Advanced Search
in vitro Fluorescence Resonance Energy Transfer (FRET) and FRET in cells
Last updated date: Dec 22, 2020 Views: 861 Forks: 0
in vitro Fluorescence Resonance Energy Transfer (FRET) and FRET in cells
Detailed protocol (in vitro FRET)
1. Transform the BL21(DE3) pLysS bacterial strain with the His-dTomato-MBD2 or His-eYFP-p66α1-206 expressing bacterial vector.
2. Incubate transformed bacteria in the shaking incubator (200 rpm) at 37°C until optical density (at 600 nm) reaches 0.4 to 0.6.
3. Induce protein expression with 0.4 mM IPTG in the shaking incubator (200 rpm) at 37°C for 2 hours.
4. Harvest bacterial cells with centrifugation (5,000 rpm) at 4°C for 15 minutes.
5. Lysis bacterial cells in the lysis buffer (25 µl/1 x 107 cells) at ice for 30 minutes.
6. Sonicate lysate 2 times with 4 pulses and place on ice for 10 seconds.
7. Add Triton-X100 to each lysate in a final concentration of 1%
8. Obtain supernatant after centrifugation (12,000 rpm) at 4°C for 20 min.
9. Incubate each lysate with Ni-NTA agarose beads at 4°C overnight.
10. Wash Ni-NTA agarose beads with washing buffer 3 times.
11. Elute His-tagged proteins with elution buffer.
12. Quantify the purified the donor (eYFP-p66α1-206) and acceptor (dTomato-MBD2) proteins with Bradford solution.
13. Mix donor and acceptor proteins at a final concentration of 1 µM in the binding buffer. Reactions are set up in duplicate.
14. Incubate the protein mixture with various concentration of the inhibitor (ABA or APC) at 37°C for 30 minutes. Mix well by pipetting but avoid forming bubbles.
15. Transfer the mixture (50 µl) to a transparent flat bottom 96 well plate.
16. Measure the fluorescence intensity using the plate reader using the following settings: Excitation spectra from 480 nm with a bandwidth 2 nm,
Emission spectra from 500 to 600 nm with a bandwidth 2 nm.
17. Calculate the mean FRET efficiency (RFRET) as RFRET = IA/(IA + ID), where IA and ID represent acceptor and donor intensities, respectively.
Detailed protocol (FRET in cells)
1. Seed 1 x 103 293T cells on glass-bottom dishes (SPL Life Sciences, 200350)
2. Incubate cells in DMEM supplemented with 10% FBS at 37°C for 12 hours.
3. Transfect the cells with the mCherry-p66α and/or eYFP-MBD2 expressing vector(s) using Effectene reagent. Incubate transfected cells at 37°C for 24 hours.
4. After treating the inhibitor (ABA or APC) at various concentrations, incubate cells at 37°C for 24 hours.
5. Replace the culture medium with phenol red free DMEM (each inhibitor should be included).
6. Quantify the fluorescence intensity using the plate reader at following settings:
Excitation wavelength: 488 nm / Emission wavelength: 588 nm
7. Calculate the mean FRET efficiency (RFRET) as RFRET = IA/(IA + ID), where IA and ID represent acceptor and donor intensities, respectively.
8. Take FRET image under the confocal microscope at following settings:
Donor fluorescence
Excitation wavelength: 488 nm / Emission filter: 525/561 nm
Acceptor fluorescence
Excitation wavelength: 550 nm / Emission filter: 570/613 nm
FRET measurement
Excitation wavelength: 488 nm / Emission filter 570/613 nm
9. Process FRET image and calculate relative FRET ratios (FRETcomp/FRETmock) with the ImageJ.
Solutions
1. Lysis buffer; 50 mM Tris- HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM DTT, and 1 mM PMSF
2. Washing buffer; 50 mM NaH2PO4, 300 mM NaCl, 20 mM Imidazole, and 1 mM PMSF
3. Elution buffer; 50 mM NaH2PO4, 300 mM NaCl, 250 mM Imidazole, and 1 mM PMSF
4. Binding buffer; 100 mM KCl, 10 mM Tris–HCl, pH 7.9, 1 mM EDTA, 1 mM DTT, 4% glycerol
Equipment
1. Plate reader: Varioskan Flash Spectral Scanning Multimode Reader (Thermo Scientific)
2. Confocal microscope: C2si (Nikon)
Software
1. Image J (ver. 1.51)
2. Microsoft Excel
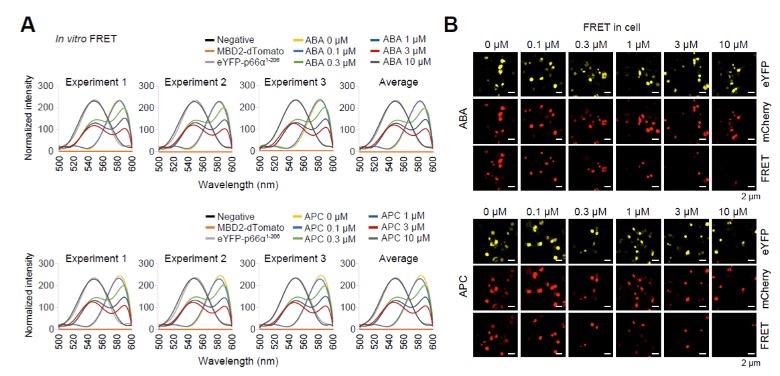
(A) Graphs show the distribution of the in vitro FRET experiments.
(B) Representative immunofluorescence microscopic photos of FRET in cells experiments.
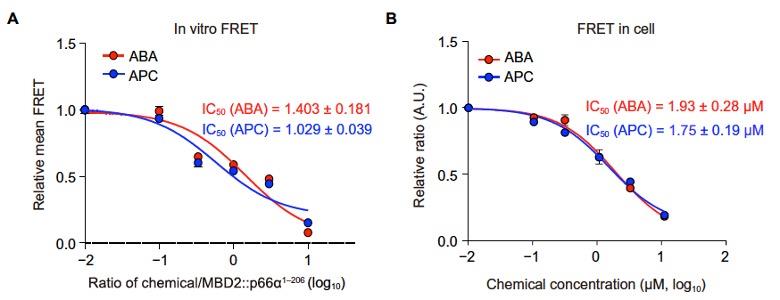
(A) Relative mean FRET values for the in vitro FRET experiments.
(B) Relative mean FRET values for the FRET in cells experiments.
Related files
 Bioprotocol (FRET in vitro and in cell) 20201222.docx
Bioprotocol (FRET in vitro and in cell) 20201222.docx - Son, S and Kim, C(2020). in vitro Fluorescence Resonance Energy Transfer (FRET) and FRET in cells. Bio-protocol Preprint. bio-protocol.org/prep715.
- Kim, M. Y., Na, I., Kim, J. S., Son, S. H., Choi, S., Lee, S. E., Kim, J., Jang, K., Alterovitz, G., Chen, Y., van der Vaart, A., Won, H., Uversky, V. N., Kim, C. G. and Vaart, A. V. D.(2019). Rational discovery of antimetastatic agents targeting the intrinsically disordered region of MBD2 . Science Advances 5(11). DOI: 10.1126/sciadv.aav9810
Category
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
