- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Generation and Selection of Transgenic Olive Plants
Published: Vol 7, Iss 22, Nov 20, 2017 DOI: 10.21769/BioProtoc.2611 Views: 9874
Reviewed by: Scott A M McAdamMoritz BomerAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Agrobacterium-mediated Transformation of Japonica Rice Using Mature Embryos and Regenerated Transgenic Plants
Ammar Elakhdar [...] Takahiko Kubo
Sep 20, 2021 6220 Views

Agrobacterium-mediated Genetic Transformation of Cotton and Regeneration via Somatic Embryogenesis
Alka Srivastava [...] Praveen C. Verma
May 20, 2023 4333 Views

A Novel Gene Stacking Method in Plant Transformation Utilizing Split Selectable Markers
Guoliang Yuan [...] Xiaohan Yang
Feb 20, 2025 1969 Views
Abstract
Olive (Olea europaea L.) is one of the most important oil crops in the Mediterranean basin. Biotechnological improvement of this species is hampered by the recalcitrant nature of olive tissue to regenerate in vitro. In previous investigations, our group has developed a reliable Agrobacterium-mediated transformation protocol using olive somatic embryos as explants (Torreblanca et al., 2010). Embryogenic cultures derived from radicles of matured zygotic embryos are infected with Agrobacterium tumefaciens, AGL1 strain, containing a binary plasmid with the gene of interest and the nptII selection gene. After a meticulous selection procedure, carried out using solid and liquid media supplemented with paromomycin, the putative transformed lines are established. A preliminary confirmation of their transgenic nature is carried out through PCR amplification. Afterwards, plants can be obtained through an efficient regeneration protocol, whose main characteristics are the use of a low-ionic-strength mineral formulation, a phase in liquid medium for synchronization of cultures and the use of semi-permeable cellulose acetate membranes for embryo maturation (Cerezo et al., 2011). Final confirmation of transgene insertion is carried out through Southern or Northern analysis using leaf samples of regenerated plants.
Keywords: Olea europaeaBackground
The protocol developed by Torreblanca et al. (2010) differs from the previous olive transformation protocol, developed by Rugini et al. (2000), in several aspects; mainly, kind of explant, Agrobacterium strain and the selection method used. Rugini et al. (2000) used embryogenic masses as explants, which were incubated in a bacterial suspension of LBA4404 Agrobacterium strain for 48 h. After the infection, the explants were rinsed in water and cultured in embryogenic medium supplemented with 250 mg/L cefotaxime; however, the selection of transgenic embryos was not started until 30 days after the infection, with the addition of 100 mg/L kanamycin. To speed up the process, the explants were transferred to liquid medium in light, and the embryos which turned green were selected and cultured in isolation on solid multiplication medium with 150 mg/L kanamycin. Later on, the plant regeneration process was carried out without kanamycin. In contrast, Torreblanca et al. (2010) used globular somatic embryos as explants and the AGL1 Agrobacterium strain, with an incubation period of only 2 h and 2 days co-culture. Afterwards, the explants were transferred to selection medium with 200 mg/L paromomycin, and re-cultured onto fresh selection medium weekly during the first month and bi-weekly thereafter. In addition, a 3 weeks selection period in liquid medium supplemented with 50 mg/L paromomycin was included. The selection process and the use of somatic embryos as explants solved the problems of chimaeric transgenic embryos appearance, and higher transformation efficiencies were obtained. The protocol for olive plant regeneration published by Cerezo et al. (2011) improved whole plant recovery (shoots and roots) from 1.5% up to 50%. Both protocols together, have allowed the development of a reliable regeneration and transformation procedure in olive, recently used in flower induction studies (Haberman et al., 2017). Indeed, these protocols have been employed to analyze the effect of overexpression of MtFT1 gene in olive.
Materials and Reagents
- Biological material
- Embryogenic olive cultures, formed by callus and globular embryo structures of yellow-creamed colour
- Agrobacterium tumefaciens AGL1 strain harbouring a binary vector, containing the gene of interest and the nptII selection gene
- Embryogenic olive cultures, formed by callus and globular embryo structures of yellow-creamed colour
- Chemicals and materials
- Sterile filter paper cut 10 x 10 cm (Filtros Anois, FILTER-LAB®, catalog number: RM13054252 )
- Petri plates (90 cm) (J. D. CATALAN, S. L.)
- Mesh, 3 x 3 (ALBUS Suministros de Laboratorio)
- Active charcoal (Sigma-Aldrich, catalog number: C9157 )
- Assay tubes (25 x 150 mm) (Kimble Chase Life Science and Research Products, catalog number: 73500-20150 )
- Dialysis tubing cellulose membrane (Sigma-Aldrich, catalog number: D9777-100FT )
- Jiffystrips 5-50 peat pots square, 4.5 x 4.5 cm (Jiffy, catalog number: 110007 )
- Plant pots (12.5 and 20 cm)
- 1:1 peat moss:perlite substrate (Projar professional)
- Agrobacterium liquid growth medium (LB medium) (AppliChem, catalog number: 414753 )
- 10 mM magnesium sulphate (MgSO4) (AppliChem, catalog number: 131404 )
- Antibiotics:
- ¼ OM (Cañas and Benbadis, 1988) macroelements
- ¼ MS (Murashige and Skoog, 1962) microelements
- ½ OM Vitamins
- Myo-inositol (Sigma-Aldrich, catalog number: I5125 )
- Sucrose (D(+)-Saccharose) (VWR, catalog number: 27478.467 )
- L-Glutamine (Biowest, catalog number: P1012 )
- Casein hydrolysate (N-Z-Amine® A) (Sigma-Aldrich, catalog number: C0626 )
- Mannitol (Sigma-Aldrich, catalog number: M9647 )
- Plant hormones:
- Olive cyclic embryogenesis medium (ECO) (see Recipes)
- Germination medium (see Recipes)
- Shoot proliferation medium (see Recipes)
- Plant rooting medium (see Recipes)
- Sterile filter paper cut 10 x 10 cm (Filtros Anois, FILTER-LAB®, catalog number: RM13054252 )
Equipment
- Culture flasks (125 ml) (Nalgene)
- Autoclave (JP SELECTA, model: Autester MOD 437-G )
- Constant temperature/orbital shaker incubator (Optic Ivymen System)
- Laminar flow hood (Telstar, model: BH-100 )
- Laboratory centrifuge (Sigma Laborzentrifugen, model: 3K30 )
- Spectrophotometer (JP SELECTA, model: UV-2005 )
- Walk in plant growth cabinet with controlled light and temperature conditions
Procedure
Note: All this protocol must be conducted under strictly sterile conditions in a laminar flow hood; except B7 step and molecular analysis.
- Agrobacterium-mediated transformation of olive embryogenic callus
- Obtain an embryogenic olive culture, derived from radicle of mature seed (Orinos and Mitrakos, 1991). Embryogenic friable callus (Figure 1A), containing globular embryos, is maintained and multiplied on ECO medium (see Recipes), transferring to fresh medium at 4-week intervals in darkness at 25 ± 2 °C (Pérez-Barranco et al., 2009).
- Grow Agrobacterium tumefaciens AGL1 strain, containing a binary plasmid harbouring the gene of interest and the selection gene nptII (pBINUbiGUSInt as example; containing uidA and nptII genes), in LB medium at 28 °C and 250 rpm for 24 h (with suitable antibiotics for the plasmid used), to obtain a culture of 40 ml at 0.5 OD600 nm. Then, centrifuge the culture at 4,000 x g, wash the pellet with 10 mM MgSO4 without shaking and dilute it in liquid ECO medium, keeping a final 0.5 OD600 nm.
- Inoculate globular somatic embryos 1-2 mm diameter, isolated from embryogenic callus, in the diluted Agrobacterium suspension for 20 min under mild agitation; about 20 embryos into 10 ml of Agrobacterium suspension with a total of 80 embryos, approximately (Figure 1B). Then, dry the embryos out on sterile filter paper (Figure 1C).
- Co-culture the infected somatic embryos on ECO solid medium for 48 h in darkness, 15-20 embryos per plate (Figure 1D).
- Afterwards, wash the somatic embryos with ECO liquid medium supplemented with 250 mg/L cefotaxime and 250 mg/L timentin (Figure 1E). Blot the embryos dry on filter paper (Figure 1F) and transfer them onto selection medium; i.e., ECO solid medium supplemented with 250 mg/L cefotaxime, 250 mg/L timentin and 200 mg/L paromomycin (Figure 1G). Re-culture the explants onto fresh selection medium weekly during the first month and bi-weekly thereafter.
- When the explants show proliferation of paromomycin resistant embryogenic callus on selection medium (Figure 1H), after 3 months of culture approximately, transfer those calli individually to 250 ml culture flasks with 40 ml of ECO liquid medium supplemented with 25-50 mg/L paromomycin (Figure 1I). Incubate the suspensions in an orbital shaker at 120 rpm for 3 weeks in darkness.
- Afterwards, sieve the suspensions through a 3 x 3 mm screen (Figure 1J) and culture the small globular embryos separately in a plate on ECO selection medium with 200 mg/L paromomycin. After two months, proliferating callus is transferred to test tubes (Figure 1K).
- Calli growing on selection medium can be analysed through PCR to obtain a preliminary verification of transformation (Figure 1L).
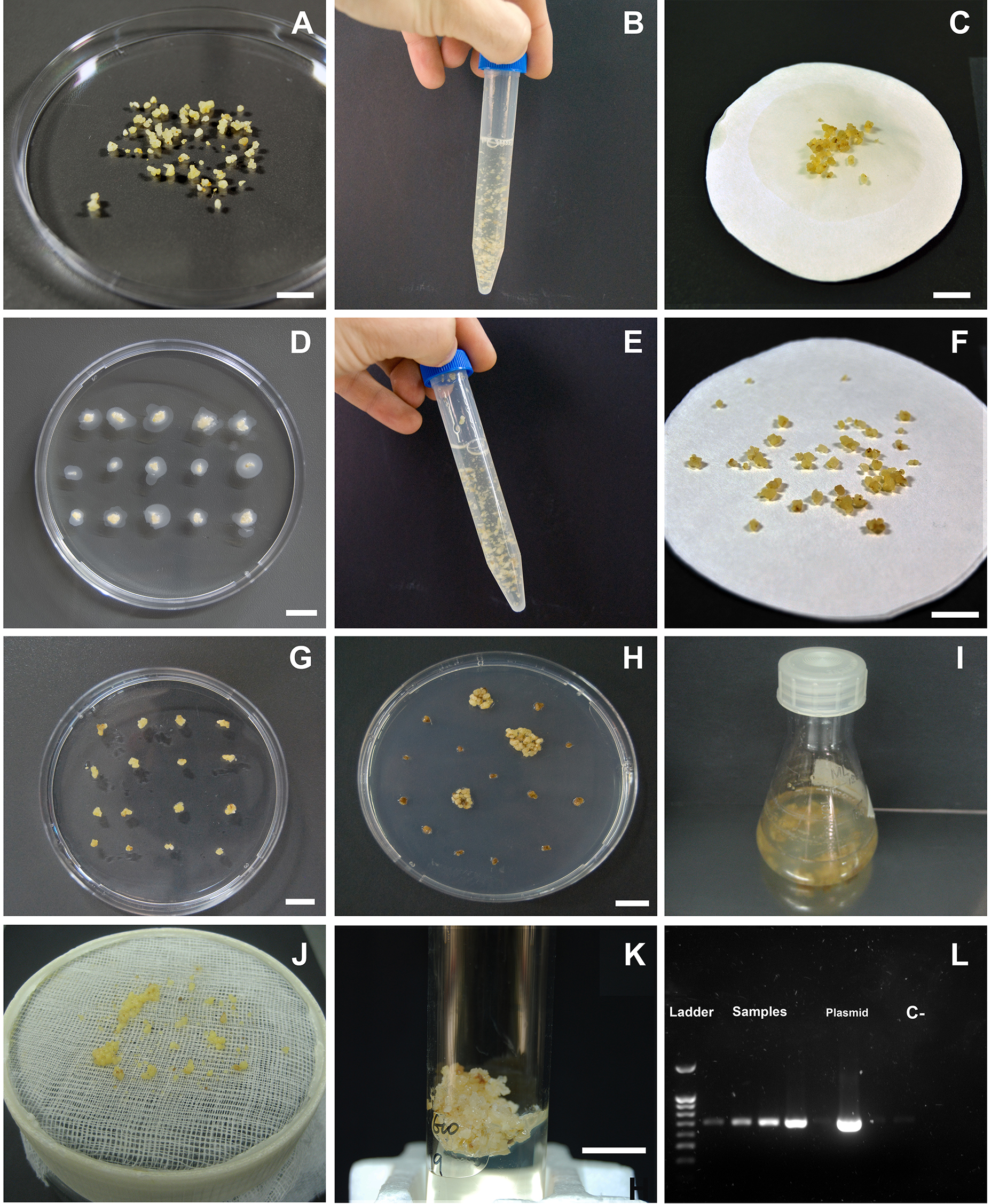
Figure 1. Sequence of olive somatic embryos transformation via Agrobacterium tumefaciens. A. Isolated somatic embryos; B. Inoculation of somatic embryos in a diluted Agrobacterium tumefaciens suspension; C. Somatic embryos drying onto sterile paper after inoculation; D. Co-culture of somatic embryos with Agrobacterium on solid medium; E. Somatic embryos washing in liquid medium supplemented with antibiotics; F. Somatic embryos drying onto filter paper after washing; G. Culture of somatic embryos onto selection medium, supplemented with antibiotics; H. Growth of resistant embryogenic callus on selection medium; I. Incubation of the resistant embryogenic callus in liquid selection medium; J. Obtainment of the fine fraction of the embryogenic callus (1-3 mm); K. Growth of putative transgenic callus on solid selection medium for multiplication; L. PCR verification in an electrophoresis gel. Scale bars = 10 mm.
- Obtain an embryogenic olive culture, derived from radicle of mature seed (Orinos and Mitrakos, 1991). Embryogenic friable callus (Figure 1A), containing globular embryos, is maintained and multiplied on ECO medium (see Recipes), transferring to fresh medium at 4-week intervals in darkness at 25 ± 2 °C (Pérez-Barranco et al., 2009).
- Regeneration of transformed olive plants
- Once the transgenic nature of the paromomycin resistant embryogenic calli has been confirmed by PCR, the process of transgenic plants regeneration from these calli can be started, following the protocol described by Cerezo et al. (2011). Firstly, grow each independent embryogenic calli in ECO liquid medium to obtain globular embryos of small size, 1-3 mm, e.g., culture 0.5 g of callus into 50 ml of medium at 120 rpm for 4 weeks in darkness and later filter through a 3 x 3 mm screen (Figures 2A-2C).
- Culture the isolated small globular embryos onto ECO maturation medium for a 4 weeks period (without hormones or cefotaxime and supplemented with 1 g/L active charcoal) in Petri dishes. Incubate the embryos in darkness, 20 globular embryos per plate (Figure 2D).
- Transfer the embryos directly on top of 4 x 4 cm dialysis tubing cellulose membrane sections lied onto fresh ECO maturation medium and incubate them for another 4 weeks period in darkness (Figure 2E). These membranes are prepared according to the manufacturer’s instructions and sterilized by autoclave.
- Afterwards, culture mature embryos at cotyledonary stage in germination medium (see Recipes) under light conditions (16 h photoperiod, 40 µmol m-2 sec-1 irradiance level). (Figure 2F)
- Transfer the shoots obtained to medium supplemented with 5.6 μM ZR (Figure 2H) for further proliferation.
- To induce rooting, transfer shoots with two or three nodes (2-3 cm) to DKW rooting medium (see Recipes) supplemented with 0.5 μM IBA (Figures 2G-2I).

Figure 2. Sequence of regeneration of olive transformed plants. A. Growth of transformed embryogenic callus on selection medium; B. Incubation of the transgenic callus in liquid medium; C. Obtaining a fine fraction embryogenic culture (1-3 mm); D. Culture of small transformed isolated embryos onto ECO maturation medium; E. Culture of transformed pre-maturated embryos onto cellulose acetate membrane lied on ECO maturation medium; F. Culture of transformed maturated embryos in germination medium; G. Transformed olive plantlets; H. Transformed plant growing in multiplication medium; I. Transformed olive plant in rooting medium. Scale bars = 10 mm.
- Acclimate rooted plants to ex vitro conditions in a greenhouse. Wash roots with distilled water and transfer the plants to a jiffy tray with 1:1 peat moss:perlite substrate and cover the tray with a translucent plastic top. Gradually, adapt the plants to ex vitro conditions by increasing exposure to ambient humidity opening the cover. Transfer the plants to larger pots as they grow (Figures 3A-3D).
- Carry out a Southern or Northern analysis to confirm the transgenic nature of regenerated plants.
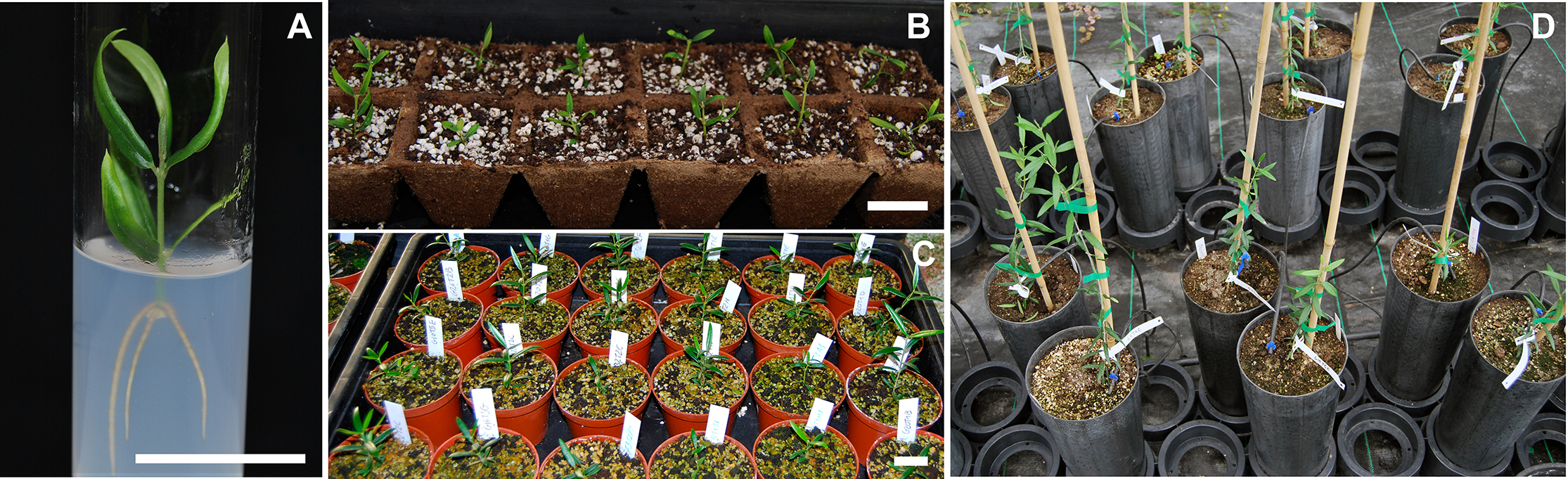
Figure 3. Sequence of recovery of transformed olive plants in a greenhouse. A. A well-developed transformed olive plant; B. Transformed olive plants acclimated into a jiffy tray with 1:1 peat moss:perlite substrate; C. Transformed olive plants in 12.5 cm pots; D. Transformed olive plants in 15 cm pots. Scale bars = 25 mm.
- Once the transgenic nature of the paromomycin resistant embryogenic calli has been confirmed by PCR, the process of transgenic plants regeneration from these calli can be started, following the protocol described by Cerezo et al. (2011). Firstly, grow each independent embryogenic calli in ECO liquid medium to obtain globular embryos of small size, 1-3 mm, e.g., culture 0.5 g of callus into 50 ml of medium at 120 rpm for 4 weeks in darkness and later filter through a 3 x 3 mm screen (Figures 2A-2C).
Data analysis
At least 50 explants were used per each transformation experiment, 3 replicates of each one. Transformation efficiency was estimated as the percentage of explants growing on selection medium, in the presence of paromomycin, after selection in liquid medium.
Notes
The selection pressure used after the infection is genotype dependent; e.g., the concentration of paromomycin may vary between 150-200 mg/L in solid medium and 25-50 mg/L in liquid medium. In addition, for some genotypes, an increasing selection pressure is advisable, starting with 50 mg/L paromomycin in solid medium and increasing up to 100, 150 and finally, 200 mg/L.
Recipes
Note: The pH of all media has been adjusted to 5.7 using NaOH or HCl.
- Olive cyclic embryogenesis medium (ECO) (Pérez-Barranco et al., 2009)
¼ OM (Cañas and Benbadis, 1988) macroelements
¼ MS (Murashige and Skoog, 1962) microelements
½ OM Vitamins
0.05 g/L myo-inositol
20 g/L sucrose
0.550 g/L glutamine
1 g/L casein hydrolysate
0.5 µM 2iP
0.44 µM BA
0.25 µM IBA
0.42 µM cefotaxime
- Germination medium (Cerezo et al., 2011)
Modified MS medium
⅓ macroelements
0.1 g/L myo-inositol
10 g/L sucrose
- Shoot proliferation medium (Vidoy et al., 2012)
Modified RP medium (Roussos and Pontikis, 2002)
1 g/L myo-inositol
20 g/L mannitol
1.2 g/L glutamine
5.6 µM ZR
- Plant rooting medium (Revilla et al., 1996)
Half-strength DKW medium (Driver and Kuniyuki, 1984) salts with no vitamins or amino acids
2% sucrose
0.5 μM IBA
Acknowledgments
This protocol was developed from the following published papers: Torreblanca et al., 2010 and Cerezo et al., 2011. This work was supported by grant P11-AGR-7992-Junta de Andalucía, Spain. The authors have no conflict of interest.
References
- Cañas, L. A. and Benbadis, A. (1988). In vitro plant regeneration from cotyledon fragments of the olive tree (Olea europaea L.). Plant Sci 54: 65-74.
- Cerezo, S., Mercado, J. A. and Pliego-Alfaro, F. (2011). An efficient regeneration system via somatic embryogenesis in olive. Plant Cell Tiss Organ Cult 106 (2):337-344.
- Driver J. A. and Kuniyuki A. H. (1984). In vitro propagation of Paradox Walnut Rootstock. HortScience 19 (4): 507-509.
- Haberman, A., Bakhshian, O., Cerezo-Medina, S., Paltiel, J., Adler, C., Ben-Ari, G., Mercado, J.A., Pliego-Alfaro, F., Lavee, S. and Samach, A. (2017). A possible role for flowering locus T-encoding genes in interpreting environmental and internal cues affecting olive (Olea europaea L.) flower induction. Plant Cell Environ 40 (8): 1263-1280.
- Murashige T. and Skoog F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15: 473-497.
- Orinos, T. and Mitrakos, K. (1991). Rhizogenesis and somatic embryogenesis in calli from wild olive (Olea europaea var. sylvestris (Miller) Lehr) mature zygotic embryos. Plant Cell Tiss Organ Cult 27 (2): 183-187.
- Pérez-Barranco, G., Torreblanca R., Padilla, I.M.G., Sánchez-Romero, C., Pliego-Alfaro, F. and Mercado, J. A. (2009). Studies on genetic transformation of olive (Olea europaea L.) somatic embryos: I. Evaluation of different aminoglycoside antibiotics for nptII selection. II. Transient transformation via particle bombardment. Plant Cell Tiss Organ Cult 97: 243-251.
- Revilla, M. A., Pacheco, J., Casares, A. and Rodríguez, R. (1996). In vitro reinvigoration of mature olive trees (Olea europaea L.) through micrografting. In Vitro Cell Dev Biol Plant 32 (4): 257-261.
- Roussos, P. A. and Pontikis, C. A. (2002). In vitro propagation of olive (Olea europaea L.) cv. Koroneiki. Plant Growth Regulation 37: 295-304.
- Rugini, E., Rita, B. and Rosario, M. (2000). Olive (Olea europaea var. sativa) transformation. In: Jain, S. M. and Minocha, S. C. (Eds.). Molecular Biology of Woody Plants (vol 2). Kluwer Academic Publishers pp: 245-279.
- Torreblanca, R., Cerezo, S., Palomo-Ríos, E., Mercado, J. A. and Pliego-Alfaro, F. (2010). Development of a high throughput system for genetic transformation of olive (Olea europaea L.) plants. Plant Cell Tiss Organ Cult 103 (1): 61-69.
- Vidoy-Mercado, I., Imbroda-Solano, I., Barceló-Muñoz, A. and Pliego-Alfaro, F. (2012). Differential in vitro behaviour of the Spanish olive (Olea europaea L.) cultivars ‘Arbequina’ and ‘Picual’. Acta Horticulturae 949: 27-30.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Palomo-Ríos, E., Cerezo, S., Mercado, J. A. and Pliego-Alfaro, F. (2017). Generation and Selection of Transgenic Olive Plants. Bio-protocol 7(22): e2611. DOI: 10.21769/BioProtoc.2611.
Category
Plant Science > Plant transformation > Agrobacterium
Molecular Biology > DNA > Transformation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









