Advanced Search
Demonstration of the Stimulatory Effect of Lipopolysaccharide (LPS) on Egg Laying in Caenorhabditis elegans
Last updated date: Nov 2, 2020 Views: 800 Forks: 0
Institute of Biomedical Studies, Department of Biology, Baylor University, Waco, Texas, U.S.A. For correspondence: myeongwoo_lee@baylor.edu
Abstract
Lipopolysaccharide extracted from Salmonella enterica stimulates egg laying in the nematode Caenorhabditis elegans. To demonstrate such stimulation, this protocol, that is adapted from a previous study, allows adult hermaphrodites to lay eggs in M9 buffer for a brief period of time, before dissolved LPS is added to treat the animals for an hour. The well-studied stimulant, serotonin, is used as the positive control condition and as a reference of the level of egg-laying stimulation.
Keywords
Caenorhabditis elegans, Salmonella enterica, lipopolysaccharide, egg laying, bacteria
Background
The egg-laying defective C. elegans mutants have been identified and have enabled the dissection of the egg-laying circuit (Trent et al. 1983; Schafer 2006). Using this egg-laying protocol adapted from that of Trent et al. (Trent et al.1983), we demonstrated that Salmonella enterica LPS also stimulates egg laying (Leung et al. 2020). Future studies can investigate whether exogenous LPS applied to the animals can elicit innate immune responses as well, since intact LPS in the live bacteria is required for the activation of the p38/PMK-1-MAPK pathway (Aballay et al. 2003).
The following sections describe a method of demonstrating the egg-laying stimulatory effect of LPS in C. elegans (Fig. A). In short, well-fed adult hermaphrodites are allowed to lay eggs in M9 buffer briefly in a 96-well plate, followed by an 1-hour LPS or serotonin treatment. Eggs (see Fig. B) are counted before and after the treatment.
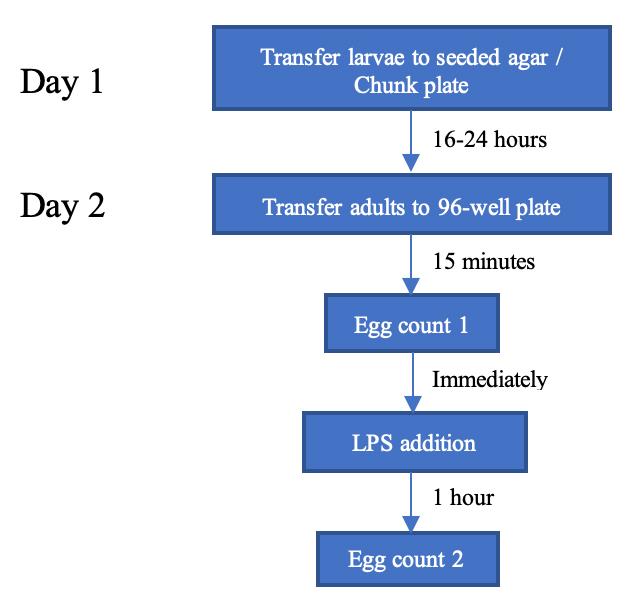
Fig. A. Experimental timeline for demonstrating LPS-stimulated egg laying in C. elegans
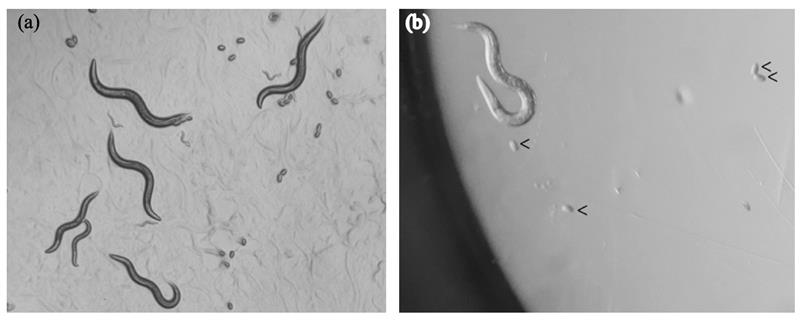
Fig. B. Eggs laid on seeded agar and in liquid in a 96-well plate. (a) Well-fed adults and the eggs they laid on an OP50-seeded NGM agar. Adults like these are suitable for egg-laying assays since they are capable of laying eggs in the one-hour long experiment. (b) One adult is placed in M9 buffer in a 96-well plate and is then allowed to lay eggs over one hour of LPS treatment. Eggs (arrowed) have well-defined edges and are as shiny as those on agar; other small things (without arrows) are not eggs.
Materials and Reagents
60 x 15 mm Petri dish (VWR, catalog number: 25384-090)
90% platinum, 10% iridium wire (Tritech Research, catalog number: PT-9010)
10 μl micropipette tips (VWR, catalog number: 76322-528)
200 μl micropipette tips (VWR, catalog number: 76322-150)
1000 μl micropipette tips (VWR, catalog number: 76322-156)
6. C. elegans: N2 strain (University of Minnesota, Caenorhabditis Genetic Center)
Escherichia coli OP50 (University of Minnesota, Caenorhabditis Genetic Center)
1.5 ml microcentrifuge tubes (VWR, catalog number: 525-0990)
96-well flat-bottom microtiter plates (VWR, catalog number: TPU9296)
25 ml pipetting reservoir (USA Scientific, catalog number: 2330-2220)
Pasteur Pipet as worm pick handle (VWR, catalog number: 53283-916)
Sodium chloride (NaCl) (BDH, catalog number: BDH9286)
Peptone (VWR, catalog number: J636)
Bacteriological agar (Lab Scientific, catalog number: A466)
Double-distilled water (ddH2O)
Cholesterol (Baker, catalog number: F676-05)
Salmonella enterica serotype typhimurium LPS (Sigma-Aldrich, catalog number: L6511)
Serotonin creatinine sulfate complex (Sigma-Aldrich, catalog number: H7752)
Calcium chloride, desiccant, anhydrous (CaCl2) (EMD, catalog number: CX0156-1)
Magnesium sulfate (MgSO4) (BDH, catalog number: BDH0246)
Sodium phosphate dibasic (Na2HPO4) (Sigma-Aldrich, catalog number: S3264)
Potassium phosphate monobasic (KH2PO4) (Ward’s Science, catalog number: 470302-250)
Potassium phosphate dibasic (K2HPO4) (Biosciences, catalog number: RC-081)
5 mg/ml cholesterol (see Recipes)
1 M CaCl2 stock solution (see Recipes)
1 M MgSO4 stock solution (see Recipes)
1 M KPO4, pH 6.0 stock solution (see Recipes)
Nematode growth media (NGM) plates (see Recipes)
1X M9 buffer (see Recipes)
5 mg/ml lipopolysaccharide (LPS) stock solution (see Recipes)
20 mg/ml serotonin stock solution (see Recipes)
Equipment
Pipettors (Gilson, model: Pipetman classic pipettes, catalog numbers: U51380N, U55166K, and T05212K)
4 L glass conical flask (VWR, catalog number: 89000-372)
Autoclave (Primus Sterilizer)
Microscope (Nikon, model: SM2645)
Vortex mixer (VWR, catalog number: 97043-564)
Software
JMP® PRO 14.0.0 (SAS Institute, Inc., Cary, NC, U.S.A.)
Procedure
A. Preparation of adult hermaphrodites
1. Prepare NGM plates.
2. Seed NGM plates with overnight-grown E. coli OP50 under sterile conditions. Allow the plates to dry at room temperature for approximately 24 h.
3. Transfer L4 hermaphrodites onto NGM plates seeded with E. coli OP50. Allow the worms to grow for 16 to 24 hours at 21 to 23oC. Alternatively, chunk a non-starved N2 plate (Stiernagle 1999).
B. Egg-laying assay
1. Fill the wells in a 96-well plate each with 100 µl M9 buffer.
2. Transfer a mature gravid adult (see Notes 1 and 2) to each well (see Note 3).
3. Allow the adults to lay eggs for 15 minutes.
4. Record the number of eggs laid.
5. Add 2 µl of 5 mg/ml LPS in M9 to each well gently without disturbing the animal to achieve a final concentration of 0.1 mg/ml, or add 5 µl of 20 mg/ml serotonin in M9 to achieve a final concentration 1 mg/ml, or skip this step for the M9-only animals (see Note 3).
6. Allow the animal to lay eggs for 1 hour.
7. Record the total number of eggs in each well. Count all eggs in the well no matter whether they were counted in step 4 or not (see Note 5).
Data analysis
1. Open a new data sheet in JMP® PRO 14.0.0. Each row represents one animal.
2. Right click Column 1 > Column Info
3. Change the column properties (data type: character; modeling type: nominal).
4. Input the treatment condition (M9, LPS or serotonin).
5. In Column 2, input the difference between the two egg counts of each well. The default column properties (data type: numeric; modeling type: continuous) can remain unchanged.
6. To compare two treatment conditions at a time, exclude the irrelevant rows (Right click > Exclude/Unexclude).
7. Analyze à Fit Y by X.
8. Drag “Column 2” to “Y, Response.”
9. Drag “Column 1” to “X, Factor.”
10. Click “OK.”
11. In the results window, click the red arrow.
12. Nonparametric > Wilcoxon Test (the software would carry out Mann-Whitney test; see Note 6).
13. Obtain the p-value under “2-sample test, normal approximation.”
Notes
1. The gravid worms used need to be mature enough to lay eggs readily but also not too old, with more than one string of eggs visible in their bodies and without any observable signs of aging.
2. If the gravid worms come from a chunked plate, the resulting plate should contain a good amount of eggs, which indicates that the adults will be capable to respond within an hour to any egg-laying stimulants, i.e. LPS and serotonin as in this experiment (see Fig. B).
3. The animals in each column of eight wells in the 96-well plate can be regarded as one batch for timing in the experiment and can receive the same treatment. Replicates of such are needed.
4. The LPS stock solution (5 mg/ml in M9 buffer) is hazy, as in any other solvents, according to the manufacturer.
5. The eggs usually sink to the bottom of the well, may or may not be at the same level as the adult. Focusing on different horizontal planes and searching for the eggs at the rim of the well are usually needed to locate all the eggs (see Fig. C).
6. Since the M9-only condition usually results in no egg-laying stimulation, it is appropriate to carry out the non-parametric test.
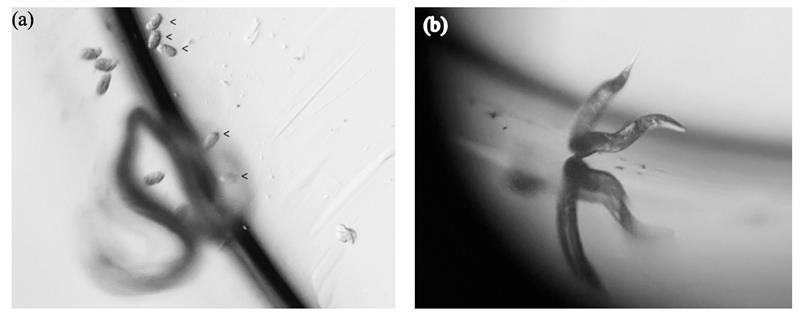
Fig. C. Eggs and adult in a well of a 96-well plate observed under a dissecting microscope at different levels. (a) The eggs (arrows) can be very close to the rim and at a different level than than the adult (blurred). On the left is the mirror image on the wall of the well. (b) The same adult is focused, demonstrating the fact that the eggs sometimes cannot be seen when the adult is focused.
Recipes
1. 5 mg/ml cholesterol
a. Dissolve 2.5 g of cholesterol in 500 ml of 100% ethanol
b. Mix at 30 °C for 15-30 min to dissolve
c. Store at room temperature
2. 1 M CaCl2 stock solution
a. Dissolve 111.0 g of CaCl2 anhydrous in 1 L of ddH2O and sterilize for 30 min
b. Store at room temperature
3. 1 M MgSO4 stock solution
a. Dissolve 60.2 g of MgSO4 in 500 ml of ddH2O and sterilize for 30 min
b. Store at room temperature
4. 1 M KPO4, pH 6.0 stock solution
a. Dissolve 68.0 g of KH2PO4 in 500 ml of ddH2O (1M KH2PO4)
b. Dissolve 52.3 g of K2HPO4 in 300 ml of ddH2O (1M K2HPO4)
c. Combine 86.8 ml 1M KH2PO4 with 13.2 ml 1M K2HPO4
d. Aliquot and sterilize for 30 min
e. Store at room temperature
5. NGM plates
a. Add 6 g of NaCl, 5 g of peptone, 34 g of bacteriological agar, and a magnetic stirring bar to a 4 L Erlenmeyer flask
b. Add to 2 L of ddH2O
c. Sterilize for 30 min
d. Let the NGM cool to 55-60 °C.
e. Add 2 ml of 5 mg/ml cholesterol, 2 ml of 1 M CaCl2, 2 ml of 1 M MgSO4, and 50 ml of 1 M KPO4, pH 6.0
f. Thoroughly mix the medium in the flask on a stir plate
g. Dispense 13.5 ml per 60 x 15 mm Petri dish and allow them to solidify for 24 h
6. 1x M9 buffer
a. Add 6 g of Na2HPO4, 3 g of KH2PO4, and 5 g of NaCl to a 1 L storage bottle
b. Add 1 L of ddH2O
c. Aliquot 200 ml into 500 ml storage bottles and sterilize for 30 min
d. Add 200 μl of 1 M MgSO4 to each bottle
7. 5 mg/ml lipopolysaccharide (LPS) stock solution
a. Dissolve 5 mg LPS in 1 ml 1X M9 buffer
b. Vortex until all powder dissolves (see Note 4).
8. 20 mg/ml serotonin stock solution
a. Dissolve 20 mg serotonin creatinine sulfate complex in 1 ml 1X M9 buffer
b. Vortex until all powder dissolves
Acknowledgments
This work was supported by the Institute of Biomedical Studies and the Department of Biology of Baylor University.
Competing interests
The authors declare no competing interests.
References
Aballay, A., E. Drenkard, L. R. Hilbun, and F. M. Ausubel (2003). Caenorhabditis elegans innate immune response triggered by Salmonella enterica requires intact LPS and is mediated by a MAPK signaling pathway. Curr Biol 13: 47-52.
Leung, A. C., C. Hughes, J. Gunderson, and M. Lee (2020). Lipopolysaccharide stimulates egg laying in Caenorhabditis elegans. MicroPubl Biol 2020.
Schafer, W. F. (2006). Genetics of egg-laying in worms. Annu Rev Genet 40: 487-509.
Stiernagle, T. (1999). Maintenance of C. elegans. C. elegans 2: 51-67.
Trent, C., N. Tsung, and H. R. Horvitz (1983). Egg-laying defective mutants of the nematode C. elegans. Genetics 104:619-647.
Related files
 bio-protocol_Leung and Lee.2020.Nov.pdf
bio-protocol_Leung and Lee.2020.Nov.pdf - Leung, A and Lee, M(2020). Demonstration of the Stimulatory Effect of Lipopolysaccharide (LPS) on Egg Laying in Caenorhabditis elegans. Bio-protocol Preprint. bio-protocol.org/prep591.
- Ching-Yee, A., Hughes, C., Gunderson, J. and Lee, M.(2020). Lipopolysaccharide stimulates egg laying in Caenorhabditis elegans. microPublication Biology. DOI: 10.17912/micropub.biology.000308
Category
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
