Advanced Search
Microinjections into Zebrafish Embryos
Last updated date: Jul 14, 2021 Views: 1028 Forks: 0
Microinjections into zebrafish embryos
Introduction
Here we provide a detailed protocol for the zebrafish microinjection procedure used in:
Liu PH, Shah RB, Li Y, Arora A, Ung PM-U, Raman R, Gorbatenko A, Kozono S, Zhou XZ, Brechin V, Barbaro JM, Thompson R, White RM, Aguirre-Ghiso JA, Heymach JV, Lu KP, Silva JM, Panageas KS, Schlessinger A, Maki RG, Skinner HD, de Stanchina E, and Sidi S. An IRAK1-PIN1 signaling axis drives intrinsic tumour resistance to radiation therapy. Nat Cell Biol 21:203-213, 2019.
1. Set up
To line up the embryos, we place a slide onto one side of a 10 mm petri dish (Fisher, #FB0875713). The embryos are placed near the edge of the slide using a polyethylene transfer pipettes (VWR, #16001-188). The embryos can be lined up in one or two columns for injection (Figure 1). Use extended fine tip polyethylene transfer pipettes (Samco Scientific, #14670-339) to remove excess water (do not remove all water). This will help keep embryos in position throughout the procedure.
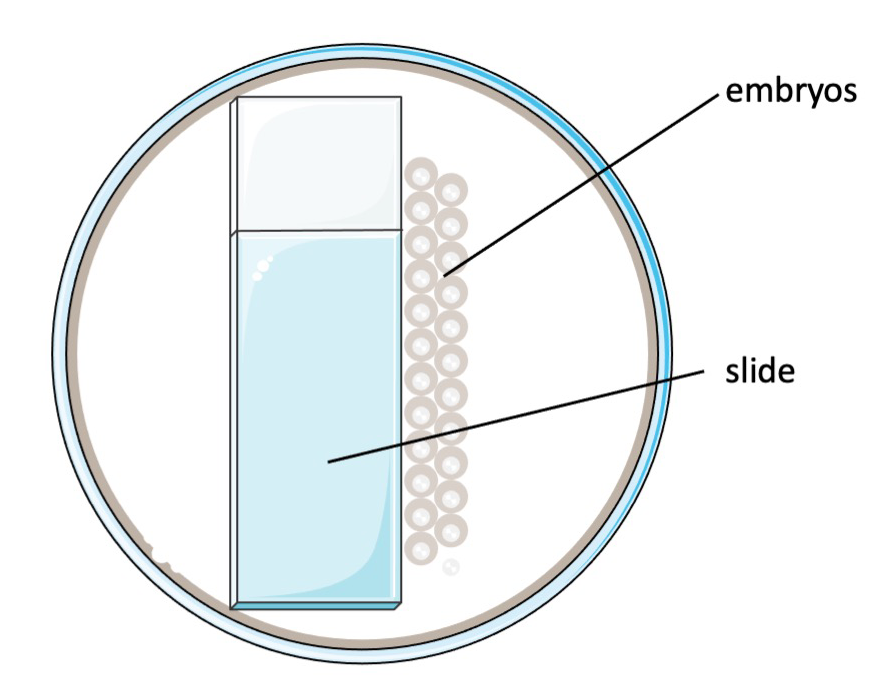
Figure 1. Schematic of the injection set-up.
2. Stage of injection
Embryos can be injected up to the four-cell stage to ensure uniform morpholino or mRNA distribution, but it is preferable to inject at the one cell stage (see Figure 2 below).
3. Needle preparation
We custom prepare our microinjection needles using the P-97 Flaming/Brown Micropipette Puller (Sutter Instrument Co.), setting the needle pulling parameters as follows:
P=500 (Pressure setting) HEAT=754 PULL=75 (pull strength) VEL.=80 (Velocity) TIME=150 |
Turn on the power and select the program. Loosen both clamping knobs then load a piece of the borosilicate glass (Sutter Instrument Co., #BF 100-50-10, O.D.: 1.00 mm, I.D.: 0.5 mm, 10 cm length) into the puller. Position the glass in the center and tighten down both clamping knobs. Press the <PULL> key. Each procedure will generate two needles. Loosen the clamping knobs and remove the needles from the puller bars. Store needles on top of a small piece of clay in a Petri dish (this will avoid dust accumulation on and in the needles) until use.
4. Injection procedure
We use the NARISHIGE IM 300 Microinjector with the following parameters:
Step1: INJECT Step2: CLR.HOLD Step3: BALANCE Step4: HOLD Step5: FILL Step6: INJECT | Step7: REL.HOLD Step8: CLR.HOLD Step9: REL.BAL Step10: CLEAR Step11: AUTO-SEQ Step12: FILL |
FILL: 26 (~20) psi INJ: 9.6 psi (5-12 psi) BALN: 3.2 psi HOLD: 0.0 -iH2O | |
CLR: 0.5 sec CLRH: 0.4 sec FILL: 0.3 sec INJ: 0.3 sec | |
To limit cellular disruption, we prefer to inject morpholinos and synthetic mRNAs in the yolk in area immediately adjacent to (below) the cytoplasm (Figure 2). The injected solution can be made visible by adding phenol red (Sigma) to working MO or mRNA solution (in which case, adjust dilution volume accordingly). We load 2 mL of mRNA or MO solution (see below) into the needle with a microloader (Eppendorf, Catalog #: 930001007). After injection, place the embryos into a 10 cm Petri dish with egg water and incubate them at 28.5°C.
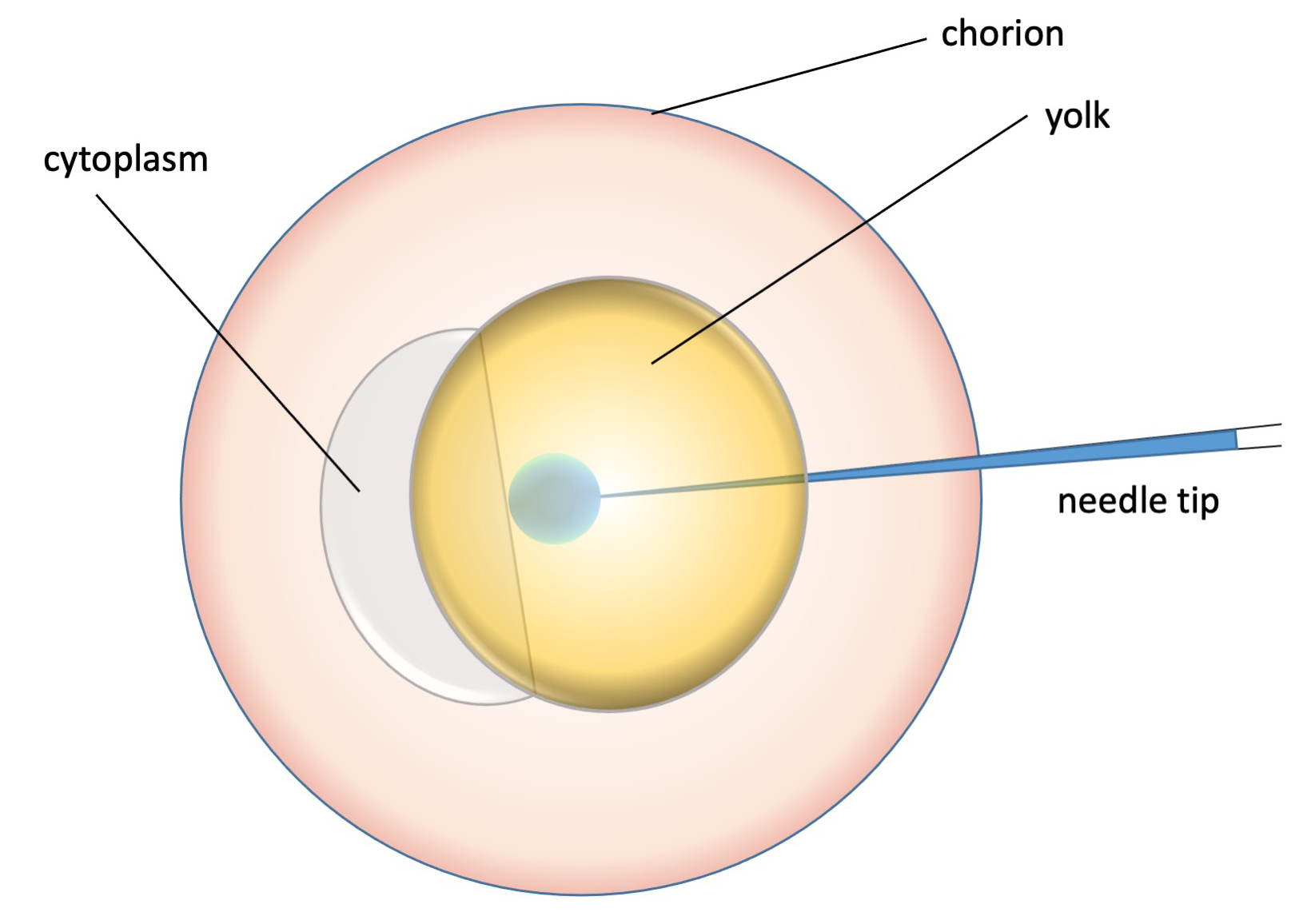
Figure 2. Schematic of the injection procedure (injection volume to scale).
5. MO/mRNA concentrations:
In the Liu et al. study (Liu et al., Nat Cell Biol 2019), we injected 25 pg (+/- 5 pg) mRNA per embryo. With the Microinjector parameters set as above, the injected volume is approximately 1 nL. Therefore, we worked with mRNA solutions at final concentrations of 25 ng/mL.
For the irak1, irak4 and pin1 MOs, we used 1 mL of a 2 mM stock diluted in 4 mL H2O. These 1:5 dilutions of the stock (0.4 mM final concentration) were the solutions used for injection. Because the injection volume is approximately 1 nl (see above), the MO dose delivered to each embryo was approximately 0.4 pmol. For the myd88 MO, the working concentration was 0.8 mM (1:2.5 dilution) for a final dose of 0.8 pmol injected per embryo.
6. Protocol summary:
Evening prior to the injection:
- 2 Male and 2 female fish are separated by divider in a mating tank around 5:00 PM (at least 30 min after feeding). More mating tanks can be set up if large numbers of embryos are needed.
Day of the injection:
- Prior to the injection, dilute the MO to the working concentration using distilled water (DEPC water may damage MO). To improve resuspension, heat the vial at 65 ℃ for 5 min and vortex, then keep at room temperature. Dilute mRNA in Nuclease-Free water and keep the vial on dry ice.
- Pull the required amount of needles (needles can also be prepared in advance).
- Remove the divider from the mating tank. Check back every 10-20 mins and collect embryos.
- Open the nitrogen tank till the pressure to about 20-40 psi.
- Turn on the injector, selected injection program and press <BALANCE>. The injection pressure is approximately 10 psi.
- Load the needle. Load 2 mL working solution into the needle using a microloader. Make sure the working solution is loaded at the tip-end of the needle and remove any bubbles by gently tapping the needle.
- Cut the needle. Use high magnification under the microscope and use a ruler to ensure cutting around 2 mm-tip with a clean blade and cut a sharp end. Place the needle in the injector needle holder.
- Place the collected embryos in a petri dish as shown in Figure 1. Remove the extra water and change the placements of misaligned embryos with extended fine tip polyethylene transfer pipettes.
- Hold the needle holder by hand, find the needle tip under the microscope. Inject the embryos one by one as shown in Figure 2.
- Once every embryo is injected, wash the embryos with the 0.1% Methylene blue egg water. Use polyethylene transfer pipettes to transfer the embryos into a new 10 cm Petri dish. Label with embryo genotype, injected solution and date.
- Incubate the embryos in a 28.5 ℃ incubator. Remove dead and unfertilized embryos around 4-6 PM (shield stage).
Solutions:
- Egg water: 12g sea salt (Instant Ocean) in 20 L H2O
- 0.1 Methylene blue stock: 100 mg Methylene blue in 100 mL H2O.
Morpholinos used in Liu et al. Nat Cell Biol, 2019:
| Morpholino | Sequence | Working concentration |
| irak1 | AATCCTGCAACACAACAGCCACATT | 0.4 mM |
| irak4 | GTGAACAGGTAAAGCCTCACAGGAT | 0.4 mM |
| pin1 | ACTCTCTCTGCTCACTCTGGATGAG | 0.4 mM |
| myd88 | GTTAAACACTGACCCTGTGGATCAT | 0.8 mM |
Prior to ordering the Mos, it is important to sequence the MO target sequences in the zebrafish stocks used in your laboratory. If SNPs are detected in the genetic background, MO sequences should be changed accordingly.
- Sidi, Y and Li, S(2021). Microinjections into Zebrafish Embryos. Bio-protocol Preprint. bio-protocol.org/prep1293.
- Liu, P. H., Shah, R. B., Li, Y., Arora, A., Ung, P. M., Raman, R., Gorbatenko, A., Kozono, S., Zhou, X. Z., Brechin, V., Barbaro, J. M., Thompson, R., White, R. M., Aguirre-Ghiso, J. A., Heymach, J. V., Lu, K. P., Silva, J. M., Panageas, K. S., Schlessinger, A., Maki, R. G., Skinner, H. D., Stanchina, E. D. and Sidi, S.(2019). An IRAK1-PIN1 Signalling Axis Drives Intrinsic Tumour Resistance to Radiation Therapy. Nat Cell Biol 21(2). DOI: 10.1038/s41556-018-0260-7
Category
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
