Advanced Search
GeneWeld: a method for efficient targeted integration directed by short homology
Last updated date: Nov 7, 2020 Views: 1568 Forks: 0
Jordan M. Welker1, Wesley A. Wierson1, Maira P. Almeida1, Carla M. Mann1, Melanie E. Torrie1, Stephen C. Ekker6, Karl J. Clark6, Drena L. Dobbs1, Jeffrey J. Essner1*, Maura McGrail1*
- Department of Genetics, Development and Cell Biology, Iowa State University, IA, USA
- Department of Biochemistry and Molecular Biology, Mayo Clinic, Rochester, MN, USA
*Corresponding authors: jessner@iastate.edu and mmcgrail@iastate.edu
GeneWeld protocol for targeted Integration in zebrafish using CRISPR/Cas9 and pGTag vectors.
This protocol is associated with the publication Wierson, Welker, Almeida et al., Efficient targeted integration directed by short homology in zebrafish and mammalian cells. 2019 eLife.
Written by J.M. Welker
Table of Contents
- Introduction to GeneWeld Targeting strategy (Figure 1)
- Selection of a CRISPR/spCas9 target site in the gene of interest
- Ordering sgRNAs and in vitro synthesis of spCas9 mRNA
- Injection of sgRNA and spCas9 mRNA
- Testing for indel production/mutagenesis
- Design of short homology arms
- One Pot Cloning of Homology Arms into pGTag Vectors
- Injection of GeneWeld reagents (spCas9 mRNA, Universal sgRNA (UgRNA), genomic sgRNA and pGTag short homology vector) into 1-cell zebrafish embryos
- Confirmation of targeted integration by fluorescence microscopy and junction analysis
A. Introduction
The GeneWeld strategy and pGTag vector series are designed for straightforward assembly of vectors containing short homology arms for efficient CRISPR/Cas9 directed recovery of germline precision targeted integration alleles.
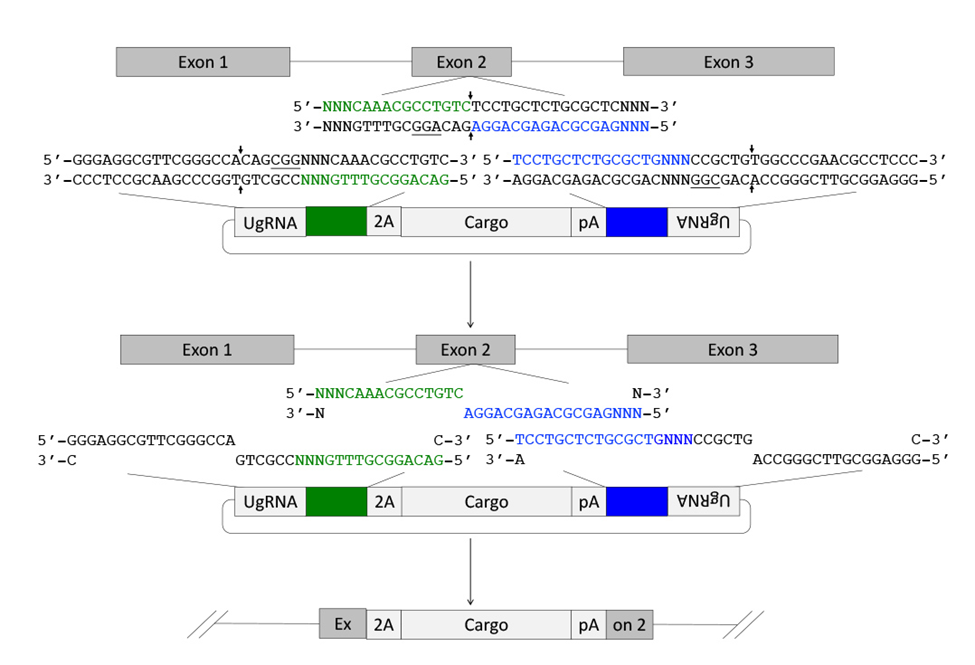
Figure 1. Targeted integration of pGTag vector cargo DNA into a 5’ coding exon. Short homology arms complementary to the 5’ (green) and 3’ (blue) sequences of the genomic target site are cloned on the 5’ and 3’ sides of the vector cargo DNA. The short homology arm cargo cassette is flanked by two universal guide RNA UgRNA sites. CRISPR/Cas9 simultaneously targets double strand breaks at the sgRNA genomic target site and at the UgRNA sites flanking the cargo on the plasmid donor. Exonuclease end resection liberates single stranded DNA in the vector homology arms that is complementary to the resected strands on the 5’ and 3’ sides of the genomic double strand break. The complementary sequences direct homology mediated end joining integration of the cargo DNA at the exon target site. PAM sequences are underlined and small black arrows indicate Cas9 cut sites in the genome and vector.
B. Selection of a CRISPR/spCas9 target site downstream of the first AUG in the gene of interest
Zebrafish wild type strains in common use are polymorphic. It is highly recommended to first sequence the target exon in the genomic DNA from your fish strain and use this sequence to design sgRNAs.
sgRNA site selection and guide ordering
1. To identify an exon in a gene for targeted integration, view the gene model on a genome browser, and download the gene sequences.
a. At <ensemble.org> Search for the gene name of interest for the species of interest and open the Transcript page.
b. In the left-hand side bar click on ”Exons” to find the first coding exon and initiation ATG. If there are alternative transcripts for the gene, make sure there are not alternative initiation ATGs. If there are alternative start codons, target the first exon that is conserved in all transcripts to generate a strong loss of function allele.
c. Download the cDNA and target exon as separate sequence files.
d. Using ApE <http://biologylabs.utah.edu/jorgensen/wayned/ape/> or SnapGene annotate the coding sequence with the exons.
e. Design primers to amplify the target exon from fin clip genomic DNA and sequence the amplicon. Use this sequence to identify sgRNA sites.
2. This updated protocol no longer includes in vitro synthesis of sgRNAs. Our standard protocol now uses guides ordered from a company, such as Synthego or IDT, which are highly efficiency and eliminate the need for a GG or G at the 5’ end of the sgRNA. Moreover, Hoshijima and Grunwald have shown that addition of 5’ GG to sgRNAs that do not have 5’ GG at the genomic target site significantly inhibits DSB activity (Hoshijima et al., 2019).
sgRNA sites can be identified by manual inspection of genomic DNA or other selective software such as CRISPR Design Tool (https://www.synthego.com/products/bioinformatics/crispr-design-tool) that searches independent of the 5’ GG criteria for in vitro synthesis.
If in vitro synthesis will be used for guide RNA synthesis, CRISPRScan (http://www.crisprscan.org/) (Moreno-Mateos et al., 2015) can be used to identify exon target sites:
a. Select the “Predict gRNAs” on the right-hand side of the home page of the CRISPRScan website.
b. Paste the exon sequence into the indicated box. If the exon is very large, start with a small amount of sequence, ~200 bp around the desired target region in the exon.
c. Select “Zebrafish (Danio rerio)” as the species.
d. Select “Cas9 – nGG” as the enzyme.
e. Select “In vitro T7 promoter”.
f. Click on “Get sgRNAs.” Examine the output. The generated targets are ranked by CRISPRScan from high to low. Select a target site (the 20 bp that are capitalized in the oligo column) from those given by CRISPRScan using the following criteria (The best gRNAs will have all of these):
- An exact match to the genomic locus. Clicking on an oligo will display additional information to the right. The section “Site Type” aligns the genomic sequence with the gRNA which has GG at the 5’ end necessary for in vitro transcription. ßSelection of sgRNAs with 5’ GG or G is not required if guides are ordered from Synthego, IDT or other.
- The target is in the desired location of the gene.
- The target is on the reverse (template) strand. Reverse strand guides are more favorable, but either will work.
- A high CRISPRScan score, and a lower CFD score. However, lower score sgRNA targets may work fine.
g. Annotate the selected target sequence and NGG PAM in the cDNA sequence files.
3. Design ~20 bp DNA primers for a PCR amplicon of ~130 bp of DNA surrounding the sgRNA target site. These primers will be used to amplify genomic DNA from embryos after injection of CRISPR reagents to test for mutagenesis at the target site. The presence of indels at the target site can be detected in the PCR products in multiple ways, including gel electrophoresis to visualize heteroduplex formation, resistance to restriction enzyme digestion at a site overlapping the sgRNA target, or direct sequencing followed by ICE analysis (https://www.synthego.com/products/bioinformatics/crispr-analysis).
- Primer 3 is used for primer design: http://biotools.umassmed.edu/bioapps/primer3_www.cgi
- Paste DNA sequence surrounding the target site into the web interface. It is recommended to use 160 – 300 bp of exon sequence centered on the cut site for primer design. Intron sequence can be used, but this often contains polymorphisms that can lead to amplification failure.
Locate the target sequence, including the PAM sequence (italicied below), and predict the cut site (3 bp into the target sequence from the PAM represented here by the ‘x’). Mark the targeted exon sequence approximately 65-150 bp on both sides of the cut site by putting [square brackets] around it. Primer3 will design primers outside this sequence. This design allows the primers to be used for both checking of mutagenesis and for junction fragment analysis when checking for integration.
Example:
CGGCCTCGGGATCCACCGGCC[AGAATCGATATACTACGATGAACAGAGCAAATTTGTGTGTAATACGGTCGCCACCATGGCCTxCCTCGGTTTGCTACGATGCATTTGCACCACTCTCTCATGTCCGGTTCTGGG]AGGACGTCATCAAGGAGTTCATGCGCTTCAAGGTGCGCATGGAGGGCTCCGTGAAC- Set the “Primer Size” variables to Min = 130, Opt = 170, and Max = 300. Everything else can be left at the defaults.
- Click on “Pick Primers”
- Select primers from the output. Note the “product size” expected and the “tm” or melting temperature of each primer/pair. Smaller product sizes are easier to visualize mutagenesis.
Preparation of SpCas9 mRNA
- Digest ~5-10 μg pT3TS-nCas9n plasmid with Xba1 (plasmid Addgene #46757 (Jao et al., 2013)).
- Purify digested DNA with Qiagen PCR cleanup kit or Promega PureYield Plasmid Miniprep System. Elute in RNF-water.
- Run 100-500 ng on 1.2% agarose gel in 1X TAE to confirm the plasmid is linearized.
- Use 100 ng to 1 μg DNA as template for in vitro transcription reaction.
- Use mMESSAGE mMACHINE T3 kit Life Technologies (AM1348) and follow the instructions in the manual.
- Use the miRNeasy Qiagen kit for purification of nCas9n mRNA according to the manufacturer’s instructions.
- Verify mRNA integrity by mixing 1 uL of purified Cas9, 4 μL of RNF water, 5 μL glyoxl dye (Ambion).
- Heat mixture at 50 oC for 30 minutes, then place on ice.
- Clean the gel box, comb and tray with RNase Away, rinse with DI water.
- Run all 10 μL of RNA mixture on 1.2% agarose gel in 1X TAE at 100 V for 1 hour as above. One band should be visible at 4.5 kb.
- Nanodrop the RNA sample to determine the concentration. Concentrations between 0.45 and 1 μg/μL are expected.
- Aliquot and store RNA at -80 °C.
C. Injection of sgRNA and spCas9 mRNA
The injections are designed to deliver 25 pg of sgRNA and 300 pg of Cas9 mRNA in a 2 nL volume to embryos at the one-cell stage.
Injection trays are cast with 1.2% agarose with 1X embryo media (Zebrafish Book; zfin.org) in polystyrene petri dishes (Fisher No. FB0875713). Injection trays can be used multiple times and stored at 4*C for up to three weeks between use.
- Trays are pre-warmed to 28.5 oC prior to injection by placing them in a 28.5 oC incubator for 20 minutes. Try to mitigate tray cooling while not in use.
Microcapillary glass needles are pulled from Kwik-Fil borosilicate glass capillaries (No. 1B100-4) on a Sutter Instruments Flaming/Brown Micropipette Puller (Model P-97).
Injection samples contain the following diluted in RNF water or injection buffer (final concentration: 12.5 mM HEPES pH 7.5, 25 mM Potassium Acetate, 37.5 mM Potassium Chloride, 0.0125 % glycerol, 0.025 mM DTT ph 7.5)a. 12.5 ng/μL of genomic sgRNA
b. 150 ng/μL of mRNA for Cas9- Needles are backloaded with sample using microloader pipet tips (Eppendorf) and attached to a micro-manipulator (Narishige). Microinjections are performed using nitrogen gas and a Harvard Apparatus PLI - 90 Pico-injector set to 40 PSI with an injection time of 100-200 msec.
- Needles are calibrated by breaking the end of the tip off with sterile tweezers, ejecting and capturing 10 droplets in a 1 μL capillary tube (Drummond No. 1-000-0010), and measuring the distance from the end of the capillary to the meniscus of the liquid. This distance is converted to volume (where 1 mm = 30 nL, or 2/3 of a mm = 20nl). The volume of each droplet is adjusted by changing the injection time in order to achieve 2 nL/droplet. There is a linear relationship between volume and time at a set pressure. Avoid injection times less than 100 msec and over 400 msec.
- One-cell embryos are transferred from collection petri dishes to the wells on the pre-warmed agarose injection tray.
- Use the micro-manipulator and microscope to pierce through the chorion and into the embryo. Inject 2 nL of sample at the center of the interface/boundary between the one cell and the yolk.
- After embryos have been injected, wash the embryos from the injection tray into a clean petri dish with embryo media.
- Keep 20 - 40 embryos separate to use as uninjected controls. Treat and score the control embryos in the same way as the injected embryos.
- At 3 - 5 hrs post injection remove any unfertilized or dead embryos from the dishes.
D. Testing for indel production/mutagenesis
Phenotypic scoring of embryos
- The sgRNA itself may be toxic to the developing embryos. Injection toxicity can be estimated by the number of dead embryos from a round of injection compared to the un-injected control dish. Count and remove any brown/dead embryos from injected and un-injected dishes. If there are significantly more dead embryos in the injected dish then the guide may be toxic, impure, or very effective at disrupting a required gene. Reducing the amount of guide or Cas9 mRNA injected may help reduce toxicity.
- Score and document embryonic phenotypes on days 1 - 4 post fertilization (dpf). Under a dissection microscope examine the un-injected controls and injected embryos, and sort the embryos into categories.
- Scoring categories
- Severe- These embryos show severe morphological defects such as embryos that lack a head, eyes, trunk, or have an unnaturally contorted or asymmetric shape.
- Mild- These embryos appear mostly normal, but have slight defects such as small eyes, pericardial edema, shortened trunk/tail, or curled/twisted tails.
- Normal- morphology appears normal and growth rate is similar to controls.
Digestion of embryos for isolation of genomic DNA for mutagenesis analysis
Genomic DNA (GDNA) can be isolated from zebrafish embryos aged between 1 and 5 dpf using this protocol. Embryos can be analyzed as individuals or as pools (maximum 5) from the same injection.
- Dechorionate embryos, if they have not emerged from the chorion.
- It is recommended to screen a minimum of 3 embryos from each scoring category for mutagenesis. Place each embryo, including controls, into separate PCR tubes. Remove as much of the fish water as possible. If needed, spin briefly and remove additional water.
- Add 20 μL of 50 mM NaOH per embryo.
- Heat the embryos at 95oC in a thermocycler for 15 minutes.
- Vortex samples for 10 seconds. Be sure that the tubes are sealed to prevent sample loss while vortexing.
- Spin samples down and heat for an additional 15 min at 95 oC in a thermocycler.
7. Vortex samples and then spin the tubes down again. The embryos should be completely dissolved.
- Neutralize the samples by adding 1 μL of 1 M Tris pH 8.0 per 10 μL NaOH. Mix by vortexing then spin down.
- Genomic DNA should be stored at -20oC.
Analysis of CRISPR/Cas9 mutagenesis efficiency at targeted gene locus.
- Remove genomic DNA samples from -20oC and place on ice to thaw. Keep thawed genomic DNA on ice at all times.
Set up the following PCR reactions for each tube of embryo digested genomic DNA using the primers designed at the end of section A, page 10.
12.5 μL of 2x GoTaq Mastermix1 μL of Forward Primer (10 uM)
1 μL of Reverse Primer (10 uM)
1 μL of gDNA template (digested embryos)
9.5 μL of nuclease-free water
25 μL total
- Flick the tubes to mix and briefly spin down the PCR reactions.
- Run the following PCR program to amplify the targeted locus.
95oC 2 minutes
95oC 30 seconds ]
55oC* 30 seconds ] x 35 cycles
72oC 30 seconds ]
72oC 5 minutes
4oC hold - Run up to 7 μL of PCR product on a 3.0% agarose gel, 1X TAE, for 1 hr at 80-100V.
- Analyze the gel for DNA bands that appear diffuse or different in size from the control lane which will show a single band. This indicates heteroduplex formation in the PCR product caused by the presence of indels mutations at the CRISPR target site in the gene of interest.
- Alternatively, clone and sequence PCR products or sequence directly to verify the presence of indels.
E. Design short homology arms
Homology directed gene targeting allows the seamless integration of exogenous DNA into the genome with precise repair events at the target site. However, designing and cloning individual targeting vectors and homology arms for each gene of interest can be time consuming. The pGTag vector series and webdesign tools provide versatility and ease to generate knockout alleles (Figure 3). The vectors contain BfuAI and BspQI type II restriction enzymes for cloning of short homology arms (24 or 48 bp) using Golden Gate cloning. The pGTag vectors require in-frame integration for proper reporter gene function. The reporter gene consists of several parts. A 2A peptide sequence causes translational skipping, allowing the following protein to dissociate from the locus peptide. The eGFP, TagRFP, or Gal4VP16 reporter coding sequences have a number of options for localization signals, including cytosolic (no signal), a nuclear localization signal (NLS), or a membrane localization CAAX sequence. Finally, translation is terminated by one of two different transcription termination polyadenylation (pA) sequences; the 3’UTR region of the zebrafish b-actin gene or the SV40 viral pA sequence.
For many genes, the level of endogenous gene expression is not high enough to produce a detectable fluorescence signal from the integrated reporter gene. The Gal4VP16 pGTag vector in combination with a transgenic Tol2<14XUAS/RFP> reporter line allows for amplification of the signal. A 14XUAS/RFP Tol2 plasmid is provided to make a transgenic line for use with the Gal4VP16 vector.
Plasmid sequence maps can be downloaded at www.genesculpt.org/gtaghd/
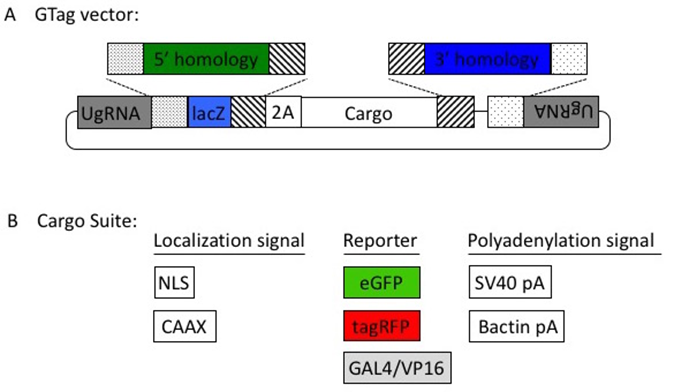
Figure 3. The pGTag vectors allow one step cloning of homology arms.
All vectors can be obtained through Addgene (www.addgene.org). Because the pGTag plasmids contain repeated sequences, vector recombination can occur in bacteria. We suggest using NEB Stable E. coli. Bacteria should be grown at 30oC to reduce further the possibility of vector recombination. It is also recommended that preps of vector be checked by digesting with diagnostic enzymes followed by separation on an agarose gel.
Homology Arm Design at GTagHD www.genesculpt.org/gtaghd/
The web tool GTagHD www.genesculpt.org/gtaghd/ allows for quick design of oligos to generate short 24 or 48 bp homology arms complementary to the target site in a gene of interest.
Two complementary oligos with overhangs are annealed to generate the double stranded homology arm for cloning into the pGTag vector.
To use the tool, choose the "Submit Single Job" tab. Follow the instructions in the tab.
The sequences of two pairs of complementary oligos will be returned, one pair for the 5’ homology arm, the other pair for the 3’ homology arm.
If there are problems with the sequences and values that were entered, the web page will display the errors and advice on how to fix them.
Manual Homology Arm Design
The following protocol describes how to design homology arm oligos manually. This is also recommended to double check your output from above.
*Note* In the following section, orientation of target sites and homology is in the context of the reading frame of the genetic locus of interest. Example: A 5’ template strand CRISPR means that the target site for the CRISPR is on the template strand at the locus and is toward the 5’ end of the gene. Upstream homology domains are 5’ of the CRISPR cut site and downstream homology domains are 3’ of the cut site with respect to the gene being targeted. Also note: Upper case and lower case bases are not specially modified; this is simply a visual marker of the different parts of the homology arms.
Upstream Homology Arm Design
1) Open the sequence file for the gene of interest and identify the CRISPR site. (In this example it is a Reverse CRISPR target in Yellow, the PAM is in Orange, coding sequence is in purple)
Copy the 48 bp 5’ of the CRISPR cut (the highlighted section below) into a new sequence file; this is the upstream homology.
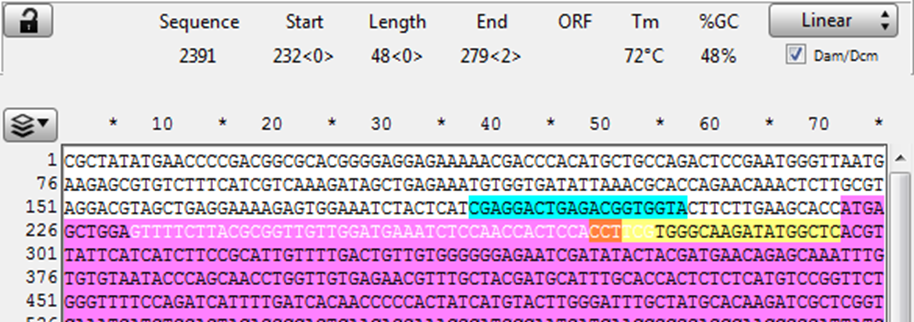
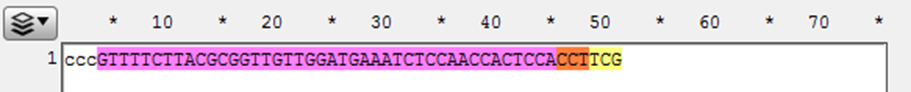
2) Observe the next three bases immediately upstream of the 48 bp of homology, and pick a base not present to be the 3 bp spacer between the homology and the Universal PAM in the vector. (Here the three bases are “GGA” so “ccc” was chosen for the spacer)
Add the spacer to the new file 5’ (in front) of the homology, see below. The spacer acts a non-homologous buffer between the homology and the eventual 6 bp flap from the universal guide sequence that will occur when the cassette is liberated and may improve intended integration rates over MMEJ events.
3) Determine where the last codon is in the homology. Here the 3’ G in the homology domain is the first base in the codon cut by this CRISPR target. Complete the codon by adding the remaining bases (called padding on GTagHD) for that codon from your sequence to ensure your integration event will be in frame.

4) Add the BfuAI enzyme overhang sequences for cloning, to the ends of the homology domain. 5’-GCGG and 3’-GGAT. (Here both overhangs are added to prevent errors in copying sequence for the oligos in the next two steps.)

5) The Upstream Homology Oligo A will be this sequence from the beginning to the end of the last codon (see highlighted below). Copy and paste this sequence into a new file and save it. In this example this oligo sequence is 5’-GCGGcccGTTTTCTTACGCGGTTGTTGGATGAAATCTCCAACCACTCCACCTTCGtg-3’.

6) The Upstream Homology Oligo B will be the reverse complement of this sequence from beginning of the spacer to the end of the sequence (see highlighted below). Copy the reverse complement, paste it into a new file, and save it. In this example this oligo sequence is 5’-ATCCcaCGAAGGTGGAGTGGTTGGAGATTTCATCCAACAACCGCGTAAGAAAACggg-3’.

Downstream Homology Arm Design
7) Open sequence file for the gene of interest and identify the CRISPR site. (Reverse CRISPR target in Yellow, PAM in Orange, coding sequence is in purple)
Copy the 48 bp 3’ of the CRISPR cut into a new sequence file; this is the downstream homology.
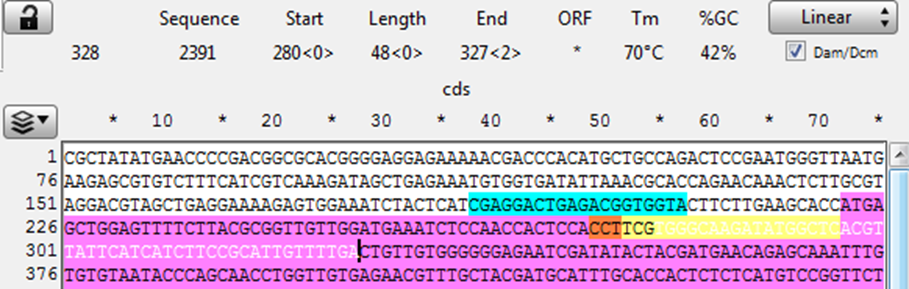
8) Observe the next three bases downstream of the 48 bp of homology, and pick a base not present to be the 3 bp spacer between the homology and the Universal PAM in the vector. (Here the bases are “CTG” so “aaa” was chosen for the spacer.)
Add the spacer to the new file 3’ of (after) the homology.

9) Add the BspQI enzyme overhang sequences for cloning, to the ends of the homology domain. 5’-AAG and 3’-CCG. (Here both overhangs are added to prevent errors in copying sequence for the oligos in the next two steps.)

10) The Downstream Homology Oligo A will be this sequence from the beginning of the sequence to the end of the spacer (see highlighted below). In this example this oligo sequence is 5’-AAGTGGGCAAGATATGGCTCACGTTATTCATCATCTTCCGCATTGTTTTGAaaa-3’.

11) The Downstream Homology Oligo B (will be the reverse complement of this sequence from the beginning of the homology to the end of the sequence (see highlighted below). In this example this oligo sequence is 5’-CGGtttTCAAAACAATGCGGAAGATGATGAATAACGTGAGCCATATCTTGCCCA-3’

F. One Pot Cloning of Homology Arms into pGTag Vectors
**Note if the homology arm oligos contain either the sequence “5’-ACCTGC-3’” or “5’-GAAGAGC-3’” (or their complements) the cloning reaction will be less efficient.
*Note some sequences just don’t work very well.
If One Plot cloning is unsuccessful, the 5’ and 3’ homology arms can be cloned sequentially into the vector using gel purified linear plasmids digested with the appropriate enzyme.
- Homology Arm Annealing
Anneal upstream and downstream homology oligo pairs separately:
4.5 μL oligo A at 10 uM
4.5 μL olio B at 10 uM
4 μL 10x Buffer 3.1 from NEB
27 μL dH20
total = 40 μL
Incubate at 98oC for 4 min, 98oC 45 sec x 90 steps decrementing temp 1oC/cycle,
4oC hold
(Alternatively heat in 95-98oC water for 5 minutes, and then remove the boiling beaker from the heat source and allow it to cool to room temp for 2 hours, before placing samples on ice.) 1-Pot Digest
Assemble the following:
4.0 μL dH2O2 μL Plasmid at 50 ng/uL
1 μL 10x Buffer 3.1 from NEB
1 μL 5' annealed homology arm
1 μL 3' annealed homology arm
0.5 μL BfuAI enzyme from NEB
0.5 μL BspQI enzyme from NEB
10 uL total
Incubate at 50oC for 1 hr, place on ice.Ligation
Add the following:
3 uL 5x T4 quick ligase buffer1.5 uL dH2O
0.5 uL T4 quick ligase
15 uL total
Incubate 8-10 min at room temperature (to overnight). Store at -20 oC,
- Transformation – To prevent recombination at repetitive elements in the plasmid, grow transformations and overnight cultures at 30oC. NEB Stable Competent E. coli (C3040H) cells are recommended for cloning and propagation of the GeneWeld pGTag and pPRISM plasmid series.
a. On ice, thaw 1 (one) vial competent cells (50 μL) for every 2 ligation reactions. (approx. 5 min). It is recommended to use NEB Stable Competent E. coli (C3040H) cells to limit recombination.
b. While cells are thawing, label the microcentrifuge tubes for each ligation and put on ice.
c. Once the cells are thawed, use a pipette to transfer 25 μL of the competent cells into each labeled tube.
d. Add 1.5 μL of a ligation reaction into competent cells to transform.
a. Amount of ligation reaction added should be less than 5% of volume of competent cells.
e. Mix by tapping the tube several times or gently mixing with the pipet tip.
a. Do NOT mix by pipetting, this will lyse the cells.
f. Incubate on ice for 5 to 20 minutes.
g. Heat shock the cells by submerging the portion of the tube containing the cells in a 42oC water bath for 40 - 50 seconds.
h. Incubate cells on ice for 2 minutes.
i. Add 125 μL of room temperature LB to each transformation.
j. Incubate cells at 30oC for 1- 1.5 hour(s) in a shaking incubator.
k. While the transformed cells are recovering, spread 40 μL of X-Gal solution, and 40 μL IPTG 0.8 M on LB Kanamycin selection plates.
a. X-Gal is lethal to cells while wet, it is recommended to first label the plates and then place them in a 30oC incubator to dry.
l. After recovery and the X-Gal is dry, Plate 150 μL of each transformation on the corresponding correctly labeled plate.
m. Incubate plates overnight at 30oC. Growing colonies
Pick 3 white colonies from each plate and grow in separate glass culture tubes with 3 mL LB/Kanamycin, overnight at 30oC.
Or to pre-screen colonies by colony PCR:
a. Pick up to 8 colonies with a pipet tip and resuspend them in separate aliquots of 5 μL dH2O. Place the tip in 3 ml of LB/Kan, label, and store at 4oC.
b. Make a master mix for your PCR reactions containing the following amounts times the number of colonies you picked.7.5 μL 2x GoTaq mastermix
5.5 μL dH2O
0.5 μL primer at 10 uM “F3'-check” 5'- GGCGTTGTCTAGCAAGGAAG -3'
0.5 μL primer at 10 uM “3’_pgtag_seq”5'-ATGGCTCATAACACCCCTTG-3'
14 μL totalc. Aliquot 14 μL of mixed master mix into separate labeled PCR tubes.
d. Add 1 μL of colony to each reaction as template.
or 20 ng purified plasmid as control.
e. Cycle in a thermocycler
95oC 2 minutes
95oC 30 seconds ]
57oC 30 seconds ] x 35 cycles
72oC 30 seconds ]
72oC 5 minutes
4oC holdf. Run 5 μL of PCR product on a 1% agarose gel. You should get bands that are a different size than the control.
- Mini Prep Cultures
Follow Qiagen Protocol - Sequencing of Plasmids
The 5’ homology arm can be sequenced by the 5'_pgtag_seq primer:
5'-GCATGGATGTTTTCCCAGTC-3’.
The 3’ homology arm can be sequenced with the “3’_pgtag_seq”primer:
5'-ATGGCTCATAACACCCCTTG-3'.
G. Injection of GeneWeld Reagents (spCas9 mRNA, Universal sgRNA (UgRNA), genomic sgRNA and pGTag homology vector) into 1-cell zebrafish embryos
Prepare and collect the following reagents for injection
- Prepare nCas9n mRNA from pT3TS-nCas9n (Addgene #46757 from (Jao et al., 2013)) as described above (page 14).
- Synthesize UgRNA and purify as described above (page 11) using the following oligo A:
5’TAATACGACTCACTATAGGGAGGCGTTCGGGCCACAGGTTTTAGAGCTAGAAATAGC-3'
Corresponding to the universal target sequence: GGGAGGCGTTCGGGCCACAG
Alternatively, the UgRNA can be directly ordered form IDT and resuspended in RNF water.
5’-GGGAGGCGUUCGGGCCACAGGUUUUAGAGCUAGAAAUAGCAAGUUAAAAUAAGG
CUAGUCCGUUAUCAACUUGAAAAAGUGGCACCGAGUCGGUGCGGAUC-3’ - The pGTag homology vectors should be purified a second time prior to microinjection under RNase free conditions with the Promega PureYield Plasmid Miniprep System beginning at the endotoxin removal wash. Plasmid DNA is eluted in RNF water.
Embryo Injections for Integration of pGTag vectors
Injections are performed into single cell embryos at a volume of 2nl per embryo containing the following concentration of RNAs and vector:
Injection mixture: Final amount per embryo:
75 pg/nl of nCas9n mRNA 150 pg of nCas9n mRNA
12.5 pg/nl of genomic sgRNA 25 pg of genomic sgRNA
12.5 pg/nl of UgRNA 25 pg of UgRNA
5 pg/nl of pGTag DNA 10 pg of pGTag DNA
H. Examine embryos for fluorescence and integrated vector-genomic junction fragments
Embryos are examined for fluorescence under a Zeiss Discovery dissecting microscope with a 1X objective at 70-100X magnification.
The type of light source used for fluorescent protein activation significantly affects the ability to visualize fluorescence signals. The Excite metal halide and LED square minicube light sources both work well to visualize fluorescence after somatic targeting.
If weak signals are observed, embryos are manually dechorionated, and viewed on glass depression well slides. Embryos displaying widespread fluorescence in expression domains consistent with the targeted gene are examined for junction fragments by PCR or raised to adulthood for outcrossing. If no or weak signals are observed, integration of pGTag-Gal4VP16 can be used to amplify reporter expression in the 14XUAS-RFP transgenic line (Balciuniene et al., 2013).
Analysis of F0 junction fragments between the genomic locus and the integrated targeting vector is carried out by isolating DNA from individual embryos followed by PCR. The following primers are used for junction fragment analysis and must be paired with gene specific primers (5’ to 3’):
5’ pGTag junctions:
R-Gal4-5'juncM GCCTTGATTCCACTTCTGTCA plus gene specific forward primer
R-RFP-5'junc CcttaatcagttcctcgcccttagA “
R-eGFP-5'-junc gctgaacttgtggccgttta “
3’ pGTag junctions:
F-Gal4-3'juncM GCAAACGGCCTTAACTTTCC plus gene specific reverse primer
F-Gal4-3'juncJ CTACGGCGCTCTGGATATGT “
F-RFP-3'junc cgacctccctagcaaactgggg “
F-eGFP-3'junc Acatggtcctgctggagttc “
To control for PCR amplification artifacts as described in (Won and Dawid, 2017), embryos injected with targeting reagents minus the genomic sgRNA are also analyzed by junction fragment PCR.
F-Gal4-3'juncM and F-Gal4-3'juncJ are alternate primers for amplification of junction fragments from the Gal4 cassette which may increase primer specificity, depending on the target gene.
7.5 μL 2x GoTaq mastermix
5.5 μL dH2O
0.5 μL primer at 10 uM genomic primer
0.5 μL primer at 10 uM pGTag primer
14 μL total
- Aliquot 14 μL of mixed master mix into separate labeled PCR tubes.
- Add 1 μL of genomic DNA to each reaction as template.
- Cycle in a thermocycler with the following steps:
95oC 2 minutes
95oC 30 seconds ]
55oC 30 seconds ] x 35 cycles
72oC 30 seconds ]
72oC 5 minutes
4oC hold
- Run 5 μL of PCR product on a 1.2 % agarose gel in 1XTAE. Putative junction fragments should give bands that are of predicted size.
F0 animals that are positive for reporter gene expression are raised to adulthood and outcrossed. Progeny are examined for reporter gene fluorescence as above to recover stable F1 germline alleles. The Gal4VP16 system can lead to silencing resulting in mosaic patterns in F1 embryos.
F1 fluorescence positive embryos are tested for precise transgene integration by junction fragment PCR as described above. F1 siblings are raised to adulthood and flin-clipped to identify individuals with precise targeted transgene integration. Positive F1 adults are used to establish F2 families. F1s are also sacrificed at 3 weeks post fertilization to the confirm location and precision of targeted integrations by genomic Southern Blot RFLP analysis. F0 and F1 identified fish can be incrossed or crossed to a known indel allele to gain an initial assessment of the mutagenicity of the targeted integration allele. It is recommended that established lines are maintained by outcrossing to wild type.
References:
Balciuniene, J., Nagelberg, D., Walsh, K.T., Camerota, D., Georlette, D., Biemar, F., Bellipanni, G., and Balciunas, D. (2013). Efficient disruption of Zebrafish genes using a Gal4-containing gene trap. BMC Genomics 14, 619.
Hoshijima, K., Jurynec, M.J., Klatt Shaw, D., Jacobi, A.M., Behlke, M.A., and Grunwald, D.J. (2019). Highly Efficient CRISPR-Cas9-Based Methods for Generating Deletion Mutations and F0 Embryos that Lack Gene Function in Zebrafish. Dev Cell 51, 645-657 e644.
Jao, L.E., Wente, S.R., and Chen, W. (2013). Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc Natl Acad Sci U S A 110, 13904-13909.
Moreno-Mateos, M.A., Vejnar, C.E., Beaudoin, J.D., Fernandez, J.P., Mis, E.K., Khokha, M.K., and Giraldez, A.J. (2015). CRISPRscan: designing highly efficient sgRNAs for CRISPR-Cas9 targeting in vivo. Nat Methods 12, 982-988.
Won, M., and Dawid, I.B. (2017). PCR artifact in testing for homologous recombination in genomic editing in zebrafish. PLoS One 12, e0172802.
Related files
 Welker et al Protocol.docx
Welker et al Protocol.docx - McGrail, M and Essner, J(2020). GeneWeld: a method for efficient targeted integration directed by short homology. Bio-protocol Preprint. bio-protocol.org/prep610.
- Wierson, W. A., Welker, J. M., Almeida, M. P., Mann, C. M., Webster, D. A., Torrie, M. E., Weiss, T. J., Kambakam, S., Vollbrecht, M. K., Lan, M., McKeighan, K. C., Levey, J., Ming, Z., Wehmeier, A., Mikelson, C. S., Haltom, J. A., Kwan, K. M., Chien, C., Balciunas, D., Ekker, S. C., Clark, K. J., Webber, B. R., Moriarity, B. S., Solin, S. L., Carlson, D. F., Dobbs, D. L., McGrail, M. and Essner, J.(2020). Efficient targeted integration directed by short homology in zebrafish and mammalian cells. eLife. DOI: 10.7554/eLife.53968
Category
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link

