Advanced Search
Stable Transformation of Japonica Rice Callus with GFP-Tagged Overexpression Vector and Transient Transformation Method
Last updated date: Nov 8, 2024 Views: 130 Forks: 0
Reagent
Blotting paper
Filter paper
Sterile large petri dishes
Sterile small petri dishes
Japonica rice seeds
Agrobacterium tumefaciens strain EHA105 (carrying the vector with the target gene)
95% Ethanol
Glucose
Antibiotics (Hygromicin, G418, or herbicide Basta)
Sealing tape
KT (Kinetin) (Sigma, catalog number: K-0753)
6-BA (6-BenzylaminoPurine) (Sigma, catalog number: B-5898)
IAA (Indole-3-acetic acid) (Sigma, catalog number: I-5148)
NAA (Napthalene acetic acid) (Sigma, catalog number: N-0640)
2,4-D (2,4-Dichlorophenoxyacetic acid) (Sigma, catalog number: D-8407)
CH (Casein Enzymatic Hydrolysate) (Sigma, catalog number: C-7290)
Kanamycin (USB, catalog number: 17924)
Cn (Carbenicillin) (GiBco BRL, catalog number: 10177-012)
Hn (hygromycin B) (GiBco BRL, catalog number: 10687-010)
AS (Acetosringone) (Aldrich chem., CO 01531 EG)
Pyridoxine HCl (Sigma, catalog number: P-8666)
Nicotinic acid (Sigma, catalog number: N-0765)
Inositol (Sigma, catalog number: I-3011)
Thiamine HCl (VB1) (Sigma, catalog number: T-3902)
Phytagel HCl (VB6) (Sigma, catalog number: P-8169)
Dimethyl Sulfoxide-DMSO (Sigma, catalog number: D-5879)
X-gluc (5-bromo-4-chloro-3-indolyl-D-galactoside) (Sigma, catalog number: B-3783)
NH4NO3
KH2PO4
KNO3
MgSO4·7H2O
CaCl2
CaCl2·2H2O
MnSO4·4H2O
ZnSO4·7H2O
KI
H3BO3
Na2MoO4·2H2O
CoCl2·6H2O
CuSO4·5H2O
(NH4)SO4
MnSO4·H2O
FeSO4·7H2O
Na2·EDTA·2H2O
KCl
NaH2PO4
NaMoO4·2H2O
KOH
HgCl2
Proline
Sucrose
MSmax Stock Solution (10x) (see solution recipe)
MSmin Stock Solution (100x) (see solution recipe)
N6max Stock Solution (10x) (see solution recipe)
N6min Stock Solution (100x) (see solution recipe)
Fe2+-EDTA Stock Solution (100x) (see solution recipe)
Vitamin Stock Solution (100x) (see solution recipe)
AAmax Stock Solution (10x) (see solution recipe)
AAmin Stock Solution (100x) (see solution recipe)
6-BA Stock Solution (1 mg/ml) (see solution recipe)
KT Stock Solution (1 mg/ml) (see solution recipe)
2,4-D Stock Solution (1 mg/ml) (see solution recipe)
100 mM AS Stock Solution (see solution recipe)
IAA Stock Solution (1 mg/ml) (see solution recipe)
NAA Stock Solution (1 mg/ml) (see solution recipe)
1 N KOH Stock Solution (see solution recipe)
0.15% HgCl2 (see solution recipe)
Induction Medium (see solution recipe)
Subculture Medium (see solution recipe)
Pre-culture Medium (see solution recipe)
Co-culture Medium (see solution recipe)
Suspension Culture Medium (see solution recipe)
Selection Medium (see solution recipe)
Differentiation Medium (see solution recipe)
Rooting Medium (see solution recipe)
Laminar Flow Cabinet
100 ml, 250 ml, 500 ml Triangular Flasks
Rooting Vials
Pipette Forceps
Small Iron Spoon
Alcohol Burner
Water Rinse Cup
Scissors
28°C Shaking Incubator
19°C Incubator
Experimental Procedure
1.Construct pMDC43-X Vector
1.1 PCR Amplification of X Linker Sequence X Sequence Synthesis Rules:
X-F: gcggccgctctagaa + first 20 bp of X CDS
X-R: tgaactatacaaagg + last 20 bp of X CDS (reverse complement)
1.2 Configuration of PCR Amplification System with Linker Gene X (Using KOD FX, TOYOBO KFX-101)
Before preparing the reaction mixture, please thoroughly mix all reagents except for KOD FX (enzyme solution). Frozen reagents should be completely thawed on ice before use.
2x PCR buffer 25 μl
2mM dNTPs 10 μl
X-F Primer 1.5 μl
X-R Primer 1.5 μl
Plant cDNA 0.2 μg
KOD FX (1.0U/μl) 1 μl
ddH2O up to 50 μl Please add KOD FX (enzyme solution) last, and mix the reaction mixture thoroughly with a Vortex or similar device, then spin down before proceeding with PCR.
1.3 PCR Amplification of Gene X with Linkers
Subsequently, use a temperature-controlled PCR machine with the following program:
Predenature 94℃, 2 min.
Denature 98℃, 10 sec
Annealing (Tm-5)℃, 30 sec
Extension 68℃, 1kb/min Set Denature to Extension for 33 cycles.
Final extension 68℃, 7 min. After the reaction is complete, transfer to a 4℃ refrigerator for storage.
1.4 Take the pGBKT7 plasmid out of the -20℃ freezer, place 10x rCutSmart buffer on ice, and after complete dissolution, configure the following system:
10x rCutSmart buffer 5 μl
Asc I-HF 1 μl
Spe I-HF 1 μl
pMDC43 Plasmid DNA 1 μg
ddH2O up to 50 μl Subsequently, use a temperature-controlled PCR machine with the following program:
37℃ 45 min
65℃ 45 sec After the reaction is complete, transfer to a 4℃ refrigerator for storage.
1.5 Agarose Gel Electrophoresis and Gel Recovery (EasyPure Quick Gel Extraction Kit, TransGen Biotech, EG101)
(The method can refer to Wang, S, Huang, Z, Liu, Y, Shao, S, Li, L and Ma, M(2024). Application of the Nicotiana Allergic Necrosis Assay for the Validation of Protein-Protein Interactions between Fungal Effectors and Plant Receptor Kinases. Bio-protocol Preprint. bio-protocol.org/prep2729.)
1.6 Construct pMDC43-X Vector by Homologous Recombination (ClonExpress® Ultra One Step Cloning Kit, Vazyme, C115)
Calculate the dosage of linearized vector and insert fragment:
Optimal cloning vector dosage = [0.02 × cloning vector base pairs] ng (0.03 pmol)
Optimal insert fragment dosage = [0.04 × insert fragment base pairs] ng (0.06 pmol) Note: Calculate the required DNA amount for the recombination reaction according to the formula. To ensure the accuracy of pipetting, dilute the linearized vector and insert fragment appropriately before preparing the recombination reaction system, with each component's volume not less than 1μl. Prepare the following reaction system on ice:
Linearized vector pMDC43 X μl
Insert fragment X μl
2 × ClonExpress Mix 5 μl
ddH2O to 10 μl Gently pipette to mix (do not vortex), briefly centrifuge to collect the reaction liquid at the bottom of the tube. Subsequently, use a temperature-controlled PCR machine with the following program:
50℃ 30 min After the reaction is complete, transfer to a 4℃ refrigerator for storage.
1.7 After sequencing of pMDC43-X, scale up the E. coli culture and extract the plasmid. (The method can refer to Wang, S, Huang, Z, Liu, Y, Shao, S, Li, L and Ma, M(2024). Application of the Nicotiana Allergic Necrosis Assay for the Validation of Protein-Protein Interactions between Fungal Effectors and Plant Receptor Kinases. Bio-protocol Preprint. bio-protocol.org/prep2729.)
2. Agrobacterium Transformation of pMDC43-X Recombinant Plasmid
2.1 Take 5μl of the final pMDC43-X plasmid DNA (approximately 1-2μg) and add it to 100μl of Agrobacterium tumefaciens competent GV3101 cells, then mix well.
2.2 Incubate on ice for 30 minutes, then quickly freeze in liquid nitrogen for 5 minutes, followed by a 37°C water bath for 5 minutes, and immediately place on ice for 2 minutes.
2.3 Add 800μl of liquid LB medium, and culture at 28°C with shaking at 200 rpm for 4 hours.
2.4 Take 200μl of the bacterial culture and spread it on solid LB agar plates containing 50μg/ml Kanamycin and 50 μg/ml Rifampicin, then incubate at 28°C for 48 hours.
3. Genetic Transformation of Japonica Rice Mediated by Agrobacterium tumefaciens
Note: All procedures in this experiment must be performed under sterile conditions.
3.1. Seed Sterilization and Induction of Callus Tissue
Prepare the induction medium (see solution recipe), dispense it into 100 ml triangular flasks (40-50 ml of medium per flask), seal with parafilm, and sterilize at 121°C under high pressure for 15 minutes, then cool and set aside. Japonica rice seeds (Nipponbare) are dehulled and first washed with 75% ethanol for 1 minute (do not exceed this time), then sterilized with 0.15% HgCl2 for 15-20 minutes, and finally rinsed with sterile dH2O 4-5 times. Inoculate 8-12 sterilized Nipponbare seeds into each bottle of induction medium and culture in the dark at 30°C for 40-45 days to induce callus formation.
3.2. Subculturing of Callus Tissue
3.2.1 Prepare the subculture medium (see solution recipe) three days in advance, dispense 25-30 ml of medium into each 50 ml triangular flask, seal with parafilm, and sterilize at 121°C under high pressure for 15 minutes, then cool and set aside. Preparing the medium three days in advance allows the surface of the medium to be relatively dry when used, as too wet a medium is not conducive to callus growth.
3.2.2 Select pale yellow, granular, dry, and vigorous callus tissue from the induced callus and transfer it into the subculture medium for dark culture for 20 days; during the first subculture, ensure to remove any attached tissues such as endosperm and buds thoroughly. Callus tissue that has been subcultured once can be used for Agrobacterium infection for transformation; the maximum number of subcultures for callus used for transformation should not exceed two. Multiple subcultures can lead to somatic mutations in the callus tissue and reduce transformation efficiency.
3.3. Pre-culture
3.3.1 Prepare an appropriate amount of sterile pre-culture medium in a 500 ml triangular flask in advance (see solution recipe), seal with parafilm, and sterilize at high temperature and pressure for 12 minutes; before use, melt with a microwave, wait until it cools to around 55°C, add 300 μl AS and 5 ml of 50% glucose to every 250 ml of medium, mix well, and pour into 8-10 dishes.
3.3.2 Select pale yellow, granular, dry, and vigorous callus tissue from the subcultured callus and transfer it into the pre-culture medium, inoculating 60-80 pieces the size of green pea seeds per dish. If the callus pieces are too large, they can be crushed with tweezers. Pre-culture in the dark at 28°C for 3-4 days.
3.4. Infection and Co-cultivation
3.4.1 Two days before the experiment, streak the Agrobacterium strain with pMDC43-X on LA plates containing the appropriate antibiotics and incubate at 28°C for 2 days. Prepare suspension medium (100 ml per segment) (see solution recipe) and co-cultivation medium (250 ml per segment) (see solution recipe), seal with parafilm, and sterilize at 121°C under high temperature and pressure for 12 minutes. Prepare several 250 ml triangular flasks, lined with several sheets of blotting paper and filter paper, large and small petri dishes, sterilize at 121°C under high temperature and pressure for 30 minutes, and dry at 80°C before use.
3.4.2 Take the Agrobacterium plate streaked with pMDC43-X, use an inoculation loop to transfer about one loop of Agrobacterium into 100 ml of suspension medium (see solution recipe), add 100 μl AS and 2 ml of 50% glucose, place at a constant temperature shaker at 28°C, and culture with shaking at 200 rpm for 30 minutes. The Agrobacterium suspension concentration should be approximately OD600 = 0.3 (slightly turbid when observed by eye).
3.4.3 While the Agrobacterium suspension of pMDC43-X is being cultured with shaking, collect the subcultured callus tissue into a 250 ml sterile triangular flask.
3.4.4 Pour the prepared Agrobacterium suspension from step 4.3 into the triangular flask containing the callus tissue until all the callus tissue is submerged, and let it stand for 10 minutes.
3.4.5 Pour off the bacterial solution. Take a sterilized small petri dish lined with blotting paper and filter paper, open the small dish and invert the triangular flask with callus onto the filter paper of the small dish, drain the bacterial solution as much as possible, then spread the callus onto the filter paper of a sterile large dish, cover with a sterilized filter paper, press the filter paper gently with tweezers to absorb the bacterial solution on the surface of the callus, remove the filter paper after it absorbs the moisture, and repeat this process four times for both the top and bottom filter papers. Finally, cover the callus with a filter paper, close the large dish, and air-dry for 1-2 hours.
Note: It is not recommended to open the dish lid to dry at this step, as it may lead to excessive drying and dehydration of the callus tissue.
3.4.6 While the callus tissue is drying, heat the co-cultivation medium in a microwave to melt, wait until it cools to around 55°C, add 300 μl AS and 5 ml of 50% glucose solution, mix well, and pour into 8-10 dishes.
3.4.7 Use tweezers (or a spoon) to transfer the dried callus particles onto the co-cultivation medium (do not move the callus after transferring it to the co-cultivation medium to reduce contact between the medium and the callus surface, preventing excessive growth of Agrobacterium).
3.4.8 Seal with parafilm and co-cultivate in the dark at 19°C for 3 days.
3.5. Washing and Screening (S1)
3.5.1 Prepare sterile distilled water, large and small petri dishes (containing multiple sheets of blotting paper and filter paper), and several 250 ml triangular flasks, sterilize at 121°C under high temperature and pressure for 30 minutes. After sterilization, place the large and small petri dishes in a 80°C oven to dry. Prepare the screening medium (see solution recipe), seal with parafilm, and sterilize at high temperature and pressure for 15 minutes.
3.5.2 Transfer the callus tissue after co-cultivation into a water wash cup, pour in sterile distilled water to completely submerge the callus tissue, cover and agitate for 20-30 seconds, then discard the sterile distilled water, repeating this washing process 3-4 times. Add sterile distilled water to completely submerge the callus tissue again, cover and agitate for 20-30 seconds, then let it stand for 3-5 minutes before discarding the sterile distilled water, repeating this washing process 3-4 times. Observe the water in the wash cup; if it is clear, it indicates that the Agrobacterium has been mostly cleaned off, otherwise continue washing. Finally, discard the sterile distilled water and add sterile distilled water containing 500 mg/L Cefotaxime (Cn), let it stand for 30 minutes.
3.5.3 Pour off the sterile distilled water containing 500 mg/L Cefotaxime (Cn), and refer to section 4.5 for the subsequent drying of the callus tissue. While drying the callus tissue, melt 250 ml of screening medium in a microwave, wait until it cools to around 55°C, add 400 μl Cefotaxime, 250 μl Hygromycin (Hn), and 5 ml of 50% glucose, mix well, and pour into 8-10 dishes. After pouring, open the dish lids on a laminar flow hood and use sterile air to blow for 1.5-2 hours (the surface of the screening medium should not be too wet, otherwise it is not conducive to the inhibition of Agrobacterium and the growth of resistant callus during screening).
3.5.4 After the callus is dried, use tweezers to transfer the callus particles onto the screening medium (suggested inoculation density is 20-25 pieces of callus/dish), and seal with parafilm.
3.5.5 Place in a dark incubator for screening culture for 20 days (first screening S1).
3.6. Second Screening (S2)
3.6.1 Prepare the S2 stage screening medium (see solution recipe), seal with parafilm, and sterilize at high temperature and pressure for 15 minutes; heat 250 ml of the screening medium in a microwave until it cools to around 55°C, then add 300 μl CN, 250 μl Hn, and 5 ml of 50% glucose in sequence, mix well, and pour into 8-10 dishes; after pouring, open the dish lids on a laminar flow hood and use sterile air to blow for 1.5-2 hours (the surface of the screening medium should not be too wet, otherwise it is not conducive to the inhibition of Agrobacterium and the growth of resistant callus during screening).
3.6.2 Select dry, Agrobacterium-free callus from the S1 medium and transfer it to the S2 medium (inoculate 20 to 35 pieces of callus per dish).
3.6.3 Incubate in the dark for 20 days, observe whether new, tender, yellow resistant callus grows. If no resistant callus has grown, continue to transfer to S3 screening medium (except for the CN added to the medium which can be appropriately reduced to 200 μl/250 ml, the rest of the preparation and sterilization method is the same as S1 and S2). Generally, after two screenings, that is, at the S2 stage, resistant callus can grow in most japonica rice varieties.
3.7. Differentiation
3.7.1 Prepare the differentiation medium (see solution recipe) 3-4 days in advance, add 40-50 ml of differentiation medium to a 100 ml triangular flask, seal with parafilm, and sterilize at 121°C under high temperature and pressure for 15 minutes.
3.7.2 Select pale yellow, dense, dry, and vigorously growing small pieces of resistant callus, pick only one resistant callus from each clump (since callus tissues from the same clump are mostly genetically identical), and be careful not to pick callus with Agrobacterium growth. Place 3-4 small pieces of resistant callus evenly in each bottle of differentiation medium, as callus cells will continue to grow on the differentiation medium, and placing them too densely can lead to different callus pieces growing together and becoming indistinguishable.
3.7.3 Incubate under light (28°C, 14 hours of light/10 hours of darkness) for 30-40 days, and when the differentiated seedlings are 3-5 cm tall, proceed to root induction. During the light incubation period, promptly remove any contaminated materials.
3.8. Rooting
3.8.1 Prepare the rooting medium (see solution recipe), add 4-5 cm of medium to each rooting tube, seal with parafilm, and sterilize at 121°C under high temperature and pressure for 15 minutes. Prepare 4-5 sterilized empty petri dishes.
3.8.2 Use tweezers to remove the differentiated seedlings from the differentiation medium, place them in sterilized empty petri dishes, take 1 healthy seedling from each callus, clean the seedlings with scissors (trim dead or yellowing leaves and roots that have grown on the differentiation medium), and transfer them into the rooting tubes, with 1 seedling per tube.
3.8.3 Incubate in the light for root induction for 15-20 days, and after the new roots have grown sufficiently, proceed to transplanting.
3.9. Transplanting
3.9.1 Unseal the rooting tubes, add some tap water, and continue to grow in the light incubator (hardening) for 3-4 days.
3.9.2 During the hardening period, take small leaf samples for transgene positive detection.
3.9.3 Remove the transformed seedlings from the rooting tubes, wash off the attached medium from the roots, and transplant them into prepared soil in pots or buckets.
Notes:
All processes, except for transplanting, must be carried out under sterile conditions to prevent contamination.
Contaminated materials should be removed promptly to prevent the spread of contamination.
Try to select callus tissue with good growth and strong vitality for the experiment.
4.Transient Transformation of Tobacco with Exogenous Proteins containing GFP
Note: Transient transformation can be achieved using either protoplasts or tobacco leaves; transformation via protoplasts can refer to Wang, S, Huang, Z, sijia, T, Feng, S, Liu, X, Shu, Y, Liang, Y and Chen, Z(2024). A buffer formulation and application for efficient protoplast extraction and transformation of rice. Bio-protocol Preprint. bio-protocol.org/prep2723.;while transient transformation using tobacco leaves can refer to Wang, S, Huang, Z, Liu, Y, Shao, S, Li, L and Ma, M(2024). Application of the Nicotiana Allergic Necrosis Assay for the Validation of Protein-Protein Interactions between Fungal Effectors and Plant Receptor Kinases. Bio-protocol Preprint. DOI: 10.21769/p2729.
eg: Transient Transformation of Tobacco Leaves with Exogenous Proteins containing GFP
4.1.1 Inoculation of Agrobacterium Single Colonies:Select single colonies of Agrobacterium containing the final vectors pMDC43-X and inoculate them into 5 ml of LB medium containing 50 μg/ml Kanamycin and 50 μg/ml Rifampicin. Cultivate at 28°C with 200 rpm shaking for 2 days. (Freshly transformed Agrobacterium single colonies can be cultured overnight in 3 ml of medium until day 1.)
4.1.2 Liquid Culture and Expansion: Transfer 1 ml of the cultured Agrobacterium liquid to 20 ml of liquid LB medium containing 50 μg/ml Kanamycin and 50 μg/ml Rifampicin for expanded culture. This LB medium also contains 15 μM acetosyringone. Cultivate at 28°C with 200 rpm shaking until the Agrobacterium reaches the logarithmic growth phase (OD600 = 0.5-0.6).
4.1.3 Collection and Resuspension of Bacterial Cells:Centrifuge at 5,000 rpm for 10 minutes at room temperature to collect the bacterial cells. Resuspend the Agrobacterium cells in infiltration buffer X (containing 10 mM MgCl2, 10 mM MES, 150 μM acetosyringone, pH = 5.6) to an OD600 of 1.0. Allow the cells to stand at room temperature for 2 hours.
4.1.4 Mixture of Bacterial Cultures and Infiltration: Use a 1 ml needle to gently make a small incision on the abaxial side of a Nicotiana benthamiana leaf (be careful not to pierce through). Then, use a needle without a syringe toabsorb the bacterial suspension and inject it into the leaf through the wound. Mark the area on the leaf with a marker.
4.1.5 Cultivation and Phenotypic Observation: Cultivate the injected plants in the dark at approximately 25°C for 48-72 hour, then observe for phenotypes in the areas of the tobacco leaves infiltrated with Agrobacterium, and save photographs using a camera.
Supplementary: Solution Formulas
0.5 M MES (pH 5.6): Weigh out 9.75 g of anhydrous MES and dissolve in deionized water. Adjust the pH to 5.6 with NaOH, bring to a final volume of 100 ml, filter sterilize, and store at 4°C.
150 mM Acetosyringone: Weigh out 2.943g of acetosyringone and dissolve in 5 ml of DMSO (dimethyl sulfoxide). Add deionized water to bring to a final volume of 10 ml, filter sterilize, and store at -20°C.
Note:1 mM=1000 μM
Solution Recipes
I. Solution Preparation
MSmax Stock Solution (10x)
16.5 g NH4NO3
1.7 g KH2PO4
19.0 g KNO3
3.7 g MgSO4·7H2O or 4.4 g CaCl2·2H2O Add two-thirds volume of dH2O, then dissolve the above reagents one by one, and finally add sterilized distilled water to make up to 1,000 ml, store at room temperature.
MSmin Stock Solution (100x)
2.23 g MnSO4·4H2O
0.86 g ZnSO4·7H2O
0.083 g KI
0.62 g H3BO3
0.025 g Na2MoO4·2H2O
0.0025 g CoCl2·6H2O
0.0025 g CuSO4·5H2O Add dH2O to make up to 1,000 ml, store at room temperature. Note: Na2MoO4 must be dissolved separately before mixing with other components.
N6max Stock Solution (10x)
28.3 g KNO3
4.63 g (NH4)SO4
4.0 g KH2PO4
1.85 g MgSO4·7H2O
1.25 g CaCl2 or 1.66 g CaCl2·2H2O Add two-thirds volume of dH2O, then dissolve the above reagents one by one, and finally add sterilized distilled water to make up to 1,000 ml, store at room temperature.
N6min Stock Solution (100x)
0.08 g KI
0.16 g H3BO3
0.15 g ZnSO4·7H2O
0.44 g MnSO4·4H2O or 0.3335 g MnSO4·H2O Add dH2O to make up to 1,000 ml, store at room temperature.
Fe2+-EDTA Stock Solution (100x)
Add about 300 ml dH2O and 2.78g FeSO4·7H2O to one reagent bottle; add about 300 ml dH2O to another reagent bottle and heat to 70°C, then add 3.73 g Na2·EDTA·2H2O; after both solutions are dissolved, let them cool to room temperature, mix the solutions from both bottles, and then add dH2O to make up to 1,000 ml, store at 4°C in the dark.
Vitamin Stock Solution (100x)
0.1 g Nicotinic acid
0.1 g Thiamine HCl (VB1)
0.1 g Pyridoxine HCl (VB6)
10 g Inositol
0.2 g Glycine Add dH2O to make up to 1,000 ml, store at 4°C.
AAmax Stock Solution (10x)
29.50 g KCl
2.50 g MgSO4·7H2O
1.50 g NaH2PO4
1.50 g CaCl2·2H2O Add dH2O to make up to 1,000 ml, store at room temperature in the dark.
AAmin Stock Solution (100x)
1.0 g MnSO4·H2O
0.2 g ZnSO4·7H2O
0.0025 g CuSO4·5H2O
0.3 g H3BO3
0.075 g KI
0.0025 g CoCl2·6H2O
0.025 g NaMoO4·2H2O Add dH2O to make up to 1,000 ml, store at room temperature in the dark. Note: Na2MoO4 must be dissolved separately before mixing with other components.
6-BA Stock Solution (1 mg/ml)
100 mg 6-BA Add 1.0 ml 1N KOH and shake until 6-BA is dissolved, then add dH2O to make up to 100 ml, store at room temperature.
KT Stock Solution (1 mg/ml)
100 mg KT Add 1.0 ml 1N KOH and shake until KT is dissolved, then add dH2O to make up to 100 ml, store at room temperature.
2,4-D Stock Solution (1 mg/ml)
100 mg 2,4-D Add 1.0 ml 1N KOH and shake for 5 minutes, then add 10 ml dH2O and shake until 2,4-D is dissolved, add dH2O to make up to 100 ml, store at room temperature.
100 mM AS Stock Solution
0.196 g AS
10 ml DMSO Divide into 1.5 ml centrifuge tubes and store at 4°C.
IAA Stock Solution (1 mg/ml)
100 mg IAA Add 1.0 ml 1N KOH and shake until IAA is dissolved, then add dH2O to make up to 100 ml, store at room temperature in the dark.
NAA Stock Solution (1 mg/ml)
100 mg NAA Add 1.0 ml 1N KOH and shake until NAA is dissolved, then add dH2O to make up to 100 ml, store at room temperature in the dark.
1 N KOH Stock Solution
5.6 g KOH Dissolve in 100 ml dH2O, store at room temperature.
0.15% HgCl2
1.5 g HgCl2 First, partially or completely dissolve with 1 ml anhydrous ethanol Then add dH2O to make up to 1,000 ml, stir for 4-8 hours, store at room temperature properly. Note: Mercury chloride is highly toxic.
II. Medium Preparation
Note: All media must be prepared immediately before use.
Induction Medium
N6max Stock Solution (10x) 100 ml
N6min Stock Solution (100x) 10 ml
Vitamin Stock Solution (100x) 10 ml
Fe2+-EDTA Stock Solution (100x) 10 ml
2,4-D Stock Solution (1 mg/ml) 2.5 ml
CH 0.6 g
Proline 0.3 g
Sucrose 30 g
Phytagel 3 g Adjust pH to 5.9, add dH2O to 1,000 ml
Subculture Medium
N6max Stock Solution (10x) 100 ml
N6min Stock Solution (100x) 10 ml
Vitamin Stock Solution (100x) 10 ml
Fe2+-EDTA Stock Solution (100x) 10 ml
2,4-D Stock Solution (1 mg/ml) 2.0 ml
CH 0.6 g
Proline 0.5 g
Sucrose 30 g
Phytagel 3 g Adjust pH to 5.9, add dH2O to 1,000 ml
Pre-culture Medium
N6max Stock Solution (10x) 12.5 ml
N6min Stock Solution (100x) 1.25 ml
Fe2+-EDTA Stock Solution (100x) 25 ml
2,4-D Stock Solution (1 mg/ml) 0.75 ml
CH 0.15 g
Sucrose 5 g
Agar 1.75 g Adjust pH to 5.4, add dH2O to 250 ml
Co-cultivation Medium
N6max Stock Solution (10x) 12.5 ml
N6min Stock Solution (100x) 1.25 ml
Vitamin Stock Solution (100x) 2.5 ml
Fe2+-EDTA Stock Solution (100x) 25 ml
2,4-D Stock Solution (1 mg/ml) 0.75 ml
CH 0.2 g
Sucrose 5 g
Agar 1.75 g Adjust pH to 5.4, add dH2O to 250 ml
Suspension Medium
N6max Stock Solution (10x) 5 ml
N6min Stock Solution (100x) 0.5 ml
Vitamin Stock Solution (100x) 1 ml
Fe2+-EDTA Stock Solution (100x) 0.5 ml
2,4-D Stock Solution (1 mg/ml) 0.2 ml
CH 0.08 g
Sucrose 2 g Adjust pH to 5.4, add dH2O to 100 ml
Screening Medium
N6max Stock Solution (10x) 25 ml
N6min Stock Solution (100x) 2.5 ml
Vitamin Stock Solution (100x) 2.5 ml
Fe2+-EDTA Stock Solution (100x) 2.5 ml
2,4-D Stock Solution (1 mg/ml) 0.625 ml
CH 0.15 g
Sucrose 7.5 g
Agar 1.75 g Adjust pH to 6.0, add dH2O to 250 ml
Differentiation Medium
MSmax Stock Solution (10x) 100 ml
MSmin Stock Solution (100x) 10 ml
Vitamin Stock Solution (100x) 10 ml
Fe2+-EDTA Stock Solution (100x) 10 ml
6-BA 2.0 ml
KT 2.0 ml
IAA 0.2 ml
NAA 0.2 ml
Sucrose 30 g
CH 1 g
Phytagel 3 g Adjust pH to 6.0, add dH2O to 1,000 ml
Rooting Medium
MSmax Stock Solution (10x) 50 ml
MSmin Stock Solution (100x) 5 ml
Vitamin Stock Solution (100x) 10 ml
Fe2+-EDTA Stock Solution (100x) 10 ml
Sucrose 20 g
Phytagel 3 g Adjust pH to 5.8, add dH2O to 1,000 ml
Vector Name: pMDC43
Vector Resistance: Kanamycin, Chloramphenicol
Vector Length: 12460 bp
Vector Type: Gene expression
Replication Origin: ori
Host: Plants
Selection Marker: Hyg
Promoter: CaMV35S (enhanced)
Competent Cells: DB3.1
The forward primer used for sequencing: ggacaggtaatggttgtct
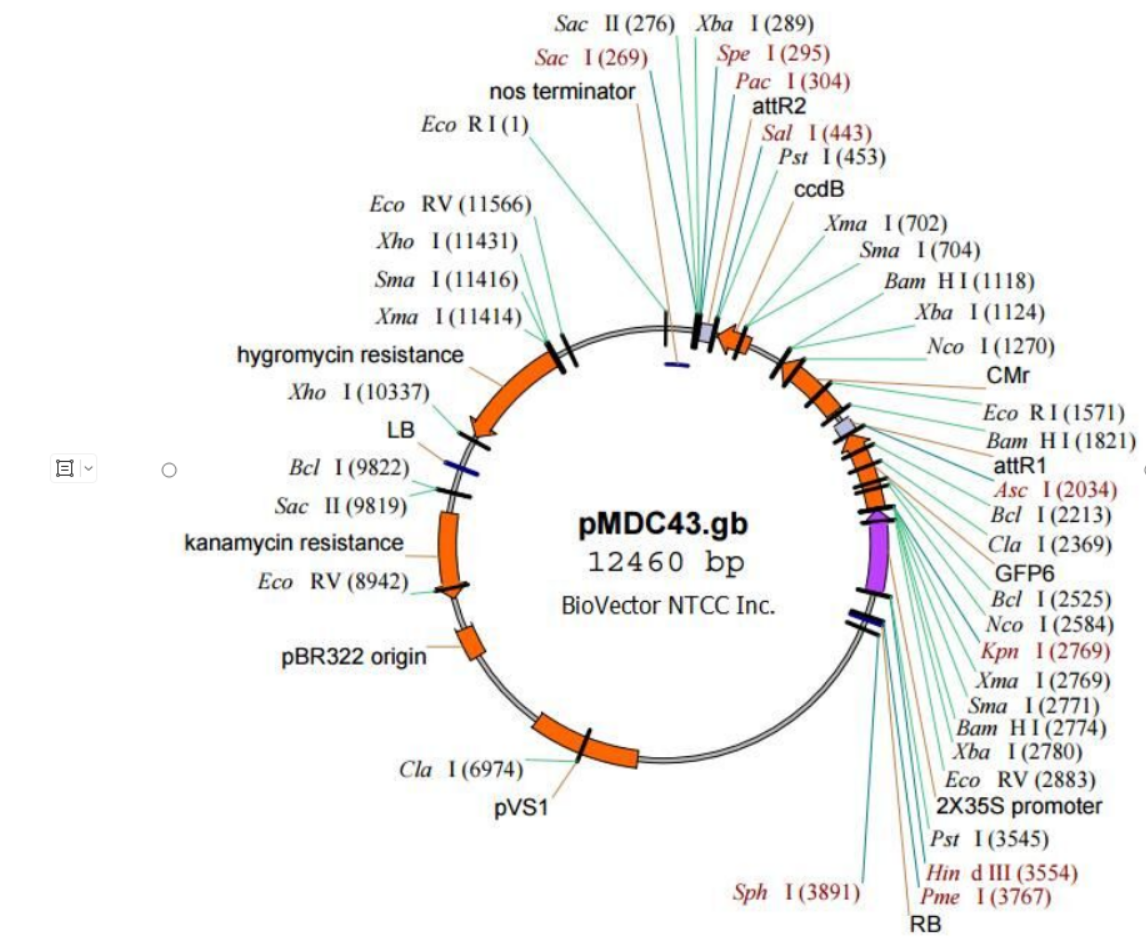
>pMDC43
AATTCAGTAACATAGATGACACCGCGCGCGATAATTTATCCTAGTTTGCGCGCTATATTTTGTTTTCTATCGCGTATTAAATGTATAATTGCGGGACTCTAATCATAAAAACCCATCTCATAAATAACGTCATGCATTACATGTTAATTATTACATGCTTAACGTAATTCAACAGAAATTATATGATAATCATCGCAAGACCGGCAACAGGATTCAATCTTAAGAAACTTTATTGCCAAATGTTTGAACGATCGGGGAAATTCGAGCTCCACCGCGGTGGCGGCCGCTCTAGAACTAGTTAATTAAGAATTATCGAACCACTTTGTACAAGAAAGCTGAACGAGAAACGTAAAATGATATAAATATCAATATATTAAATTAGATTTTGCATAAAAAACAGACTACATAATACTGTAAAACACAACATATCCAGTCACTATGGTCGACCTGCAGACTGGCTGTGTATAAGGGAGCCTGACATTTATATTCCCCAGAACATCAGGTTAATGGCGTTTTTGATGTCATTTTCGCGGTGGCTGAGATCAGCCACTTCTTCCCCGATAACGGAGACCGGCACACTGGCCATATCGGTGGTCATCATGCGCCAGCTTTCATCCCCGATATGCACCACCGGGTAAAGTTCACGGGAGACTTTATCTGACAGCAGACGTGCACTGGCCAGGGGGATCACCATCCGTCGCCCGGGCGTGTCAATAATATCACTCTGTACATCCACAAACAGACGATAACGGCTCTCTCTTTTATAGGTGTAAACCTTAAACTGCATTTCACCAGTCCCTGTTCTCGTCAGCAAAAGAGCCGTTCATTTCAATAAACCGGGCGACCTCAGCCATCCCTTCCTGATTTTCCGCTTTCCAGCGTTCGGCACGCAGACGACGGGCTTCATTCTGCATGGTTGTGCTTACCAGACCGGAGATATTGACATCATATATGCCTTGAGCAACTGATAGCTGTCGCTGTCAACTGTCACTGTAATACGCTGCTTCATAGCACACCTCTTTTTGACATACTTCGGGTATACATATCAGTATATATTCTTATACCGCAAAAATCAGCGCGCAAATACGCATACTGTTATCTGGCTTTTAGTAAGCCGGATCCTCTAGATTACGCCCCGCCCTGCCACTCATCGCAGTACTGTTGTAATTCATTAAGCATTCTGCCGACATGGAAGCCATCACAGACGGCATGATGAACCTGAATCGCCAGCGGCATCAGCACCTTGTCGCCTTGCGTATAATATTTGCCCATGGTGAAAACGGGGGCGAAGAAGTTGTCCATATTGGCCACGTTTAAATCAAAACTGGTGAAACTCACCCAGGGATTGGCTGAGACGAAAAACATATTCTCAATAAACCCTTTAGGGAAATAGGCCAGGTTTTCACCGTAACACGCCACATCTTGCGAATATATGTGTAGAAACTGCCGGAAATCGTCGTGGTATTCACTCCAGAGCGATGAAAACGTTTCAGTTTGCTCATGGAAAACGGTGTAACAAGGGTGAACACTATCCCATATCACCAGCTCACCGTCTTTCATTGCCATACGGAATTCCGGATGAGCATTCATCAGGCGGGCAAGAATGTGAATAAAGGCCGGATAAAACTTGTGCTTATTTTTCTTTACGGTCTTTAAAAAGGCCGTAATATCCAGCTGAACGGTCTGGTTATAGGTACATTGAGCAACTGACTGAAATGCCTCAAAATGTTCTTTACGATGCCATTGGGATATATCAACGGTGGTATATCCAGTGATTTTTTTCTCCATTTTAGCTTCCTTAGCTCCTGAAAATCTCGCCGGATCCTAACTCAAAATCCACACATTATACGAGCCGGAAGCATAAAGTGTAAAGCCTGGGGTGCCTAATGCGGCCGCCATAGTGACTGGATATGTTGTGTTTTACAGTATTATGTAGTCTGTTTTTTATGCAAAATCTAATTTAATATATTGATATTTATATCATTTTACGTTTCTCGTTCAGCTTTTTTGTACAAACTTGTTTGATAGCTTGGCGCGCCTTTGTATAGTTCATCCATGCCATGTGTAATCCCAGCAGCTGTTACAAACTCAAGAAGGACCATGTGGTCTCTCTTTTCGTTGGGATCTTTCGAAAGGGCAGATTGTGTGGACAGGTAATGGTTGTCTGGTAAAAGGACAGGGCCATCGCCAATTGGAGTATTTTGTTGATAATGATCAGCGAGTTGCACGCCGCCGTCTTCGATGTTGTGGCGGGTCTTGAAGTTGGCTTTGATGCCGTTCTTTTGCTTGTCGGCCATGATGTATACGTTGTGGGAGTTGTAGTTGTATTCCAACTTGTGGCCGAGGATGTTTCCGTCCTCCTTGAAATCGATTCCCTTAAGCTCGATCCTGTTGACGAGGGTGTCTCCCTCAAACTTGACTTCAGCACGTGTCTTGTAGTTCCCGTCGTCCTTGAAGAAGATGGTCCTCTCCTGCACGTATCCCTCAGGCATGGCGCTCTTGAAGAAGTCGTGCCGCTTCATATGATCAGGGTAACGGGAGAAGCACTGCACGCCGTAGGTCAGGGTGGTGACCAGGGTTGGCCATGGAACAGGTAGTTTTCCAGTAGTGCAAATAAATTTAAGGGTAAGTTTTCCGTATGTTGCATCACCTTCACCCTCTCCACTGACAGAAAATTTGTGCCCATTAACATCACCATCTAATTCAACAAGAATTGGGACAACTCCAGTGAAAAGTTCTTCTCCTTTACTCATTTTTTCTACCGGTACCCGGGGATCCTCTAGAGTCGAGGTCCTCTCCAAATGAAATGAACTTCCTTATATAGAGGAAGGGTCTTGCGAAGGATAGTGGGATTGTGCGTCATCCCTTACGTCAGTGGAGATATCACATCAATCCACTTGCTTTGAAGACGTGGTTGGAACGTCTTCTTTTTCCACGATGCTCCTCGTGGGTGGGGGTCCATCTTTGGGACCACTGTCGGCAGAGGCATCTTCAACGATGGCCTTTCCTTTATCGCAATGATGGCATTTGTAGGAGCCACCTTCCTTTTCCACTATCTTCACAATAAAGTGACAGATAGCTGGGCAATGGAATCCGAGGAGGTTTCCGGATATTACCCTTTGTTGAAAAGTCTCAATTGCCCTTTGGTCTTCTGAGACTGTATCTTTGATATTTTTGGAGTAGACAAGTGTGTCGTGCTCCACCATGTTATCACATCAATCCACTTGCTTTGAAGACGTGGTTGGAACGTCTTCTTTTTCCACGATGCTCCTCGTGGGTGGGGGTCCATCTTTGGGACCACTGTCGGCAGAGGCATCTTCAACGATGGCCTTTCCTTTATCGCAATGATGGCATTTGTAGGAGCCACCTTCCTTTTCCACTATCTTCACAATAAAGTGACAGATAGCTGGGCAATGGAATCCGAGGAGGTTTCCGGATATTACCCTTTGTTGAAAAGTCTCAATTGCCCTTTGGTCTTCTGAGACTGTATCTTTGATATTTTTGGAGTAGACAAGTGTGTCGTGCTCCACCATGTTGACCTGCAGGCACGCCAAGCTTGGCACTGGCCGTCGTTTTACAACGTCGTGACTGGGAAAACCCTGGCGTTACCCAACTTAATCGCCTTGCAGCACATCCCCCTTTCGCCAGCTGGCGTAATAGCGAAGAGGCCCGCACCGATCGCCCTTCCCAACAGTTGCGCAGCCTGAATGGCGAATGCTAGAGCAGCTTGAGCTTGGATCAGATTGTCGTTTCCCGCCTTCAGTTTAAACTATCAGTGTTTGACAGGATATATTGGCGGGTAAACCTAAGAGAAAAGAGCGTTTATTAGAATAACGGATATTTAAAAGGGCGTGAAAAGGTTTATCCGTTCGTCCATTTGTATGTGCATGCCAACCACAGGGTTCCCCTCGGGATCAAAGTACTTTGATCCAACCCCTCCGCTGCTATAGTGCAGTCGGCTTCTGACGTTCAGTGCAGCCGTCTTCTGAAAACGACATGTCGCACAAGTCCTAAGTTACGCGACAGGCTGCCGCCCTGCCCTTTTCCTGGCGTTTTCTTGTCGCGTGTTTTAGTCGCATAAAGTAGAATACTTGCGACTAGAACCGGAGACATTACGCCATGAACAAGAGCGCCGCCGCTGGCCTGCTGGGCTATGCCCGCGTCAGCACCGACGACCAGGACTTGACCAACCAACGGGCCGAACTGCACGCGGCCGGCTGCACCAAGCTGTTTTCCGAGAAGATCACCGGCACCAGGCGCGACCGCCCGGAGCTGGCCAGGATGCTTGACCACCTACGCCCTGGCGACGTTGTGACAGTGACCAGGCTAGACCGCCTGGCCCGCAGCACCCGCGACCTACTGGACATTGCCGAGCGCATCCAGGAGGCCGGCGCGGGCCTGCGTAGCCTGGCAGAGCCGTGGGCCGACACCACCACGCCGGCCGGCCGCATGGTGTTGACCGTGTTCGCCGGCATTGCCGAGTTCGAGCGTTCCCTAATCATCGACCGCACCCGGAGCGGGCGCGAGGCCGCCAAGGCCCGAGGCGTGAAGTTTGGCCCCCGCCCTACCCTCACCCCGGCACAGATCGCGCACGCCCGCGAGCTGATCGACCAGGAAGGCCGCACCGTGAAAGAGGCGGCTGCACTGCTTGGCGTGCATCGCTCGACCCTGTACCGCGCACTTGAGCGCAGCGAGGAAGTGACGCCCACCGAGGCCAGGCGGCGCGGTGCCTTCCGTGAGGACGCATTGACCGAGGCCGACGCCCTGGCGGCCGCCGAGAATGAACGCCAAGAGGAACAAGCATGAAACCGCACCAGGACGGCCAGGACGAACCGTTTTTCATTACCGAAGAGATCGAGGCGGAGATGATCGCGGCCGGGTACGTGTTCGAGCCGCCCGCGCACGTCTCAACCGTGCGGCTGCATGAAATCCTGGCCGGTTTGTCTGATGCCAAGCTGGCGGCCTGGCCGGCCAGCTTGGCCGCTGAAGAAACCGAGCGCCGCCGTCTAAAAAGGTGATGTGTATTTGAGTAAAACAGCTTGCGTCATGCGGTCGCTGCGTATATGATGCGATGAGTAAATAAACAAATACGCAAGGGGAACGCATGAAGGTTATCGCTGTACTTAACCAGAAAGGCGGGTCAGGCAAGACGACCATCGCAACCCATCTAGCCCGCGCCCTGCAACTCGCCGGGGCCGATGTTCTGTTAGTCGATTCCGATCCCCAGGGCAGTGCCCGCGATTGGGCGGCCGTGCGGGAAGATCAACCGCTAACCGTTGTCGGCATCGACCGCCCGACGATTGACCGCGACGTGAAGGCCATCGGCCGGCGCGACTTCGTAGTGATCGACGGAGCGCCCCAGGCGGCGGACTTGGCTGTGTCCGCGATCAAGGCAGCCGACTTCGTGCTGATTCCGGTGCAGCCAAGCCCTTACGACATATGGGCCACCGCCGACCTGGTGGAGCTGGTTAAGCAGCGCATTGAGGTCACGGATGGAAGGCTACAAGCGGCCTTTGTCGTGTCGCGGGCGATCAAAGGCACGCGCATCGGCGGTGAGGTTGCCGAGGCGCTGGCCGGGTACGAGCTGCCCATTCTTGAGTCCCGTATCACGCAGCGCGTGAGCTACCCAGGCACTGCCGCCGCCGGCACAACCGTTCTTGAATCAGAACCCGAGGGCGACGCTGCCCGCGAGGTCCAGGCGCTGGCCGCTGAAATTAAATCAAAACTCATTTGAGTTAATGAGGTAAAGAGAAAATGAGCAAAAGCACAAACACGCTAAGTGCCGGCCGTCCGAGCGCACGCAGCAGCAAGGCTGCAACGTTGGCCAGCCTGGCAGACACGCCAGCCATGAAGCGGGTCAACTTTCAGTTGCCGGCGGAGGATCACACCAAGCTGAAGATGTACGCGGTACGCCAAGGCAAGACCATTACCGAGCTGCTATCTGAATACATCGCGCAGCTACCAGAGTAAATGAGCAAATGAATAAATGAGTAGATGAATTTTAGCGGCTAAAGGAGGCGGCATGGAAAATCAAGAACAACCAGGCACCGACGCCGTGGAATGCCCCATGTGTGGAGGAACGGGCGGTTGGCCAGGCGTAAGCGGCTGGGTTGTCTGCCGGCCCTGCAATGGCACTGGAACCCCCAAGCCCGAGGAATCGGCGTGACGGTCGCAAACCATCCGGCCCGGTACAAATCGGCGCGGCGCTGGGTGATGACCTGGTGGAGAAGTTGAAGGCCGCGCAGGCCGCCCAGCGGCAACGCATCGAGGCAGAAGCACGCCCCGGTGAATCGTGGCAAGCGGCCGCTGATCGAATCCGCAAAGAATCCCGGCAACCGCCGGCAGCCGGTGCGCCGTCGATTAGGAAGCCGCCCAAGGGCGACGAGCAACCAGATTTTTTCGTTCCGATGCTCTATGACGTGGGCACCCGCGATAGTCGCAGCATCATGGACGTGGCCGTTTTCCGTCTGTCGAAGCGTGACCGACGAGCTGGCGAGGTGATCCGCTACGAGCTTCCAGACGGGCACGTAGAGGTTTCCGCAGGGCCGGCCGGCATGGCCAGTGTGTGGGATTACGACCTGGTACTGATGGCGGTTTCCCATCTAACCGAATCCATGAACCGATACCGGGAAGGGAAGGGAGACAAGCCCGGCCGCGTGTTCCGTCCACACGTTGCGGACGTACTCAAGTTCTGCCGGCGAGCCGATGGCGGAAAGCAGAAAGACGACCTGGTAGAAACCTGCATTCGGTTAAACACCACGCACGTTGCCATGCAGCGTACGAAGAAGGCCAAGAACGGCCGCCTGGTGACGGTATCCGAGGGTGAAGCCTTGATTAGCCGCTACAAGATCGTAAAGAGCGAAACCGGGCGGCCGGAGTACATCGAGATCGAGCTAGCTGATTGGATGTACCGCGAGATCACAGAAGGCAAGAACCCGGACGTGCTGACGGTTCACCCCGATTACTTTTTGATCGATCCCGGCATCGGCCGTTTTCTCTACCGCCTGGCACGCCGCGCCGCAGGCAAGGCAGAAGCCAGATGGTTGTTCAAGACGATCTACGAACGCAGTGGCAGCGCCGGAGAGTTCAAGAAGTTCTGTTTCACCGTGCGCAAGCTGATCGGGTCAAATGACCTGCCGGAGTACGATTTGAAGGAGGAGGCGGGGCAGGCTGGCCCGATCCTAGTCATGCGCTACCGCAACCTGATCGAGGGCGAAGCATCCGCCGGTTCCTAATGTACGGAGCAGATGCTAGGGCAAATTGCCCTAGCAGGGGAAAAAGGTCGAAAAGGTCTCTTTCCTGTGGATAGCACGTACATTGGGAACCCAAAGCCGTACATTGGGAACCGGAACCCGTACATTGGGAACCCAAAGCCGTACATTGGGAACCGGTCACACATGTAAGTGACTGATATAAAAGAGAAAAAAGGCGATTTTTCCGCCTAAAACTCTTTAAAACTTATTAAAACTCTTAAAACCCGCCTGGCCTGTGCATAACTGTCTGGCCAGCGCACAGCCGAAGAGCTGCAAAAAGCGCCTACCCTTCGGTCGCTGCGCTCCCTACGCCCCGCCGCTTCGCGTCGGCCTATCGCGGCCGCTGGCCGCTCAAAAATGGCTGGCCTACGGCCAGGCAATCTACCAGGGCGCGGACAAGCCGCGCCGTCGCCACTCGACCGCCGGCGCCCACATCAAGGCACCCTGCCTCGCGCGTTTCGGTGATGACGGTGAAAACCTCTGACACATGCAGCTCCCGGAGACGGTCACAGCTTGTCTGTAAGCGGATGCCGGGAGCAGACAAGCCCGTCAGGGCGCGTCAGCGGGTGTTGGCGGGTGTCGGGGCGCAGCCATGACCCAGTCACGTAGCGATAGCGGAGTGTATACTGGCTTAACTATGCGGCATCAGAGCAGATTGTACTGAGAGTGCACCATATGCGGTGTGAAATACCGCACAGATGCGTAAGGAGAAAATACCGCATCAGGCGCTCTTCCGCTTCCTCGCTCACTGACTCGCTGCGCTCGGTCGTTCGGCTGCGGCGAGCGGTATCAGCTCACTCAAAGGCGGTAATACGGTTATCCACAGAATCAGGGGATAACGCAGGAAAGAACATGTGAGCAAAAGGCCAGCAAAAGGCCAGGAACCGTAAAAAGGCCGCGTTGCTGGCGTTTTTCCATAGGCTCCGCCCCCCTGACGAGCATCACAAAAATCGACGCTCAAGTCAGAGGTGGCGAAACCCGACAGGACTATAAAGATACCAGGCGTTTCCCCCTGGAAGCTCCCTCGTGCGCTCTCCTGTTCCGACCCTGCCGCTTACCGGATACCTGTCCGCCTTTCTCCCTTCGGGAAGCGTGGCGCTTTCTCATAGCTCACGCTGTAGGTATCTCAGTTCGGTGTAGGTCGTTCGCTCCAAGCTGGGCTGTGTGCACGAACCCCCCGTTCAGCCCGACCGCTGCGCCTTATCCGGTAACTATCGTCTTGAGTCCAACCCGGTAAGACACGACTTATCGCCACTGGCAGCAGCCACTGGTAACAGGATTAGCAGAGCGAGGTATGTAGGCGGTGCTACAGAGTTCTTGAAGTGGTGGCCTAACTACGGCTACACTAGAAGGACAGTATTTGGTATCTGCGCTCTGCTGAAGCCAGTTACCTTCGGAAAAAGAGTTGGTAGCTCTTGATCCGGCAAACAAACCACCGCTGGTAGCGGTGGTTTTTTTGTTTGCAAGCAGCAGATTACGCGCAGAAAAAAAGGATCTCAAGAAGATCCTTTGATCTTTTCTACGGGGTCTGACGCTCAGTGGAACGAAAACTCACGTTAAGGGATTTTGGTCATGCATTCTAGGTACTAAAACAATTCATCCAGTAAAATATAATATTTTATTTTCTCCCAATCAGGCTTGATCCCCAGTAAGTCAAAAAATAGCTCGACATACTGTTCTTCCCCGATATCCTCCCTGATCGACCGGACGCAGAAGGCAATGTCATACCACTTGTCCGCCCTGCCGCTTCTCCCAAGATCAATAAAGCCACTTACTTTGCCATCTTTCACAAAGATGTTGCTGTCTCCCAGGTCGCCGTGGGAAAAGACAAGTTCCTCTTCGGGCTTTTCCGTCTTTAAAAAATCATACAGCTCGCGCGGATCTTTAAATGGAGTGTCTTCTTCCCAGTTTTCGCAATCCACATCGGCCAGATCGTTATTCAGTAAGTAATCCAATTCGGCTAAGCGGCTGTCTAAGCTATTCGTATAGGGACAATCCGATATGTCGATGGAGTGAAAGAGCCTGATGCACTCCGCATACAGCTCGATAATCTTTTCAGGGCTTTGTTCATCTTCATACTCTTCCGAGCAAAGGACGCCATCGGCCTCACTCATGAGCAGATTGCTCCAGCCATCATGCCGTTCAAAGTGCAGGACCTTTGGAACAGGCAGCTTTCCTTCCAGCCATAGCATCATGTCCTTTTCCCGTTCCACATCATAGGTGGTCCCTTTATACCGGCTGTCCGTCATTTTTAAATATAGGTTTTCATTTTCTCCCACCAGCTTATATACCTTAGCAGGAGACATTCCTTCCGTATCTTTTACGCAGCGGTATTTTTCGATCAGTTTTTTCAATTCCGGTGATATTCTCATTTTAGCCATTTATTATTTCCTTCCTCTTTTCTACAGTATTTAAAGATACCCCAAGAAGCTAATTATAACAAGACGAACTCCAATTCACTGTTCCTTGCATTCTAAAACCTTAAATACCAGAAAACAGCTTTTTCAAAGTTGTTTTCAAAGTTGGCGTATAACATAGTATCGACGGAGCCGATTTTGAAACCGCGGTGATCACAGGCAGCAACGCTCTGTCATCGTTACAATCAACATGCTACCCTCCGCGAGATCATCCGTGTTTCAAACCCGGCAGCTTAGTTGCCGTTCTTCCGAATAGCATCGGTAACATGAGCAAAGTCTGCCGCCTTACAACGGCTCTCCCGCTGACGCCGTCCCGGACTGATGGGCTGCCTGTATCGAGTGGTGATTTTGTGCCGAGCTGCCGGTCGGGGAGCTGTTGGCTGGCTGGTGGCAGGATATATTGTGGTGTAAACAAATTGACGCTTAGACAACTTAATAACACATTGCGGACGTTTTTAATGTACTGAATTAACGCCGAATTAATTCGGGGGATCTGGATTTTAGTACTGGATTTTGGTTTTAGGAATTAGAAATTTTATTGATAGAAGTATTTTACAAATACAAATACATACTAAGGGTTTCTTATATGCTCAACACATGAGCGAAACCCTATAGGAACCCTAATTCCCTTATCTGGGAACTACTCACACATTATTATGGAGAAACTCGAGCTTGTCGATCGACAGATCCGGTCGGCATCTACTCTATTTCTTTGCCCTCGGACGAGTGCTGGGGCGTCGGTTTCCACTATCGGCGAGTACTTCTACACAGCCATCGGTCCAGACGGCCGCGCTTCTGCGGGCGATTTGTGTACGCCCGACAGTCCCGGCTCCGGATCGGACGATTGCGTCGCATCGACCCTGCGCCCAAGCTGCATCATCGAAATTGCCGTCAACCAAGCTCTGATAGAGTTGGTCAAGACCAATGCGGAGCATATACGCCCGGAGTCGTGGCGATCCTGCAAGCTCCGGATGCCTCCGCTCGAAGTAGCGCGTCTGCTGCTCCATACAAGCCAACCACGGCCTCCAGAAGAAGATGTTGGCGACCTCGTATTGGGAATCCCCGAACATCGCCTCGCTCCAGTCAATGACCGCTGTTATGCGGCCATTGTCCGTCAGGACATTGTTGGAGCCGAAATCCGCGTGCACGAGGTGCCGGACTTCGGGGCAGTCCTCGGCCCAAAGCATCAGCTCATCGAGAGCCTGCGCGACGGACGCACTGACGGTGTCGTCCATCACAGTTTGCCAGTGATACACATGGGGATCAGCAATCGCGCATATGAAATCACGCCATGTAGTGTATTGACCGATTCCTTGCGGTCCGAATGGGCCGAACCCGCTCGTCTGGCTAAGATCGGCCGCAGCGATCGCATCCATAGCCTCCGCGACCGGTTGTAGAACAGCGGGCAGTTCGGTTTCAGGCAGGTCTTGCAACGTGACACCCTGTGCACGGCGGGAGATGCAATAGGTCAGGCTCTCGCTAAACTCCCCAATGTCAAGCACTTCCGGAATCGGGAGCGCGGCCGATGCAAAGTGCCGATAAACATAACGATCTTTGTAGAAACCATCGGCGCAGCTATTTACCCGCAGGACATATCCACGCCCTCCTACATCGAAGCTGAAAGCACGAGATTCTTCGCCCTCCGAGAGCTGCATCAGGTCGGAGACGCTGTCGAACTTTTCGATCAGAAACTTCTCGACAGACGTCGCGGTGAGTTCAGGCTTTTTCATATCTCATTGCCCCCCGGGATCTGCGAAAGCTCGAGAGAGATAGATTTGTAGAGAGAGACTGGTGATTTCAGCGTGTCCTCTCCAAATGAAATGAACTTCCTTATATAGAGGAAGGTCTTGCGAAGGATAGTGGGATTGTGCGTCATCCCTTACGTCAGTGGAGATATCACATCAATCCACTTGCTTTGAAGACGTGGTTGGAACGTCTTCTTTTTCCACGATGCTCCTCGTGGGTGGGGGTCCATCTTTGGGACCACTGTCGGCAGAGGCATCTTGAACGATAGCCTTTCCTTTATCGCAATGATGGCATTTGTAGGTGCCACCTTCCTTTTCTACTGTCCTTTTGATGAAGTGACAGATAGCTGGGCAATGGAATCCGAGGAGGTTTCCCGATATTACCCTTTGTTGAAAAGTCTCAATAGCCCTTTGGTCTTCTGAGACTGTATCTTTGATATTCTTGGAGTAGACGAGAGTGTCGTGCTCCACCATGTTATCACATCAATCCACTTGCTTTGAAGACGTGGTTGGAACGTCTTCTTTTTCCACGATGCTCCTCGTGGGTGGGGGTCCATCTTTGGGACCACTGTCGGCAGAGGCATCTTGAACGATAGCCTTTCCTTTATCGCAATGATGGCATTTGTAGGTGCCACCTTCCTTTTCTACTGTCCTTTTGATGAAGTGACAGATAGCTGGGCAATGGAATCCGAGGAGGTTTCCCGATATTACCCTTTGTTGAAAAGTCTCAATAGCCCTTTGGTCTTCTGAGACTGTATCTTTGATATTCTTGGAGTAGACGAGAGTGTCGTGCTCCACCATGTTGGCAAGCTGCTCTAGCCAATACGCAAACCGCCTCTCCCCGCGCGTTGGCCGATTCATTAATGCAGCTGGCACGACAGGTTTCCCGACTGGAAAGCGGGCAGTGAGCGCAACGCAATTAATGTGAGTTAGCTCACTCATTAGGCACCCCAGGCTTTACACTTTATGCTTCCGGCTCGTATGTTGTGTGGAATTGTGAGCGGATAACAATTTCACACAGGAAACAGCTATGACCATGATTACG
Reference
Wang, S., Huang, Z., Liu, Y., Shao, S., Li, L., & Ma, M. (2024). Application of the Nicotiana Allergic Necrosis Assay for the Validation of Protein-Protein Interactions between Fungal Effectors and Plant Receptor Kinases. Bio-protocol Preprint. https://doi.org/10.21769/p2729.
Wang, S., Huang, Z., Siji, T., Feng, S., Liu, X., Shu, Y., Liang, Y., & Chen, Z. (2024). A buffer formulation and application for efficient protoplast extraction and transformation of rice. Bio-protocol Preprint. https://doi.org/10.21769/prep2723.
Horsch, R. B., Fry, J. E., Hoffmann, N. L., Eichholtz, D., Rogers, S. G., & Fraley, R. T. (1985). A simple and general method for transferring genes into plants. Science, 227(4691), 1229-1231.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum, 15(3), 473-497.
Chalfie, M., Tu, Y., Euskirchen, G., Ward, W. W., & Prasher, D. C. (1994). Green fluorescent protein as a marker for gene expression. Science, 263(5148), 802-805.
Sparkes, I. A., Runions, J., Kearns, A., & Hawes, C. (2006). Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nature Protocols, 1(3), 2019-2025.
De Block, M., Herrera-Estrella, L., Van Montagu, M., Schell, J., & Zambryski, P. (1984). Expression of foreign genes in regenerated plants and their progeny. EMBO Journal, 3(7), 1681-1687.
Gamborg, O. L., Miller, R. A., & Ojima, K. (1968). Nutrient requirements of suspension cultures of soybean root cells. Experimental Cell Research, 50(1), 151-158.
Gelvin, S. B. (2000). Agarose gel for DNA and small RNA analysis. In Current Protocols in Molecular Biology (pp. A.3D.1-A.3D.7). John Wiley & Sons.
Sambrook, J., & Russell, D. W. (2001). Molecular Cloning: A Laboratory Manual (3rd ed.). Cold Spring Harbor Laboratory Press.
- Wang, S(2024). Stable Transformation of Japonica Rice Callus with GFP-Tagged Overexpression Vector and Transient Transformation Method. Bio-protocol Preprint. bio-protocol.org/prep2748.
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
