- Protocols
- Articles and Issues
- About
- Become a Reviewer
Past Issue in 2025
Volume: 15, Issue: 12
Biochemistry
Surface Plasmon Resonance for the Interaction of Capsular Polysaccharide (CPS) With KpACE
A Novel Protein Purification Approach Using Elastin-Like Polypeptides (ELP) With His-Tag Assistance
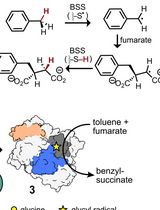
Activation of X-Succinate Synthases for Fumarate Hydroalkylation Using an In Vitro Activation Method
Expression and Purification of the Human Voltage-Gated Proton Channel (hHv1)
Protein Structural Characterization Using Electron Transfer Dissociation and Hydrogen Exchange-Mass Spectrometry
Bioinformatics and Computational Biology
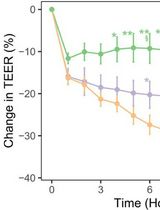
Method for Extracellular Electrochemical Impedance Spectroscopy on Epithelial Cell Monolayers
Biological Engineering
Protocol of Whey Protein Isolate–Based Microgel Targeted Delivery in Mouse Kidney
Cancer Biology
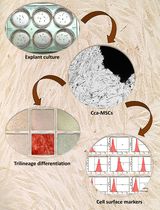
Isolation and Characterization of Cervical Cancer-Associated Mesenchymal Stem Cells From Primary Tumors Using Explant Culture
Cell Biology
Optimized Midgut Tissue Dissociation of Mosquitoes and Sandflies for High-Quality Single-Cell RNA Sequencing
Primary Mouse Choroidal Endothelial Cell Culture
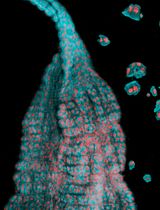
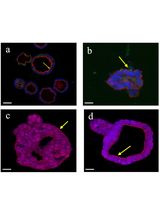
The Establishment of 3D Polarity-Reversed Organoids From Human Endometrial Tissue as a Model for Infection-Induced Endometritis
Developmental Biology
Cloning-Free Targeting of Endogenous Loci to Generate Fluorescent Reporters in Medaka
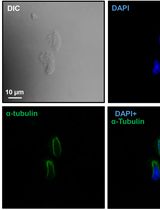
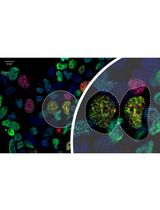
Preparation of Testicular Cells for Immunofluorescence Analysis of Manchette in Elongating Spermatids
Microbiology
In Vitro Co-culture of Bacterial and Mammalian Cells to Investigate Effects of Potential Probiotics on Intestinal Barrier Function
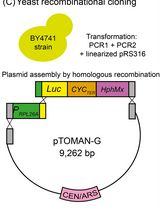
High-Throughput Indirect Monitoring of TORC1 Activation Using the pTOMAN-G Plasmid in Yeast
Molecular Biology
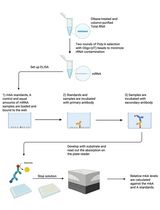
An Improved m6A-ELISA for Quantifying N6-methyladenosine in Poly(A)-purified mRNAs
Ub-POD: A Ubiquitin-Specific Proximity-Dependent Labeling Technique to Identify E3 Ubiquitin Ligase Substrates in Human Cells
An Optimized RNA Extraction Method From Micro-quantities of Guinea Pig Cartilage and Synovium for Osteoarthritis Research
Neuroscience
An Optimized Ex Vivo Protocol for Quantitative Electrophysiological Assessment of Neuromuscular Junctions and Skeletal Muscle Function Using the Aurora System
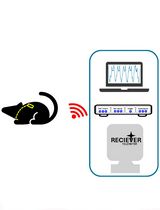
Surgical Implantation of a Telemetry-Based Pressure Sensor in the Internal Jugular Vein to Monitor Respiration Wirelessly
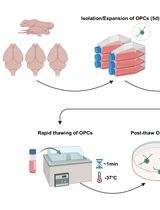
Cryopreservation of Bulk-Produced Primary Rat Oligodendrocyte Progenitor Cells
Stem Cell
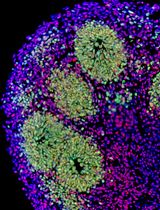
A Hybrid 2D/3D Approach for Neural Differentiation Into Telencephalic Organoids and Efficient Modulation of FGF8 Signaling