial (Corning, catalog number: 430487 )
- Pipette tips
1,000 μl (VWR, catalog number: 16466-008 )
100 μl (VWR, catalog number: 53510-070 )
10 μl (VWR, catalog number: 89368-974 ) - Glass coverslips (Corning, catalog number: 2975245 )
- Adult 6- to 8-week-old BALB/c mice (Charles Rivers Bioscience or preferred vendor)
- Bacterial stock: S. pneumoniae serotype 3 strain can be obtained from American Tissue Culture Collection (ATCC) (ATCC, catalog number: ATCC® 6303 ) or S. pneumoniae serotype 2 strain D39 can be obtained from the National Center for Tissue Collection (National Center for Tissue Collection, catalog number: NCTC 7466 )
- Influenza A virus (H1N1) can be obtained from ATCC: PR8 (ATCC, catalog number: ATCC VR-1469 ) or the 2009 pandemic virus (ATCC, catalog number: ATCC VR-1894 )
- Albumin from chicken egg white (Sigma, catalog number: A2512 )
- Blood agar plates (Fisher Scientific, catalog number: R01623 )
- Aluminum hydroxide/Rehydragel (Fisher scientific, catalog number: NC1105454 )
- 1x Phosphate Buffered Saline (PBS) (Fisher Scientific, catalog number: 20-012-027 )
- House dust mite (HDM) extract, lyophilized Dermatophagoides pteronyssinus (Stallergenes-Greer, item# B85)
- Todd Hewitt broth (BD, catalog number: 249240 )
- Glycerol (MilliporeSigma, catalog number: G7893 )
- 2 mg/ml ovalbumin (OVA) solution (see Recipes)
- 1 mg/ml HDM stock solution (see Recipes)
- S. pneumoniae bacterial stock (see Recipes)
Equipment
- 250 ml beaker (Corning, catalog number: 1003250 )
- 1-L flask (Corning, catalog number: 4980-1L )
- Stir bar (Fisher scientific, catalog number: 14-513-51 )
- E-Z Anesthesia system (Euthanex Corp)
- ABSL-2 biosafety cabinet
- Pipette set (P20, P200, P1000) (Gilson, catalog number: F167300 )
- Isoflurane liquid inhalation (Henry Schein, catalog number: 1182097 )
- Centrifuge (Eppendorf, model: 5810R )
- Microscope (Olympus, model: BX60 )
- Microbiological Incubator (New Brunswick Scientific, Excella E24 incubator shaker series)
- Dissection instruments
Forceps (Roboz surgical, catalog number: RS-8120 )
Scissors (Roboz surgical, catalog number: RS-6808 ) - -80 °C freezer
Procedure
- Induction of ovalbumin (OVA)-induced AAD
- Prepare a stock solution of OVA at 2 mg/ml in PBS (Recipe 1).
- Thaw a vial of OVA stock solution and prepare OVA/Alum cocktail (100 µl of cocktail needed per mouse). This mixture contains 10 µg of OVA and 4 mg of Alum per 100 µl inoculum.
OVA/Alum cocktail:
70% 1x PBS
25% Aluminum hydroxide
5% OVA (2 mg/ml) - Transfer OVA/Alum cocktail into a 1 ml syringe and intraperitoneally (i.p.) inject 100 µl into each mouse on days -18 and -11 (see Figure 1 and Table 1). A fresh OVA/Alum cocktail should be made for each day.
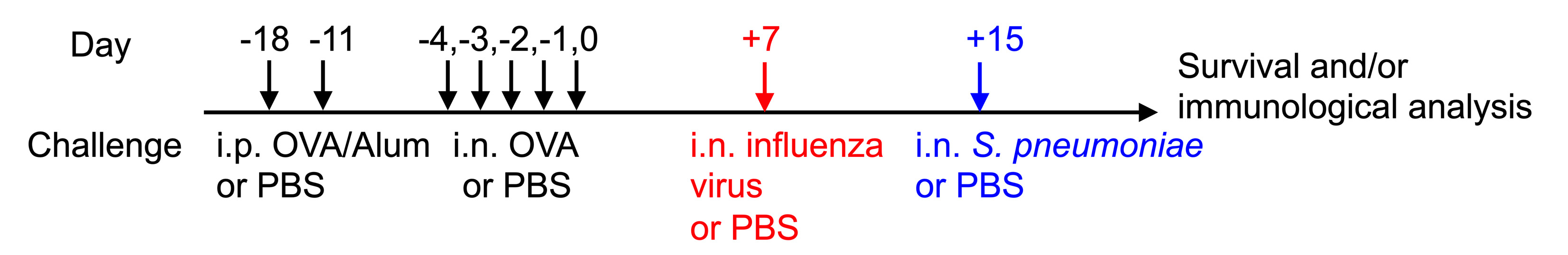
Figure 1. The ovalbumin (OVA)-induced AAD-influenza H1N1 virus–S. pneumoniae triple challenge model. The schematic diagram of OVA-induced AAD and influenza–S. pneumoniae co-infection in mice. The triple challenge model consists of treating mice intraperitoneally (i.p.) with OVA in Aluminum hydroxide (OVA/Alum) and challenging them intranasally (i.n.) with OVA followed by infection with influenza virus, Streptococcus pneumoniae (S. pneumoniae), and/or phosphate-buffered saline (PBS).
Table 1. Experimental layout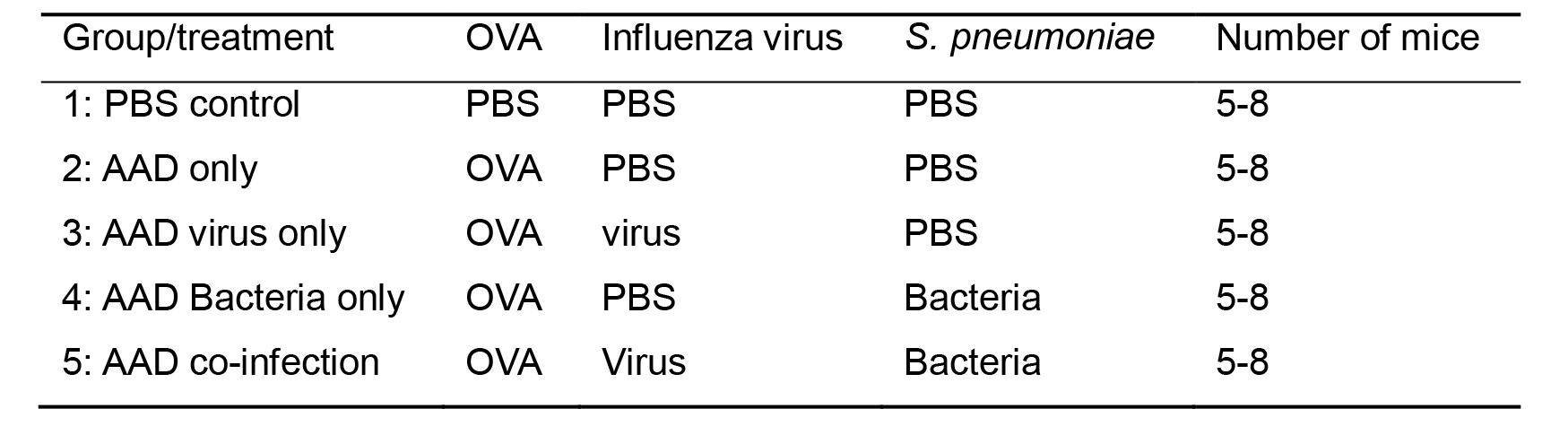
- On day -4, thaw a vial of OVA stock solution (2 mg/ml in PBS).
- Place the anesthesia chamber in the biosafety cabinet and “pre-charge” the anesthesia chamber with 3% isoflurane by following the manufacturer’s instructions.
- Place mice into the anesthesia chamber; mice are ready once their breathing has slowed. This usually occurs after 1-2 min in the chamber.
- Pick up an anesthetized mouse with one hand by grabbing the dorsal skin and positioning the mouse upright and facing you–please see Video 1 in Tibbitt and Coquet (2016).
- With the other hand, pipette 50 µl (100 µg) of the OVA stock into the nostril. Continue to hold the mouse upright for a few seconds to allow the inoculum to be completely taken up; heavy breathing indicates that the inoculum has reached the lungs.
- Repeat the intranasal (i.n.) OVA inoculation for 5 consecutive days. Excess OVA can be stored at -20 °C but discarded after 2-3 freeze-thaw cycles. Successful induction of AAD in mice can be confirmed by measuring allergic airway associated cytokines (interleukin (IL)-5 and IL-13), OVA-specific anti-IgE antibody concentrations by enzyme-linked immunosorbent assay (ELISA), cellular infiltration into the lung by flow cytometry, lung damage/inflammation by histology, and/or airway responsiveness to methacholine (as described in Roberts et al., 2019), our original paper.
We have used HDM as an alternative allergen to OVA and observed comparable results (Roberts et al., 2019). Mice are intranasally challenged with HDM for three consecutive days per week for three weeks before the influenza virus and subsequent bacterial infection (Figure 2):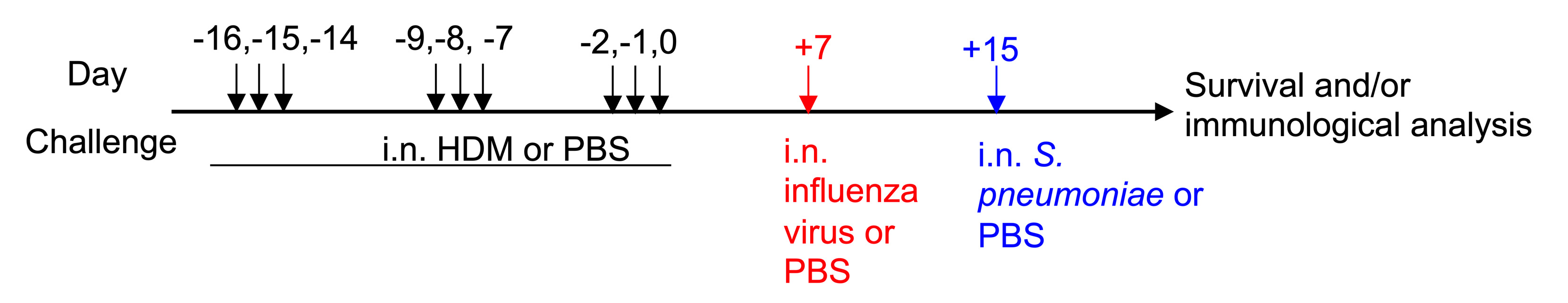
Figure 2. House-dust mite (HDM)-induced AAD and influenza-S. pneumoniae coinfection. Schematic diagram of HDM-induced AAD and subsequent influenza–S. pneumoniae coinfection.- Prepare a stock solution of HDM at 1 mg/ml in PBS (Recipe 2).
- On day -16, thaw a vial of stock solution of HDM (1 mg/ml in PBS).
- Place the anesthesia chamber in the biosafety cabinet and “pre-charge” the anesthesia chamber with isoflurane by following the manufacturer’s instructions.
- Pipette 50 µl (100 µg) of the HDM solution into the nostril of each mouse. Continue to hold mouse upright for a few seconds to allow the inoculum to be completely taken up. Excess HDM can be stored at -20 ℃ but discarded after 2-3 freeze-thaw cycles.
- Repeat HDM inoculation on subsequent days as outlined above.
- Influenza H1N1 virus and S. pneumoniae coinfection of AAD mice
Note: We produced a lab stock of influenza H1N1 A/California/4/2009 (CA04) and A/PR8/34 (PR8) in chicken eggs. See protocol for growing viruses (Cottey et al., 2001; Zhao et al., 2018) or purchase from vendors.
Influenza H1N1 virus challenge- On day 7 following the last OVA or HDM treatment, working in an ABSL-2 cabinet, dilute the influenza H1N1 virus stock to 200 plaque-forming units (PFU) per ml in sterile 1x PBS (Final dose: 10 PFU in 50 µl inoculum per mouse) and store on ice.
- Place the anesthesia chamber into the biosafety cabinet and pre-charge the anesthesia chamber with isoflurane by following the manufacturer’s instructions.
- Pipette 50 µl of the diluted influenza virus into the nostril of each mouse. Continue to hold the mouse upright for a few seconds to allow the inoculum to be completely taken up.
- Weigh mice individually or as a group either before or after influenza infection.
- Weigh daily until mice start to recover their weight.
Note: Sub-lethally infected mice will start losing weight around day 3 and will begin to recover on days 6-9. On average, mice typically lose about 10% of their starting weight with a low-dose influenza challenge.
Secondary S. pneumoniae challenge- On day 8 after influenza virus infection, in an ABSL-2 cabinet, thaw a vial of frozen S. pneumoniae stock (Recipe 3) and serially dilute it in 1.5 ml conical tubes to a sublethal dose in sterile 1x PBS and store on ice.
- For our stock of S. pneumoniae type 3 A66.1 strain, 100-500 colony forming units (CFU) is a sublethal dose for non-influenza-infected mice and is 90-100% lethal for influenza-infected mice.
- For our stock of serotype 2 D39 strain, 1 x 105 CFU is lethal for influenza-infected mice but sublethal for non-influenza-infected mice.
- To quantify the initial bacterial input, pipette 100 µl of the bacteria from the serially diluted tubes onto blood agar plates (aiming for 100-300 CFU per plate) and store in a 37 °C incubator overnight–count the number of CFU on the plates the following day to determine the actual infectious dose.
- Anesthetize mice using isoflurane, as described above.
- Infect mice with bacteria by pipetting 50 µl into the nostril.
- Monitor mice for survival or sacrifice and harvest bronchoalveolar lavage fluid and/or lungs to analyze cellularity, cytokine production, and/or histology for lung architecture.
Recipes
- 2 mg/ml OVA solution
- In a 250 ml beaker, add 200 mg of the OVA (Albumin from chicken egg) and bring the volume up to 100 ml with 1x PBS
- Place a stir bar into the mixture and stir for 10 min at room temperature
- Aliquot 1 ml into 1.5 ml conical tubes and store at -20 °C
- 1 mg/ml HDM stock solution
- In a 250 ml beaker, add 100 mg of lyophilized HDM extract and bring the volume up to 100 ml with 1x PBS
- Place a stir bar into the mixture and stir for 10 min at room temperature
- Aliquot 1 ml into 1.5 ml conical tubes and store at -20 °C
Note: Gently swirl solution during aliquoting to prevent HDM from settling.
- S. pneumoniae bacterial stock
Day 1:- Streak the frozen bacteria obtained from ATCC on a blood agar plate and incubate overnight in a 37 °C microbiological incubator
- Prepare and autoclave 500 ml of Todd-Hewitt broth by mixing 15 g of Todd-Hewitt media and distilled H2O to a final volume of 500 ml
- At 3:00 PM, create a starter culture by adding a single bacterial colony to 5 ml of Todd-Hewitt broth and allow the culture to grow without shaking at 37 °C until 5:00 PM
- At 5:00 PM, add 300 ml of Todd-Hewitt broth to a 1-L flask and inoculate with the 5 ml starter culture and allow the culture to grow overnight by placing it in a 37 °C microbiological incubator with no shaking
- Transfer the 300 ml overnight culture into 50 ml tubes and centrifuge at 1,000 x g for 35 min at 4 °C. Discard the supernatant
- Resuspend each pellet in 10 ml PBS and vortex to break up the pellet. Bring the volume up to 45 ml with PBS
- Repeat centrifugation and discard the supernatant
- Repeat steps b-c (Day 3), 2 more times
- Before the third spin, combine the aliquots (add 10 ml to each tube, vortex, then combine)
- After the final wash, add 2 ml of 15% glycerol/Todd-Hewitt broth and resuspend the pellet by gently pipetting up and down. Bring the final volume to 4 ml
Note: This typically results in a concentration of 1 x 109 -5 x 109 CFU per ml. - Add an aliquot to a coverslip and check the bacteria under the microscope to make sure that the majority are single cells and not long chains.
- Aliquot 130 μl into 1.2 ml cryovial tubes and store aliquots at -80 °C.
- To titer the bacterial stock, thaw a vial of the stock
- Make 10-fold serial dilutions in PBS in 1.5 ml conical tubes (make -1 to -8 dilutions)
- Plate the -4 to -8 dilutions on blood agar plates and incubate overnight in a microbiological incubator
- On the following day, count the plates with 100-300 colonies and calculate the concentration. For example, if there are 100 colonies on the -6 plate, the stock would be: (100 CFU counted/0.1ml plated) x 106 dilution = 1 x 109 CFU/ml stock concentration
Acknowledgments
An abbreviated protocol was previously published in Roberts et al. (2019). Y. F. was supported by the American Lung Association Biomedical Research Grant (RG341974) and is supported by American Heart Association Scientist Development Grant (17SDG33630188) and NIH (1R56AI146434-01).
Competing interests
We do not have any conflict of interest.
Ethics
Animal care and experimental protocols were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Albany Medical College (protocol number 17-03006, 05/19/2017-05/19/2020).
References
- Cottey, R., Rowe, C. A. and Bender, B. S. (2001). Influenza virus. Curr Protoc Immun 42(1): 19.11.11-19.11.32.
- Furuya, Y., Furuya, A. K., Roberts, S., Sanfilippo, A. M., Salmon, S. L. and Metzger, D. W. (2015). Prevention of Influenza Virus-Induced Immunopathology by TGF-β Produced during Allergic Asthma. PLoS Pathog 11(9): e1005180.
- Gilca, R., De Serres, G., Boulianne, N., Ouhoummane, N., Papenburg, J., Douville-Fradet, M., Fortin, É., Dionne, M., Boivin, G. and Skowronski, D. M. (2011). Risk factors for hospitalization and severe outcomes of 2009 pandemic H1N1 influenza in Quebec, Canada. Influenza Other Respir Viruses 5(4): 247-255.
- MacIntyre, C. R., Chughtai, A. A., Barnes, M., Ridda, I., Seale, H., Toms, R. and Heywood, A. (2018). The role of pneumonia and secondary bacterial infection in fatal and serious outcomes of pandemic influenza a(H1N1)pdm09. BMC Infect Dis 18(1): 637.
- Morens, D. M., Taubenberger, J. K. and Fauci, A. S. (2008). Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 198(7): 962-970.
- Nunes, C., Pereira, A. M. and Morais-Almeida, M. (2017). Asthma costs and social impact. Asthma Res Pract 3: 1.
- Roberts, S., Salmon, S. L., Steiner, D. J., Williams, C. M., Metzger, D. W. and Furuya, Y. (2019). Allergic airway disease prevents lethal synergy of influenza a virus-Streptococcus pneumoniae coinfection. MBio 10(4).
- Sanfilippo, A. M., Furuya, Y., Roberts, S., Salmon, S. L. and Metzger, D. W. (2015). Allergic Lung Inflammation Reduces Tissue Invasion and Enhances Survival from Pulmonary Pneumococcal Infection in Mice, Which Correlates with Increased Expression of Transforming Growth Factor β1 and SiglecFlow Alveolar Macrophages. Infect Immun 83(7): 2976-2983.
- Zhao, B., Shan, J., Xiong, R., Xu, K. and Li, B. (2018). H1N1 virus production and infection. Bio-protocol 8(20): e3062.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Roberts, S., Williams, C. M., Roy, S. and Furuya, Y. (2020). A Triple-challenge Mouse Model of Allergic Airway Disease, Primary Influenza Infection, and Secondary Bacterial Infection. Bio-protocol 10(8): e3583. DOI: 10.21769/BioProtoc.3583.
Category
Immunology > Animal model > Mouse
Microbiology > Microbe-host interactions > In vivo model > Mammal
Immunology > Complement analysis > Virus
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








