- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Histological Methods to Detect Early-stage Plant Defense Responses during Artificial Inoculation of Lolium perenne with Epichloë festucae
Published: Vol 11, Iss 9, May 5, 2021 DOI: 10.21769/BioProtoc.4013 Views: 6983
Reviewed by: Xiaofei LiangShweta PanchalSatyabrata Nanda

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Puromycin Proximity Ligation Assay (Puro-PLA) to Assess Local Translation in Axons From Human Neurons
Raffaella De Pace [...] Saikat Ghosh
Mar 5, 2025 3292 Views

Assay for Site-Specific Homologous Recombination Activity in Adherent Cells, Suspension Cells, and Tumor Tissues
Yuki Yoshino [...] Natsuko Chiba
Apr 5, 2025 2431 Views

Artificial Metalloenzymes in Artificial Sanctuaries Through Liquid–Liquid Phase Separation
Kaixin Wang [...] Tong Wu
Oct 5, 2025 1566 Views
Abstract
Epichloë species form agriculturally important symbioses with many cool season grasses. To study these symbioses, such as the interaction of Epichloë festucae with perennial ryegrass (Lolium perenne), host plants can be infected by artificial inoculation of etiolated seedlings. This inoculation is performed by placing mycelium into an incision in the meristem, as previously described by Latch and Christensen (1985). In recent years, this method has been broadly used to study this interaction at the molecular level using different Epichloë festucae mutants that can cause incompatible interactions. We have developed and adapted methods to study four of the most important host plant responses to infection, including cell death, callose deposition, lignin production, and hydrogen peroxide (H2O2) production, which are useful in defining the host response to infection at a very early time point.
Keywords: Endophytic fungiBackground
Although artificial inoculation of perennial ryegrass (Lolium perenne) is broadly used to study its interaction with Epichloë festucae, there exists no comprehensive protocol for the evaluation of responses to fungal infection at an early post-inoculation time point, which likely defines the future of this interaction. In our recent work (Rahnama et al., 2018), we adapted methods to study four of the most important host plant responses during plant-fungal interactions including cell death, callose deposition, lignin production, and hydrogen peroxide (H2O2) production.
In response to invading microbes, one of the early plant actions is the production of different types of reactive oxygen species (ROS), including H2O2, which act as an antimicrobial agent to protect the plant against invading microbes and are one of the first signals to induce other plant responses (Walters, 2003). In addition, by depositing callose and lignin, plants remodel their cell wall at the infection site to stop invading fungi (Luna Diez et al., 2010; Zipfel, 2009). As a late defense response, plant cells surrounding the infection site undergo programmed cell death (hypersensitive response) to limit available food for the invading microbe (Lo Presti et al., 2015). To initiate and maintain a successful symbiotic lifestyle, Epichloë endophytes must somehow suppress or avoid these different host defense responses; although, very little is known about how this occurs. Developing methods to detect these responses will aid in the understanding of this system, especially when applied to the study of mutant strains that compromise symbiosis.
To stain for callose deposition, we adapted an Aniline Blue staining method (Knox, 1979) that was originally used for the detection of callose in pollen tubes but has also been used in different fungal-plant interactions such as powdery mildew infection of Arabidopsis (Ellinger et al., 2013). Besides aniline blue, Toluidine Blue O has also been used in other systems such as guava root infection with Fusarium (Gupta et al., 2012). In addition to staining methods, there exist non-staining methods using immunofluorescence that detect callose at the micro-level in internal cell sites such as plasmodesmata (Pendle and Benitez-Alfonso, 2015).
To detect lignin deposition, we used a Safranin solution adapted from a study on maize infection by Ustilago maydis (Tanaka et al., 2014); however, Safranin is broadly used in other systems such as Fusarium infection of different cereals (Knight et al., 2011). Moreover, the Wiesner (phloroglucinol-HCl) reaction (Pomar et al., 2002) is another method to study lignin deposition in plant-fungal interactions.
To visualize cell death responses, Trypan Blue staining is the most widely used method, for which we adapted the protocol used to study Arabidopsis infection with downy mildew (Koch and Slusarenko, 1990). Recently, a non-toxic method has also been suggested, which uses red light imaging (Landeo Villanueva et al., 2021).
There are several methods to detect H2O2, one of the basic methods for which is the detection of the oxidization of small molecules, which we also used here. This method is based on visualizing the color change of 3,3’-diaminobenzidine (DAB) after being oxidized by H2O2. We adapted this method from a study on barley infection with powdery mildew (Thordal-Christensen et al., 1997). Other recent methods to study H2O2 have used genetically encoded green fluorescent protein (GFP)-based probes that react directly with H2O2 (reviewed in Winterbourn, 2018).
Here, we demonstrate that these methods are useful for defining the response of perennial ryegrass to infection with Epichloë endophyte at a very early time point. Using these methods in time-point studies, we defined the most suitable time to measure these responses post-inoculation. As such, these methods are useful for studying compatible and incompatible interactions of symbiotic fungi with their host plants during the early stages of infection (Rahnama et al., 2018 and 2019). Although these methods are routinely used for other fungal-plant interactions, our adaption of them to study the early inoculation stages of Epichloë-grass interactions is novel. These methods can be used in other grass-endophyte interactions that use a similar inoculation technique.
Materials and Reagents
1.5 ml Pierce microcentrifuge tubes (Thermo Scientific, catalog number: 69715)
Petri dishes (Fisher brand, Fisher Scientific, catalog number: FB0875712)
Microscope slides (Pearl, catalog number: 7101)
Microscope slide coverslips (Pearl)
1 ml Sterilin plastic transfer pipettes (Thermo Scientific, catalog number: 201C)
Surgical blade No.11 for metal scalpel 10621 (Feather, catalog number: 2976)
Household aluminum foil
Root trainers (Flight Plastic Ltd.)
Grass seeds (used here: Lolium perenne cv Samson seeds, endophyte-free; Agricom, New Zealand)
An Epichloë strain, E. festucae Fl1 (ATCC, catalog number: MYA-3407)
Autoclaved distilled water
Technical grade agar (Difco Laboratories, catalog number: DF0812-17-9)
Potato dextrose broth (Difco Laboratories, catalog number: DF0549-17-9)
L-lactate hydrate (C3H6O3) (Sigma-Aldrich, catalog number: L1750)
Glycerol (HOCH2CH(OH)CH2OH) (Sigma-Aldrich, catalog number: G9012)
Phenol (C6H5OH) (Thermo Scientific, catalog number: 17914)
Trypan Blue (C34H24N6O14S4Na4) (Sigma-Aldrich, catalog number: T6146)
Chloral hydrate (Cl3CCH(OH)2) (Sigma-Aldrich, catalog number: C8383)
Aniline Blue W.S. (C32H25N3Na2O9S3) (Sigma-Aldrich, catalog number: 28631-66-5)
Tripotassium orthophosphate (K3PO4) (VWR, catalog number: 700001)
Safranin O (C.I. 50240) (C20H19ClN4) (Sigma-Aldrich, catalog number: 1159480025)
3,3’-Diaminobenzidine (DAB) (Sigma-Aldrich, catalog number: D12384)
Hydrochloric acid (HCL) (Sigma-Aldrich, catalog number: D12384)
95% Ethanol (CH3CH2OH) (Sigma-Aldrich, catalog number: 11727)
Sucrose (Sigma-Aldrich, catalog number: S8501)
Boric acid (BH3O3) (Thermo Scientific, catalog number: AC315185000)
2.4% Potato dextrose agar (see Recipes)
3% Water agar (see Recipes)
Lactophenol-Trypan Blue solution (see Recipes)
Chloral hydrate solution (see Recipes)
Aniline Blue solution (see Recipes)
Pollen germination slides (see Recipes)
Safranin solution (see Recipes)
DAB solution (see Recipes)
Equipment
100 ml glass beaker
Leica DMR microscope (camera: Leica DC500)
Autoclave
Fridge
Laminar flow cabinet
Incubator (Thermo Scientific, 3110 CO2 Water-Jacketed Incubator)
Stereomicroscope (Leica, model: Leica M3Z)
Precellys 24 tissue disruptor (Bertin Technologies)
Stainless-steel forceps (Sigma-Aldrich, catalog number: Z168777)
Metal scalpel (Sigma-Aldrich, catalog number: S2646)
Procedure
Seedling preparation and inoculation (adapted from Latch and Christensen, 1985):
Seed surface sterilization:
Incubate endophyte-free seeds in 50% sulfuric acid (H2SO4) for 30 min.
Pour off the acid and soak the seeds 3 times in sterile water for 3 min each time.
Incubate the seeds in 50% commercial bleach for 20 min.
Pour off and soak the seeds 3 times in sterile water for 2 min each time.
Dry the seeds on sterile filter paper in a laminar flow hood.
Seed culture:
Using sterile forceps, transfer the sterile dried seeds to 4% water agar (10 seeds per plate).
Incubate the plates in the dark at 22°C for 7 days.
Mycelium preparation for inoculation:
Subculture the Epichloë strain onto a PDA plate.
Incubate the plate at 20°C for 7-10 days.
Seedling inoculation:
After 7-8 days, the seeds will germinate with etiolated shoots.
Using a dissecting microscope in a laminar flow hood, make a small incision in the meristem (usually appears as a faintly visible line between the mesocotyl and the coleoptile) with a scalpel.
Cut a small piece of fungal mycelium from the PDA plate (around 2 mm × 2 mm) and insert into the incision in the meristem.
Incubate the seedlings in their original water agar plates at 22°C in the dark, with the plates standing on their end and the seedlings upright.
Subsequent steps are described in detail (including videos and figures) in Becker et al. (2018) .
In all plant response tests, there are 4 inoculation treatments including seedlings with an incision inoculated with wild type E. festucae (“Wild Type”), mutant ΔvelA E. festucae (“ΔvelA”), a block of agar without fungi (“E- (cut)”), and seedlings without an incision but a piece of agar placed over the meristem (“E- (Uncut)”).
Staining for defense responses
Inoculated seedlings were incubated for the different plant response tests as follows:
Callose deposition (adapted from Knox, 1979):
Impatiens walleriana pollen tubes can be used as a positive control to test whether the Aniline Blue solution is working properly.
Sprinkle pollen over a freshly prepared pollen germination slide.
Store the slides in a moist Petri dish (containing wet tissue paper) for at least 5 h until germination occurs.
Cover the slide with a few drops of Aniline Blue solution and incubate at room temperature for 30 min.
Rinse the slides twice with distilled water.
Observe callose deposition in the pollen tubes using a fluorescence microscope (excitation 450-490 nm, emission >515 nm).
Callose deposition appears as a yellow-green color (Figure 1A).
Studying callose deposition at different time points determined that 4 days post-inoculation (DPI) was optimal for Epichloë-ryegrass associations.
At 4 DPI, place the seedlings in Aniline Blue solution for 30 min at room temperature.
Rinse the seedlings twice with distilled water.
Remove the inoculum (fungal block, or agar block in the negative controls) from the meristem and cut out the meristem section of the seedlings including the 1.5 cm above and below.
Observe callose deposition under a fluorescence microscope (excitation 450-490 nm, emission >515 nm).
Callose deposition appears as a yellow-green color (Figure 1B).
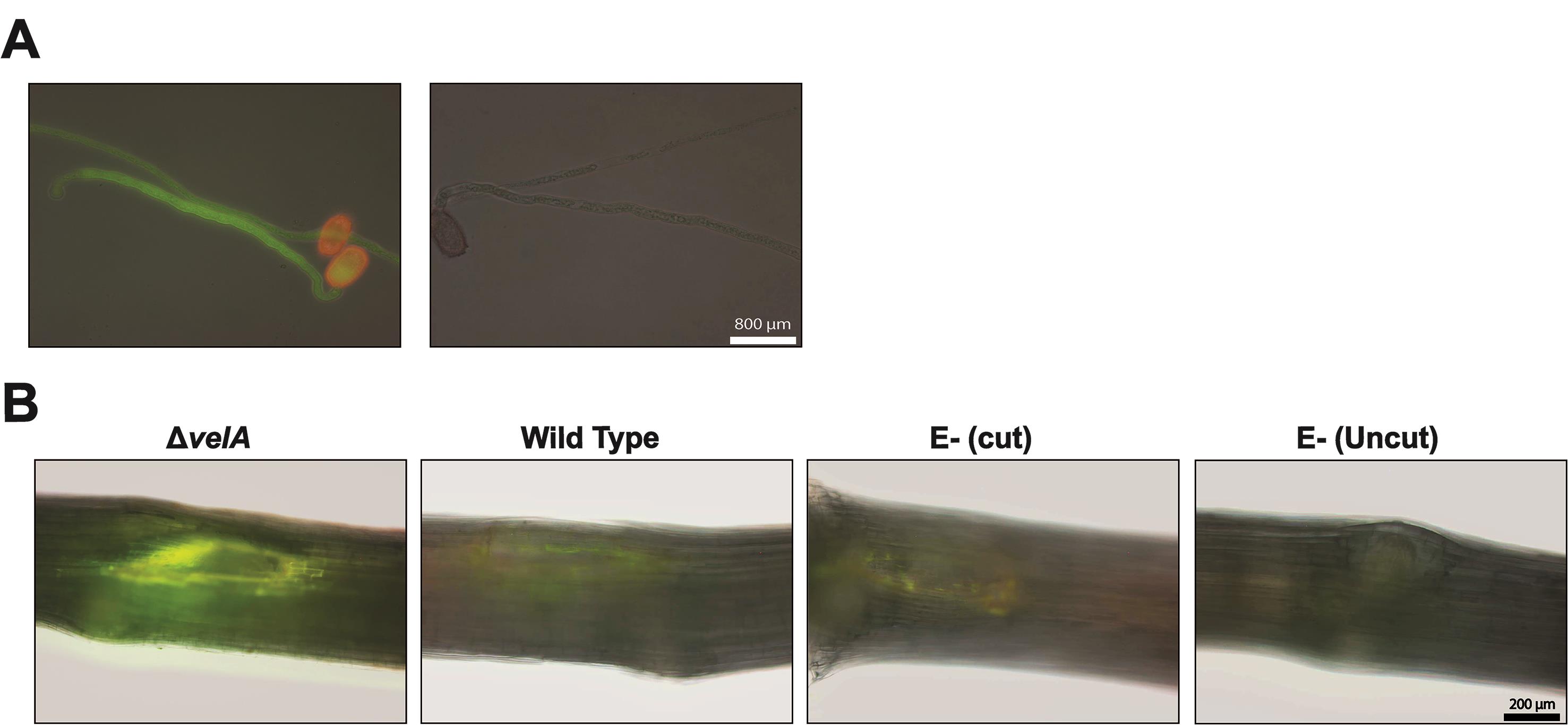
Figure 1. Callose detection. A. Using Impatiens walleriana pollen tubes as a positive control for callose detection. Left panel; pollen tubes in Aniline Blue solution, right panel; pollen tubes in only buffer without Aniline Blue. B. Callose deposition in the meristematic region of 7-d-old seedlings inoculated with wild type and ΔvelA mutant strains of E. festucae and endophyte-free cut and uncut seedlings inoculated with agar block as a control; 4 DPI stained with Aniline Blue solution. Callose deposition appears yellow-green.Lignin production (adapted from Tanaka et al., 2014):
Studying lignin production at different time points determined that 2.5 DPI was optimal for Epichloë-ryegrass associations.
At 2.5 DPI, place the seedlings in Safranin solution for 10 min in the dark at room temperature.
Rinse the seedlings twice with distilled water
Remove the inoculum (fungal block, or agar block in the negative controls) from the meristem and cut out the meristem section of the seedlings including the 1.5 cm above and below.
Observe lignin under a fluorescence microscope in brightfield.
Lignin deposition appears as a red color (Figure 2).
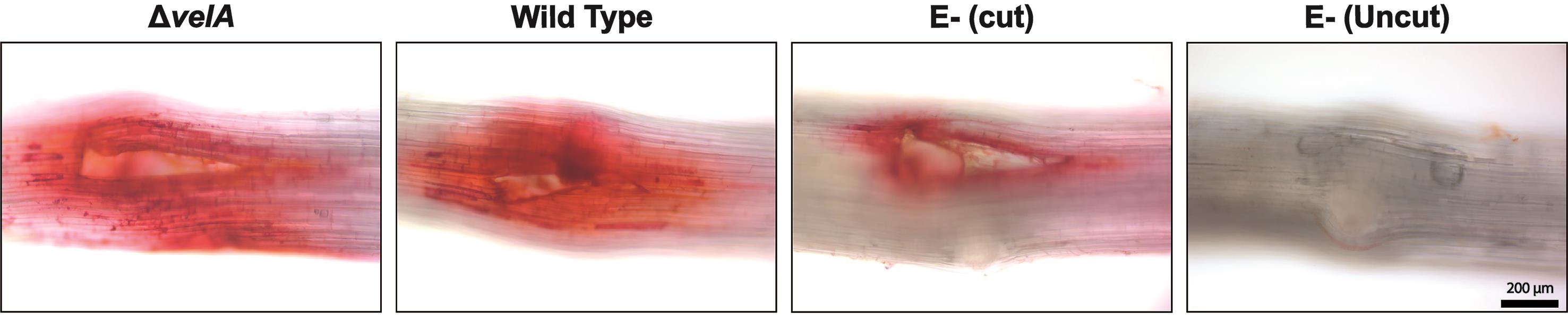
Figure 2. Lignin detection in the meristematic region of 7-d-old seedlings inoculated with wild type and ΔvelA mutant strains of E. festucae and endophyte-free cut and uncut seedlings inoculated with agar block as a control; 4 DPI stained with 1% Safranin O solution. Lignin deposition appears red.Plant cell death (adapted from Koch and Slusarenko, 1990):
The highest levels of plant cell death were observed at 7 days post-inoculation (DPI), but a time-course study can be performed from 1-12 DPI.
At 7 DPI, place whole seedlings in 20 ml boiling lactophenol-Trypan Blue for 1 min. Since this solution contains corrosive phenol and requires boiling, this step should be carried out under the laminar flow cabinet. First, warm the solution in a 100-ml glass beaker over a Bunsen burner until boiling, then add the seedlings.
Decolorize the stained seedlings by placing in 20 ml chloral hydrate solution for 30 min. Since chloral hydrate is toxic, this step should be carried out under the laminar flow cabinet.
Rinse the seedlings twice with distilled water.
Remove the inoculum (fungal block or agar block in the negative controls) from the meristem and cut out the meristem section of the seedlings including the 1.5 cm above and below.
Mount the seedling sections on slides with coverslips in water.
Observe the cell death response under a Nikon Ti-E inverted microscope (camera: Nikon DsRi1) in brightfield.
Dead cells appear dark blue (Figure 3).
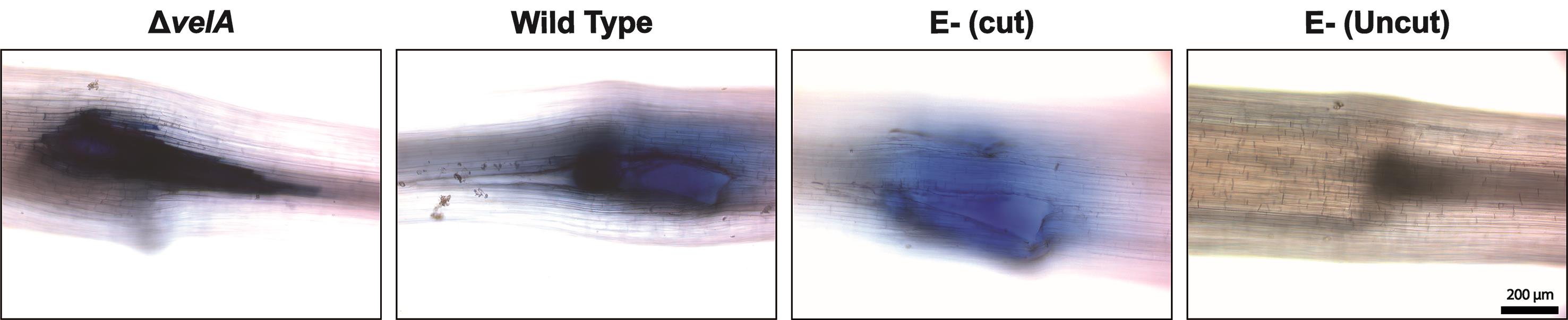
Figure 3. Cell death detection in the meristematic region of 7-d-old seedlings inoculated with wild type and ΔvelA mutant strains of E. festucae and endophyte-free cut and uncut seedlings inoculated with agar block as a control; 4 DPI stained with lactophenol-Trypan Blue solution. Dead cells appear dark blue.Hydrogen peroxide (H2O2) production (adapted from Thordal-Christensen et al., 1997):
Detecting H2O2 production after the normal inoculation procedure
Most H2O2 production should be detected immediately after inoculation.
Immediately after inoculation, place whole seedlings in freshly prepared DAB solution for 4 h in the dark at room temperature.
Rinse the seedlings twice with distilled water
Remove the inoculum (fungal block, or agar block in the negative controls) from the meristem and cut out the meristem section of the seedlings including the 1.5 cm above and below.
Observe H2O2 production under a Leica DMR microscope in brightfield.
H2O2 appears as a brown color (Figure 4A).
Detecting H2O2 production without incision
Since cutting the meristem produces a high level of H2O2, making the differentiation between treatments difficult, we optimized the methodology to measure H2O2 in the absence of an incision.
Place 1-2 mm2 fungal culture on agar blocks 1 cm above the meristem.
Immediately after inoculation, place whole seedlings in freshly prepared DAB solution for 4 h in the dark at room temperature.
Remove the inoculum (fungal block, or agar block in the negative controls) from the meristem and cut out the meristem section of the seedlings including the 1.5 cm above and below.
Observe H2O2 production under a Leica DMR microscope in brightfield.
H2O2 appears as a brown color (Figure 4B).
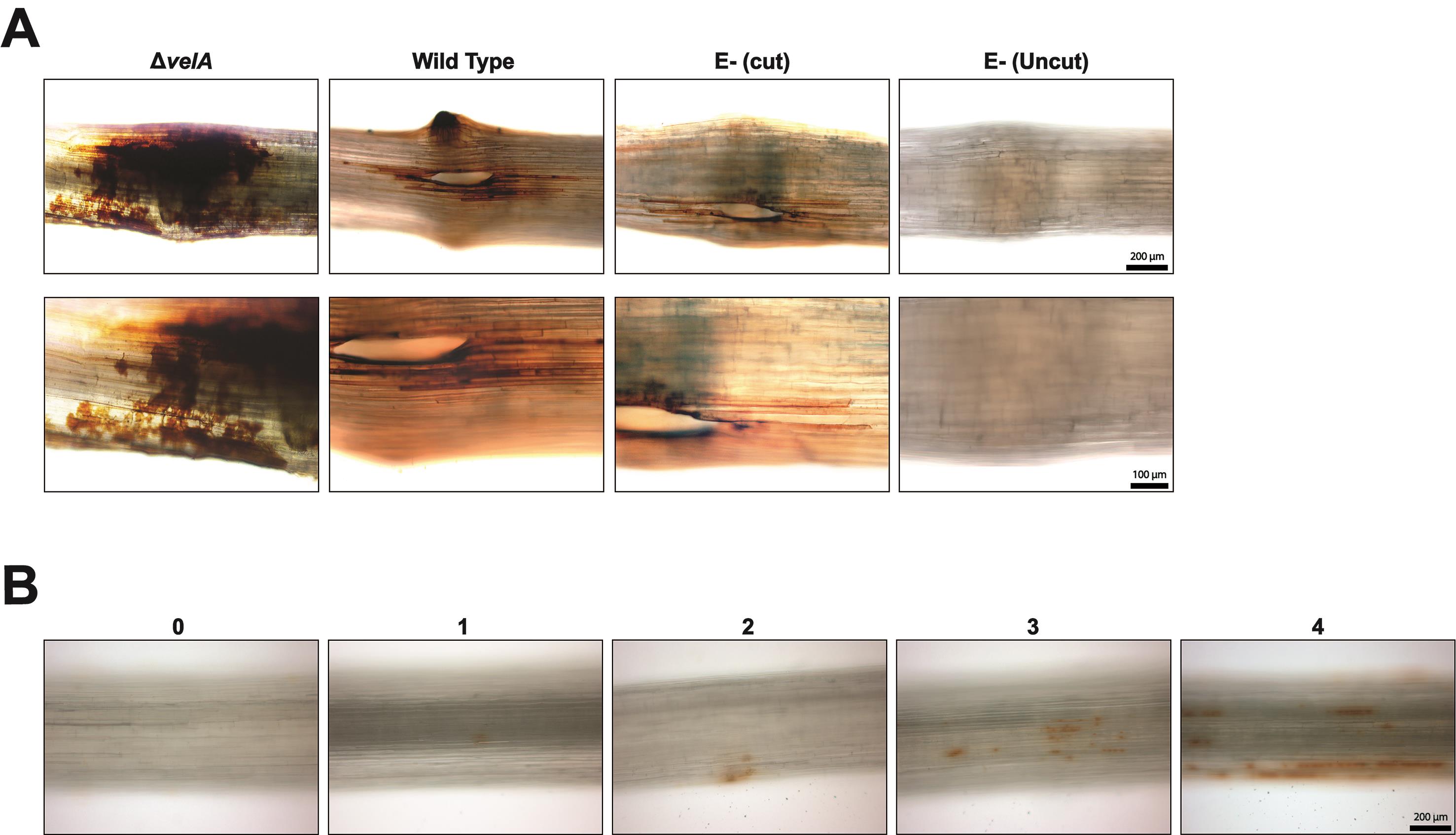
Figure 4. Hydrogen peroxide (H2O2) detection. A. H2O2 production in the meristematic region of 7-d-old seedlings inoculated with wild type and ΔvelA mutant strains of E. festucae and endophyte-free cut and uncut seedlings inoculated with agar block as a control; immediately stained with DAB solution. B. H2O2 response 1 cm above the meristem of 7-d-old seedlings was detected immediately after inoculating seedlings using method 4b (Detecting H2O2 production without incision). Arbitrary scoring system for measuring the severity of the response. H2O2 appears brown.
Data analysis
For all detection methods and for each treatment, a range of responses can be observed because ryegrass is an out-crossing species; therefore, each plant has a different genotype. As such, we used an arbitrary categorization method based on the percentage of seedlings that show the response. For all the methods (except 4b; Detecting H2O2 production without incision), data analyses are based on the percentage of seedlings showing the test response (similar to Figure 1B, first on the left). As an example, the results of callose deposition in seedlings inoculated with different strains of E. festucae are presented in Figure 5.
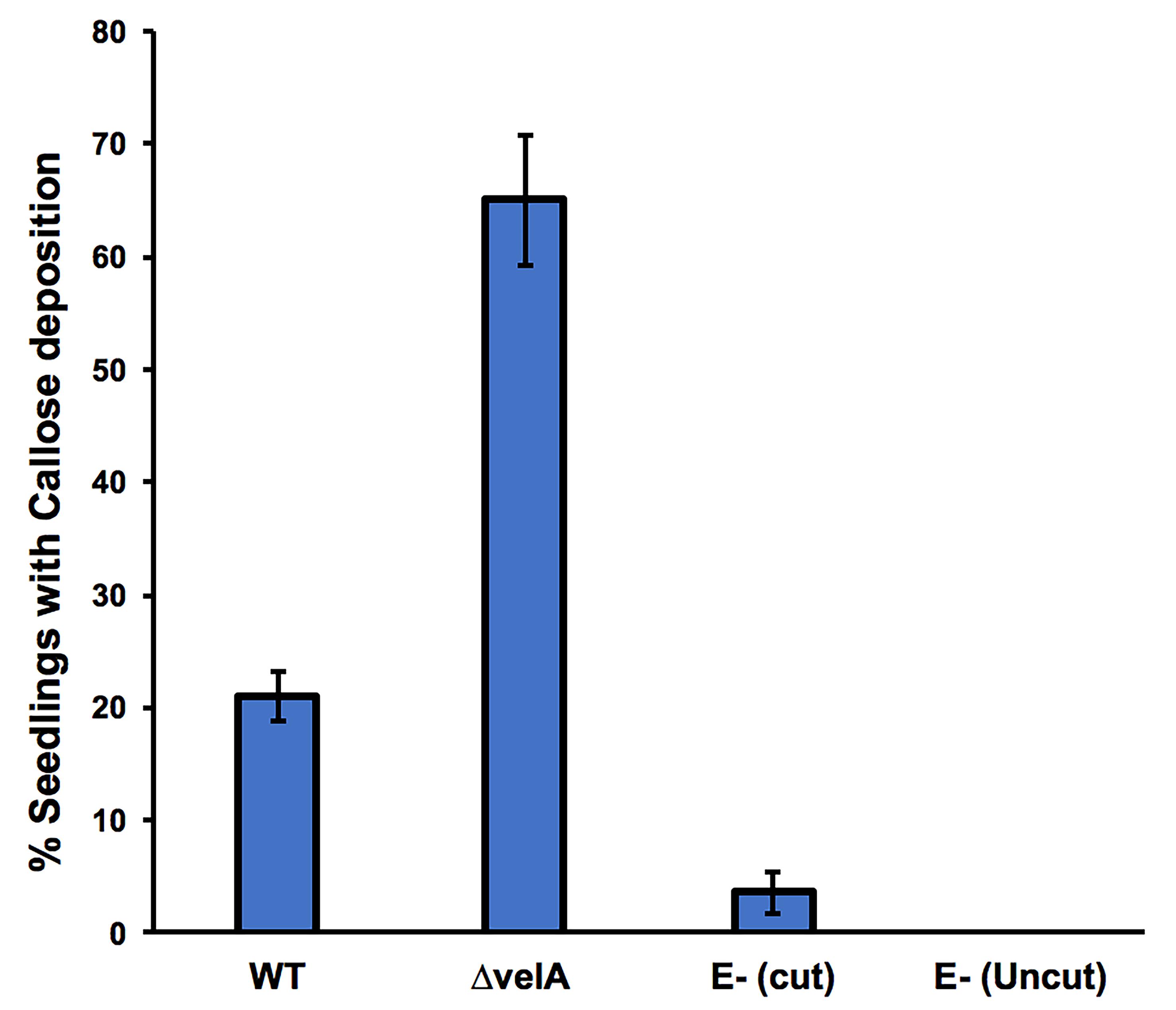
Figure 5. Percentage of 7-d-old seedlings showing callose deposition at 4 days post-inoculation. Seedlings were inoculated with wild type and ΔvelA mutant strains of E. festucae, and endophyte-free cut and uncut seedlings were inoculated with agar block as a control. In each inoculation, 30 seedlings were inoculated. Bars represent the standard error of the mean calculated from 3 independent inoculation experiments.
To detect H2O2 production without incision (method 4b), a range of 5 different responses (0 to 4) was used (Figure 4B) due to the sensitivity of the method. The percentage of seedlings showing an H2O2 response can be visualized in Figure 6.
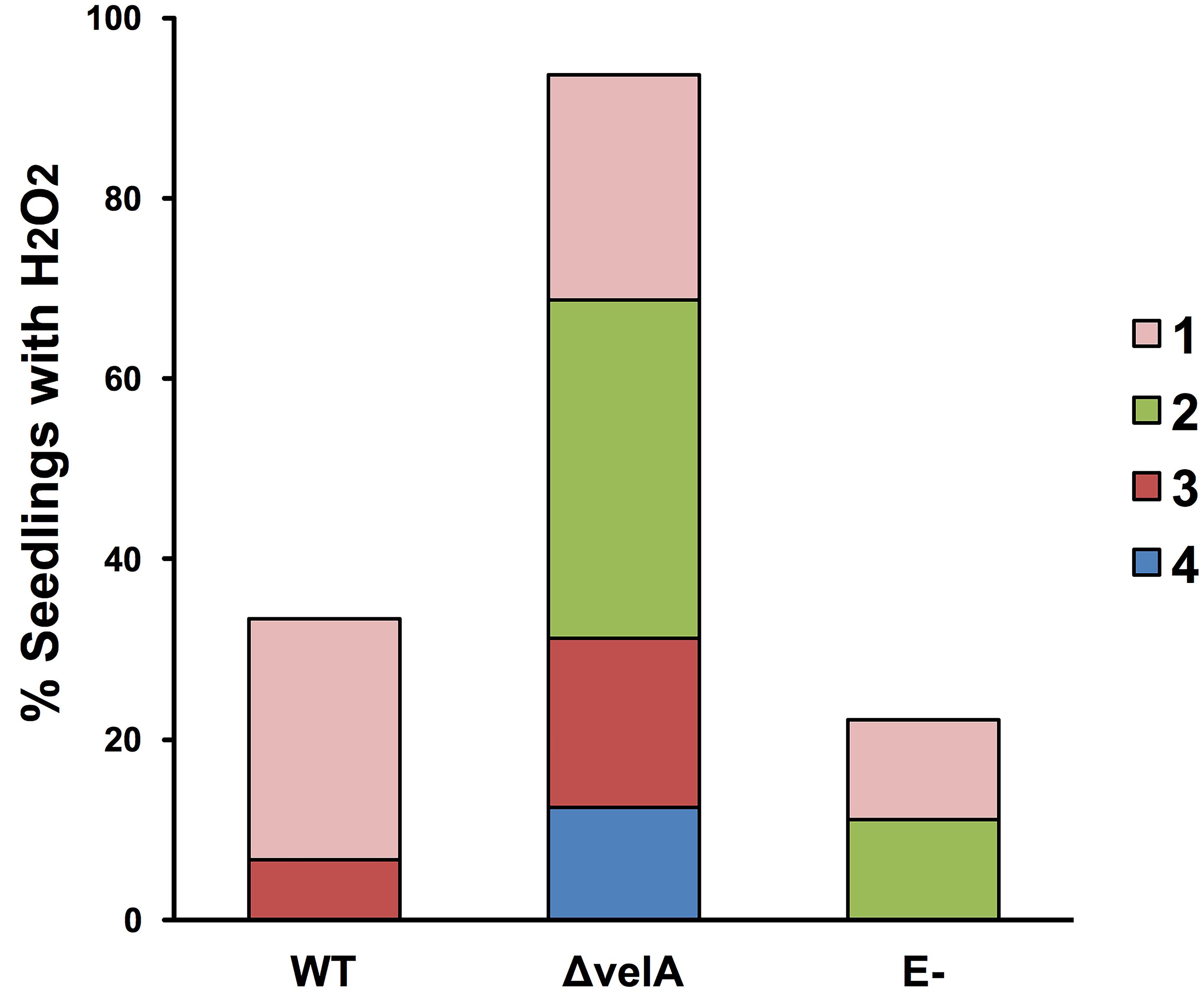
Figure 6. Percentage of 7-d-old seedlings showing the production of hydrogen peroxide (H2O2). The H2O2 response 1-cm above the meristem of 7-d-old seedlings was detected immediately after inoculating seedlings using method 4b (detecting H2O2 production without incision) with wild type (WT) and ΔvelA mutant strains of E. festucae and agar block as a control (E-). In each inoculation, 30 seedlings were inoculated. Numbers 1-4 in the legend represent arbitrary categorization of the H2O2 responses represented in Figure 4B.
Notes
Three independent inoculation tests were performed for each method.
The number of seedlings used for each test should be greater than 30, and increasing the number of seedlings can help to reduce the variability of the responses that can result from differences in genetic background of the seeds.
In all the detection methods, we normally stain whole seedlings and cut out the meristem region (region of interest) for microscopy. This reduces host responses due to wounding that can interfere with microscopy.
Recipes
2.4% potato dextrose agar
Dissolve 24 g potato dextrose broth and 15 g agar in 1 L distilled water
Autoclave for 30 min at 121°C and pour 25 ml solution per Petri dish
Plates can be stored in the fridge for up to one month
3% water agar
Dissolve 30 g agar in 1 L distilled water
Autoclave for 30 min at 121°C and pour 25 ml solution per Petri dish
Plates can be stored in the fridge for up to one month
Lactophenol-trypan blue solution
Dissolve 10 mg trypan blue in 10 ml distilled water and add 10 ml lactic acid (98%), 10 ml glycerol, and 10 ml phenol
The solution can be stored in the dark in the fridge for up to 3 months
Chloral hydrate solution
Dissolve 2.5 g chloral hydrate in 1 ml distilled water
The solution can be stored in the fridge for up to 1 month
Aniline blue solution
Dissolve 0.1 g aniline blue and 2.3 g tripotassium orthophosphate in 100 ml distilled water
Place the solution in the dark for 24 h until it becomes colorless
The solution can be stored in the dark in the fridge for up to 3 months
Pollen germination slides
Dissolve 10 g sucrose, 1 g agar, and 8 mg boric acid in 100 ml distilled water
Autoclave for 30 min at 121°C and drop on a slide to make a flat film
Slides can be stored in the fridge for up to one week
Safranin solution
Dissolve 1 g Safranin O (C.I. 50240) in 100 ml distilled water
The solution can be stored in the dark in the fridge for up to 3 months
DAB solution
Dissolve 10 mg 3,3’-diaminobenzidine (DAB) in 10 ml distilled water
Adjust the pH to 3.8 using HCl
The solution should be prepared fresh for each test
Acknowledgments
This research was funded by grants from the Royal Society of New Zealand Marsden Fund, contract AGR1002, and the New Zealand Strategic Science Investment Fund, contract A20067. We thank Adrian Turner (Microscopy & Graphics Unit, University of Auckland), C.R. Voisey, W.R. Simpson, W. Mace, and A. deBonth for technical assistance (Forage Improvement, AgResearch Grasslands), and Biotelliga Ltd. for providing laboratory space.
Competing interests
The authors declare no conflicts of interest.
References
- Latch, G. C. M. and Christensen, M. J. (1985). Artificial infection of grasses with endophytes. Ann Appl Biol 107: 17-24.
- Becker, Y., Green, K. A., Scott, B. and Becker, M. (2018). Artificial inoculation of Epichloë festucae into Lolium perenne, and visualisation of endophytic and epiphyllous fungal growth. Bio-protocol 8(17): e2990.
- Ellinger, D., Naumann, M., Falter, C., Zwikowics, C., Jamrow, T., Manisseri, C., Somerville, S. C. and Voigt, C. A. (2013). Elevated early callose deposition results in complete penetration resistance to powdery mildew in Arabidopsis.Plant Physiol 161(3): 1433-1444.
- Gupta, V. K., Misra, A. K. and Pandey, B. K. (2012). Histological changes in guava root during wilting induced by Fusarium spp. Arch Phytopathology Plant Protect 45(5): 570-573.
- Knox, R. B. (1979). Pollen and allergy. Studies in Biology. No. 107. Arnold, London.
- Koch, E. and Slusarenko, A. (1990). Arabidopsis is susceptible to infection by a downy mildew fungus. Plant Cell 2(5): 437-445.
- Lo Presti, L., Lanver, D., Schweizer, G., Tanaka, S., Liang, L., Tollot, M., Zuccaro, A., Reissmann, S. and Kahmann, R. (2015). Fungal effectors and plant susceptibility. Annu Rev Plant Biol 66: 513-545.
- Luna, E., Pastor, V., Robert, J., Flors, V., Mauch-Mani, B. and Ton, J. (2011). Callose deposition: a multifaceted plant defense response. Mol Plant Microbe Interact 24(2): 183-193.
- Pendle, A. and Benitez-Alfonso, Y. (2015). Immunofluorescence detection of callose deposition around plasmodesmata sites. In: Heinlein, M. (Ed.). Plasmodesmata. Methods in Molecular Biology (Methods and Protocols), vol 1217, pp95-104.Humana Press, New York, NY.
- Pomar, F., Merino, F. and Barcelo, A. R. (2002). O-4-Linked coniferyl and sinapyl aldehydes in lignifying cell walls are the main targets of the Wiesner (phloroglucinol-HCl) reaction. Protoplasma 220(1-2): 17-28.
- Rahnama, M., Johnson, R. D., Voisey, C. R., Simpson, W. R. and Fleetwood, D. J. (2018). The global regulatory protein vela is required for symbiosis between the endophytic fungus Epichloë festucae and Lolium perenne. Mol Plant Microbe Interact 31(6): 591-604.
- Rahnama, M., Maclean, P., Fleetwood, D. J. and Johnson, R. D. (2019). The LaeA orthologue in Epichloë festucae is required for symbiotic interaction with Lolium perenne. Fungal Genet Biol 129: 74-85.
- Tanaka, S., Brefort, T., Neidig, N., Djamei, A., Kahnt, J., Vermerris, W., Koenig, S., Feussner, K., Feussner, I. and Kahmann, R. (2014). A secreted Ustilago maydis effector promotes virulence by targeting anthocyanin biosynthesis in maize. Elife 3: e01355.
- Thordal-Christensen, H., Zhang, Z., Wei, Y. and Collinge, D. B. (1997). Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley—powdery mildew interaction. The Plant J 11(6): 1187-1194.
- Landeo Villanueva, S., Malvestiti, M. C., van Ieperen, W., Joosten, M. and van Kan, J. A. L. (2021). Red light imaging for programmed cell death visualization and quantification in plant-pathogen interactions. Mol Plant Pathol 22(3): 361-372.
- Walters, D. R. (2003). Polyamines and plant disease. Phytochemistry 64(1): 97-107.
- Winterbourn, C. C. (2018). Biological Production, Detection, and Fate of Hydrogen Peroxide. Antioxid Redox Signal 29(6): 541-551.
- Zipfel, C. (2009). Early molecular events in PAMP-triggered immunity. Curr Opin Plant Biol 12(4): 414-420.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Rahnama, M., Fleetwood, D. J. and Johnson, R. D. (2021). Histological Methods to Detect Early-stage Plant Defense Responses during Artificial Inoculation of Lolium perenne with Epichloë festucae. Bio-protocol 11(9): e4013. DOI: 10.21769/BioProtoc.4013.
Category
Plant Science > Plant biochemistry
Microbiology > Microbe-host interactions
Biochemistry > Other compound > Lignin
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









