Advanced Search
Y1HGold Yeast One-Hybrid Screening and Validation Experiment Protocol
Last updated date: Oct 18, 2024 Views: 460 Forks: 1
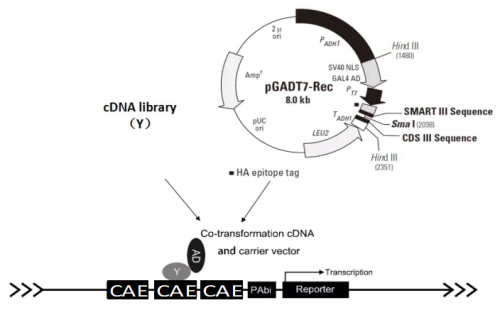
Y1HGold Yeast One-Hybrid Screening and Validation Experiment
Reagents:
1. Synthetic mutant and bait sequences.
2. pAbAi and pGADT7 plasmid DNA.
3. 10x rCutSmart buffer.
4. SacI-HF and SalI-HF enzymes.
5. ddH2O.
6. Agarose.
7. Gel-Red nucleic acid dye.
8. 6X DNA loading buffer.
9. Trans2K® Plus II DNA Marker (TransGen Biotech).
10. Gel Solubilization Buffer.
11. Wash Buffer.
12. 50x TAE (Tris-Acetate-EDTA) buffer: Tris 242.28 g, glacial acetic acid 60.05 g, EDTA 18.612 g, ddH2O up to 1 L.
13. Bstb I restriction enzyme (NEB).
14. Carrier DNA.
15. Y1, Y2, and Y3 solutions for yeast transformation.
16. cDNA library plasmid.
Equipment:
1. Programmable PCR machine.
2. UV lamp.
3. Electrophoresis apparatus and gel mold.
4. Centrifuge.
5. Water bath.
6. Gel imaging system.
7. Micropipettes and tips.
8. 1.5 mL centrifuge tubes.
Y1HGold Yeast One-Hybrid Screening and Validation Experiment Protocol
**I. Y1HGold One-Hybrid Library Screening**
1. **Synthesis of Mutant and Bait Sequences**
- Bait sequence synthesis rule: gcttgaattcgagct + 25 bp upstream sequence of the domain + domain sequence/domain sequence/domain sequence (repeated 3 times) + 25 bp downstream sequence of the domain + cctcgaggcatgtgc
- Mutant sequence synthesis rule: gcttgaattcgagct + 25 bp upstream sequence of the domain + domain sequence/domain sequence/domain sequence (repeated 3 times, but the domain sequence here is deliberately changed by 1-2 bases, used as a control) + 25 bp downstream sequence of the domain + cctcgaggcatgtgc
2. **Plasmid Preparation and Digestion**
- Thaw pAbAi plasmid DNA and 10x rCutSmart buffer on ice.
- Prepare the following system:
- 10x rCutSmart buffer 5 μl
- SacI-HF 1 μl
- SalI-HF 1 μl
- pAbAi Plasmid DNA 1 μg
- ddH2O up to 50 μl
- Use a programmable PCR instrument with the program:
- 37℃ 45min
- 65℃ 45sec
- After the reaction, transfer to a 4℃ refrigerator for storage.
3. **Agarose Gel Electrophoresis and Gel Recovery (EasyPure Quick Gel Extraction Kit, TransGen Biotech, EG101)**
- Prepare a 1.5% agarose gel by adding 1.5g agarose powder to 100mL 1×TAE (50xTAE: ddH2O = 1:49). Microwave until the agarose is completely dissolved, avoid excessive boiling that leads to liquid evaporation affecting the final concentration. Cool to about 50℃ and add 10ul of Gel-Red (10,000x) nucleic acid dye.
- Pour the gel into a mold, insert a comb at the appropriate position, and solidify at room temperature for 40 minutes. Remove the comb, place the gel in the electrophoresis tank, noting that the wells are on the negative pole side.
- Pour 1×TAE into the tank to cover the gel surface by about 1mm. Load 10μL of PCR product mixed with 2ul 6X DNA loading buffer into each well. Load 5μL Trans2K® Plus II DNA Marker into the leftmost well, and electrophorese at 120V for about 25-40 minutes.
- After electrophoresis, carefully cut out the gel area containing the target DNA fragment under a UV lamp. Place the cut gel piece into a 1.5 mL centrifuge tube, and add Gel Solubilization Buffer three times the weight of the gel. Heat in a 55℃ water bath until the gel piece is completely dissolved.
- When the melted gel solution is reduced to room temperature (high temperature weakens the binding capacity of Gel Spin Columns to DNA), add it to the Gel Spin Columns with Collection Tubes, stand for 1 minute, centrifuge at 10,000×g for 1 minute, and discard the flow-through.
- Add 650μl Wash Buffer, centrifuge at 10,000×g for 1 minute, and discard the flow-through. Centrifuge at 10,000×g for 1-2 minutes to completely remove the residual Wash Buffer.
- Place the Gel Spin Columns in a clean ep tube, open the cap and stand for 1 minute to let the residual ethanol volatilize. Add 30-50 ul of deionized water to the center of the Gel Spin Columns, stand at room temperature for 1 minute.
- Centrifuge at 10,000×g for 1 minute to elute the nucleic acid, and store the eluted nucleic acid at 4℃.
4. **Construction of pBait-AbAi and Mutant Bait pAbAi Vectors by Homologous Recombination (ClonExpress® Ultra One Step Cloning Kit, Vazyme, C115)**
- Calculate the amount of linearized vector and insert fragment:
- Optimal cloning vector amount = [0.02×vector base pairs] ng (0.03 pmol)
- Optimal insert fragment amount = [0.04×insert base pairs] ng (0.06 pmol)
- Prepare the following reaction systems on ice:
- Linearized vector pAbAi X μl
- Insert fragment Bait Y μl
- 2 × ClonExpress Mix 5 μl
- ddH2O to 10 μl
- Linearized vector pAbAi X μl
- Insert fragment Mutant Bait Y μl
- 2 × ClonExpress Mix 5 μl
- ddH2O to 10 μl
- Gently mix with a pipette (do not vortex), briefly centrifuge to collect the reaction liquid at the bottom of the tube.
- Use a programmable PCR instrument with the program:
- 50℃ 30min
- After the reaction, transfer to a 4℃ refrigerator for storage.
5. **Linearization of pBait-AbAi Series Plasmids**
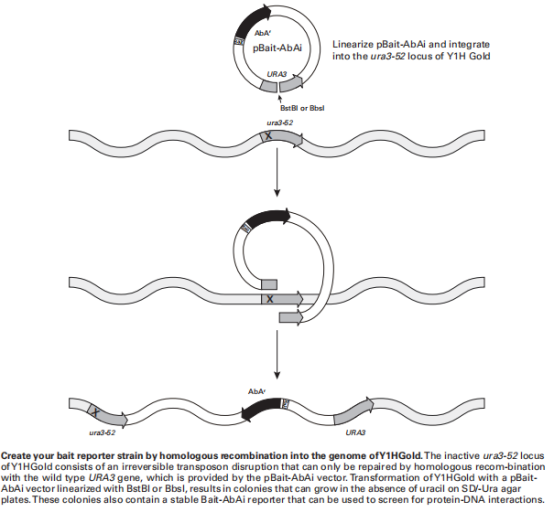
- Linearize with Bstb I restriction enzyme (NEB) (if Bstb I site exists in the connected Bait and Mutant Bait, BbsI can be used as an alternative).
- 10 x rCutSmart buffer 5 μl
- Bstb 1 μl
- pBait-AbAi Plasmid DNA 1 μg
- ddH2O up to 50 μl
- Use a programmable PCR instrument with the program:
- 65 ℃ 2 h
- After the reaction, transfer to a 4℃ refrigerator for storage.
- Run a 0.8% agarose gel to check if the vector is completely digested, and purify and recover. The concentration of the recovered plasmid should be >100ng/µL. Note: After linearization of Mutant Bait pAbAi, transform it into Y1HGold as an optional control group.
6. **Transformation of Y1HGold with pAbAi Series Plasmids**
- Take 100 µL of thawed Y1HGold competent cells (Cat. No.: CC308), add 5 μL of pre-chilled linearized plasmid (2-5 µg), 10 µL of Carrier DNA (95-100 ℃ for 5 min, quick ice bath, repeat once), and 500 µL of PEG/LiAc, mix gently, and incubate at 30 ℃ for 30 min (invert 6-8 times at 15 min for mixing).
- Place the tube in a 42 ℃ water bath for 15 min (invert 6-8 times at 7.5 min for mixing).
- Centrifuge at 10,000 rpm for 30 s, discard the supernatant, resuspend with 400 µL of ddH2O, centrifuge for 30 s, and discard the supernatant.
- Resuspend with 50 µL of ddH2O, spread on SD-/Ura plates, and incubate at 30℃ for 3-5 days. Note: At the same time, dissolve the freeze-dried Y1HGold positive
and negative strains, activate on SD plates.
7. **Identification of Bait Yeast Strains**
- Use sterile toothpicks or 10 μL pipette tips to scrape single colonies (diameter 1-2 mm, scrape 1/4) from the SD/-Ura solid medium and suspend in lysis buffer.
- Mix well by pipetting or vortexing, then use a PCR machine to lyse at 98 ℃ for 5 min, which is the lysate.
- Add 45 μL of PCR premix (with primers) to the lysate, and perform PCR amplification. PCR reaction conditions: 98℃ for 3 min; 98℃ for 10 s, 60℃ for 30 s, 72℃ for 1 min (15-30 s/kb); 72℃ for 5 min. 35 cycles.
- Run the PCR products on a 1% agarose gel and sequence to verify.
8. **Determination of the Optimal AbA Concentration for Screening Bait Yeast Strains**
- Bait yeast strains express very low levels of AbA in the absence of Prey vectors. The optimal AbA concentration varies for different bait fragments. Therefore, it is necessary to screen for the optimal AbA concentration for each bait yeast strain.
- After the above transformation verification is successful, pick fresh single colonies (2-3 mm) from the SD/-Ura plate into 1 mL of 0.9% sodium chloride solution, adjust OD600 to 0.002 (can also be cultured in SD/-Ura liquid medium until OD600=0.002).
- Take 100 μL of the culture and spread on SD/-Ura plates with different AbA concentrations (usually 0, 100 ng/mL, 200 ng/mL, 300 ng/mL, 500 ng/mL, 800 ng/mL, 1000 ng/mL), and incubate at 30℃ for 2-3 days.
- Observe the growth of bait yeast on plates with different AbA concentrations to determine the optimal AbA concentration.
- Note: On plates with different concentrations of AbA (0, 100 ng/mL, 200 ng/mL, 300 ng/mL, 500 ng/mL, 800 ng/mL, 1000 ng/mL), the appearance of the fewest or no yeast colonies indicates the optimal AbA concentration (best inhibitory concentration, minimum inhibitory concentration, background expression concentration, self-activation concentration). Generally, the self-activation AbA concentration for screening libraries should not exceed 800 ng/mL.
9. **Preparation of Y1HGold[pBait-AbAi] Competent Cells and Screening of cDNA Library**
- Yeast strain Y1HGold contains the Leu2-3 gene, which is suppressed under physiological conditions and cannot synthesize leucine, so the bait yeast strain cannot grow on leucine-deficient medium (SD/-Leu). The library plasmid pGADT7 contains the LEU2 gene, and when the library plasmid is successfully transformed into the bait yeast strain, the bait yeast can grow on leucine-deficient medium (SD/-Leu). When the prey protein in the library interacts with the bait fragment, it activates the AbA resistance gene (AUR1-C) in the bait yeast, allowing the interacting yeast strains to grow healthily on SD/-Leu/AbA medium.
- Note: Y1HGold[p53-AbAi+pGADT7-p53] and Y1HGold[p53-AbAi+pGADT7] use SD/-Leu medium, and Y1HGold[p53-AbAi] uses SD/-Ura medium.
- Pick a single colony of the identified successful Y1HGold[pBait-AbAi] and inoculate into 3 mL of YPDA liquid medium in a 15 mL shaking culture tube. Incubate at 30℃ overnight with shaking at 200 rpm.
- Transfer 3 mL of the small culture (from the previous step) to a flask containing 50 mL of liquid YPDA medium and continue to culture until OD600 reaches 0.4-0.5, centrifuge at 3000 rpm for 5 min, and discard the supernatant. (Yeast culture can be stored at 4℃ for up to 1 week, and 3 mL can be used to inoculate 50 mL of YPDA medium and cultured overnight.)
- Resuspend the pellet with 10 mL of Y1 solution, centrifuge at 3000 rpm for 5 min, and discard the supernatant.
- Add 600-1000 μL of Y2 solution to resuspend, aliquot 600 μL for library transformation and 100 μL for plasmid transformation, and use directly for transformation or freeze for storage.
- Note: Competent cells prepared should be slowly frozen before being stored in a -80℃ refrigerator for long-term storage. Place the competent cells in a programmed freezing box or wrap with multiple layers of paper and place in a foam box, store in a -80℃ refrigerator overnight, then transfer to a -80℃ refrigerator, which can be stored for up to one year. Thaw at room temperature before transformation.
- Take 600 µL of the above Y1HGold[pBait-AbAi] competent cells on ice, add 15-25 μg of pre-chilled cDNA library plasmid (about 30μL), and 25-20 μL of Y3 solution, mix gently, and incubate at 30℃ for 90 min (invert 6-8 times every 10 min for mixing). For some yeast strains, extending the incubation time can improve transformation efficiency, but do not exceed 3 hours.
- Note: The amount of library plasmid added is related to the quality of the library and can be adjusted according to the actual situation. It is recommended to supplement the volume with ddH2O, and adjust the volume of the plasmid and Y3 solution to 2.6 mL.
- Centrifuge at 3000 rpm for 5 min, discard the supernatant. Resuspend the pellet with 15 mL of 0.9% sodium chloride solution, and spread on the screening medium plate.
- Note: The above is a large-scale transformation of yeast, transforming the library plasmid into the bait yeast strain; at the same time, do a small-scale transformation to transform pGADT7 and pGADT7-53 into Y1HGold[p53-AbAi] as negative and positive controls, and spread on SD/-Leu.
- Take 50 µL of the resuspended pellet from the previous step, dilute at 1/10, 1/100, and 1/1000 ratios, and spread 100 μL of the culture on SD/-Leu and SD/-Leu/AbA plates.
- Spread the remaining resuspended pellet, 150 μL per plate, on SD/-Leu/AbA plates.
- Note: A total of 15 mL, 150 μL per plate can spread 100 plates. The amount of resuspended pellet per plate can be increased, reducing the number of plates, it is recommended to spread 35-100 plates. The AbA concentration on the screening plate should be 50 ng/mL higher than the self-activation concentration.
- Incubate at 30℃ for 3-5 days, count the number of single colonies on the SD/-Leu plate, and calculate the number of clones screened from the library. Library screening clone number = [cfu/mL on SD/-Leu]×[dilution factor]×[resuspension volume (15 mL)]
- Transformation efficiency = clone cell number×resuspension volume (mL)×dilution factor×[spread plate volume (mL)×total DNA amount (μg)]-1 Note: At least 1.0×10^6 clones should be on the SD/-Leu plate, if the number of clones is low, it will reduce the probability of screening positive clones. On the SD/-Leu/AbA plate, very few clones are often related to the bait sequence and library quality. Studies have shown that 300,000 clones can also screen positive clones.
10. **Identification of Positive Clones**
- Select clones with a diameter of about 2-3 mm for colony PCR amplification, primers:
- T7: TAATACGACTCACTATAGG
- 3AD: GAGATGGTGCACGATGCACAGT
- Or
- 5' Sequencing Primer and Sequence: 5'-CTATTCGATGATGAAGATACCCC-3'
- 3' Sequencing Primer and Sequence: 5'-GTGAACTTGCGGGGTTTTTCAG-3'
- PCR system and program refer to step 7.
- Electrophoresis bands greater than 400 bp (based on experimental purposes and actual situation) of single colonies, streak on SD/-Leu
/AbA medium, and incubate at 30℃ for 2-4 days. Single colonies with multiple electrophoresis bands (transformants containing multiple plasmids) need to be streaked and cultured on SD/-Leu/AbA* selection medium for 2-3 generations, and then single plasmid clones are selected by colony PCR method.
**Supplementary: Configuration of Y1, Y2, and Y3 Solutions in the Yeast One-Hybrid Library**
- Y1 Solution (Preparation Solution): Used for the preparation of yeast competent cells. Includes:
- 1M Lithium Acetate (LiAc)
- 10mM Tris-HCl pH 7.5
- 1mM Ethylenediaminetetraacetic Acid (EDTA)
- Y2 Solution (Freezing Solution): Used for the preservation of competent cells. Includes:
- 50% DMSO
- Y3 Solution (Transformation Solution): Used for the transformation process of plasmid DNA. Y3 solution contains single-stranded carrier DNA (Carrier DNA), which helps plasmid DNA enter yeast cells. Includes:
- 40% PEG 3350 (Polyethylene Glycol)
- 0.1M Lithium Acetate (LiAc)
- 10 µL Carrier DNA
Y1HGold-pAbAi System Yeast One-Hybrid Interaction Validation
The Y1HGold-pAbAi system interaction validation can be divided into four steps: transformation of Y1Hgold with pBait-AbAi, determination of the optimal AbA concentration for the bait yeast strain, preparation of Y1HGold[Bait] competent cells, and prey transformation for interaction validation. The transformation of Y1Hgold with pBait-AbAi and the determination of the optimal AbA concentration for the bait yeast strain have been mentioned in step one and will not be repeated here.
Supplementary Note:
Determination of the optimal AbA concentration: Y1HGold[p53-AbAi] bait yeast strain with an OD600 of 0.002 should just fail to grow on SD/-Ura with AbA (200 ng/mL) plates.

(Figure 1 from Y1HGold-pAbAi Yeast One-Hybrid Interaction Proving Kit, Coolaber: DE240914)
11. Preparation of Y1HGold[pBait] Competent Cells
11.1 Pick a single colony of the identified successful Y1HGold[pBait-AbAi] on an SD-Ura agar plate and streak, incubate at 30℃ for 3-5 days.
11.2 When the yeast single colony reaches a diameter of 2-3 mm, pick the colony and inoculate into a 15 mL shaking culture tube containing 3 mL YPDA liquid medium. Incubate at 30℃ overnight with shaking at 200 rpm.
11.3 Transfer 3 mL of the small culture (from the previous step) to a flask containing 50 mL of liquid YPDA medium and continue to culture until OD600 reaches 0.4-0.5, centrifuge at 3000 rpm for 5 min, and discard the supernatant. (Yeast culture can be stored at 4℃ for up to 1 week, and 3 mL can be used to inoculate 50 mL of YPDA medium and cultured overnight.)
11.4 Centrifuge at room temperature at 700g for 5 min, discard the supernatant, collect the cells, and resuspend the pellet in 100 mL of YPDA liquid medium.
11.5 Incubate at 30℃ for 3-5 hours; at this time, the OD value should reach 0.4-0.5; centrifuge at room temperature at 700g for 5 min, discard the supernatant, collect the cells, and resuspend the pellet in 60 mL of sterile ddH2O.
11.6 Centrifuge at room temperature at 700g for 5 min, discard the supernatant, collect the cells, and resuspend the pellet in 3 mL of 1xTE/LiAc solution.
11.7 Divide the resuspended cells into two 1.5 mL centrifuge tubes, each containing 1.5 mL; centrifuge at maximum speed of 6000g for 1 min.
11.8 Discard the supernatant, resuspend the pellet in 600 μL of 1xTE/LiAc, and place on ice or in a 4℃ refrigerator temporarily.
Note: Competent cells should be used as soon as possible (within 5-6 hours) and cannot be stored.
10 mL of 1xTE/LiAc Solution Recipe:
1 mL of 10x TE buffer (100mM Tris-HCl and 10mM EDTA).
1 mL of 1M LiAc solution.
Sterile ddH2O to make up to 10 mL.
12. Prey Transformation of Y1HGold[pBait]
12.1 Take 50 μL of the above Y1HGold[pBait-AbAi] competent cells on ice, add 2 μg of pre-chilled target plasmid, and 350 μL of Y3 solution, mix gently, and incubate at 30℃ for 60 min (invert once at 10 min for mixing). For some yeast strains, extending the incubation time can improve transformation efficiency, but do not exceed 3 hours. It is recommended to supplement the volume with ddH2O, and adjust the volume of the plasmid and Y3 solution to 0.36 mL.
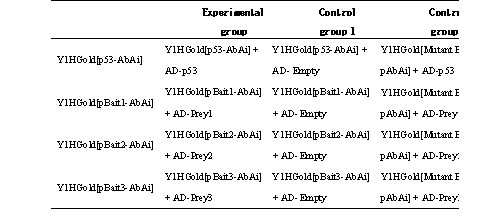
Note: Control group 1* can better reflect whether the experimental group interacts. Control group 2** the bait yeast strain Y1HGold[Mutant Bait pAbAi] can avoid false positives caused by the direct activation of the reporter gene by the prey in the absence of bait sequence mutation (or no bait), which is not very common and can be omitted.
12.2 Centrifuge at 3000 rpm for 5 min, discard the supernatant.
12.3 Resuspend the pellet in 0.5 mL of YPD Plus Liquid Medium, incubate at 30℃ with shaking for 30-60 min, centrifuge at 12000 rpm for 15 s, and discard the supernatant.
12.4 Add 50 μL of ddH2O to resuspend the cells, spread on SD-/Leu plates, and incubate at 30℃ for 3-5 days.
12.5 After the transformation is successful, pick fresh single colonies (2-3 mm) from each sample in 1 mL of 0.9% sodium chloride solution, adjust OD600 to 0.2 (can also be cultured in SD-Leu liquid medium until OD600=0.2).
12.6 Dilute with 0.9% sodium chloride solution in 10-fold, 100-fold, and 1000-fold increments (i.e., OD600=0.2, 0.02, 0.002, 0.0002).
12.7 In the order of experimental group first, then control group, spot 10 μL on the corresponding SD/-Leu with AbA* plates, refer to Figure 1.
12.8 Incubate at 30℃ for 2-3 days, observe the growth of each group of recombinant yeast on the corresponding self-activation AbA concentration plates to determine if there is an interaction.
Interaction Validation Analysis
13.1 Interaction Analysis
From the selection of the optimal AbA concentration for the bait yeast strain, it is known that the self-activation concentration for Y1HGold[p53-AbAi] is 100 ng/mL. From Figure 2 on the SD/-Leu plate, the growth of the control groups Y1HGold[p53-AbAi+pGADT7-p53] and Y1HGold[p53-AbAi+pGADT7] is the same. On the SD/-Leu with AbA (100 ng/mL) plate, the growth of Y1HGold[p53-AbAi+pGADT7-p53] is significantly better than Y1HGold[p53-AbAi+pGADT7], so p53-AbAi interacts with pGADT7-p53. Similarly, Bait1 and Prey1 also interact.
Note: The interaction between GAL4 AD-p53 and the p53 binding sequence (cis-acting element) induces the expression of the AbA resistance gene AUR1-C, so Y1HGold[p53-AbAi+pGADT7-p53] can grow on the SD/-Leu with AbA (1000 ng/mL) plate.
13.2 Prey Protein Toxicity Analysis
From the selection of the optimal AbA concentration for the bait yeast strain, it is known that the self-activation concentration for Y1HGold[pBait2-AbAi] is 100 ng/mL. From Figure 2, it can be seen that on the SD/-Leu plate, the growth of Y1HGold[pBait2-AbAi+pGADT7-Prey2] is weaker than the control group Y1HGold[pBait2-AbAi+pGADT7], which may be due to the toxicity of the Prey protein; however, on the SD/-Leu with AbA (100 ng/mL) and SD/-Leu with AbA (300 ng/mL) plates, the growth of Y1HGold[pBait2-AbAi+pGADT7-Prey2] is better than Y1HGold[pBait2-AbAi+pGADT7], so Bait2 interacts with prey2.
Note: The yeast interaction validation experiment is not suitable for very toxic Prey proteins.
13.3 No Interaction Analysis
In Figure 2, on all SD plates, the growth of Y1HGold[pBait3-AbAi+pGADT7-Prey3]/Y1HGold[pBait3-AbAi+pGADT7] and Y1HGold[pBait4-AbAi+pGADT7-Prey4]/Y1HGold[pBait4-AbAi+pGADT7] is the same, so Bait3 does not interact with prey3, and Bait4 does not interact with prey4.
Note: Y1HGold[pBait3-AbAi+pGADT7] only grows on the SD/-Leu plate, indicating that Bait3 has no self-activation. Y1HGold[pBait5-AbAi+pGADT7] can grow on the SD/-Leu with AbA (300 ng/mL) plate with the same growth, indicating that Bait5 has some self-activation.
Supplementary: Configuration of Related Media for This Experiment
YPDA Medium (Yeast Extract Peptone Dextrose Medium):
Yeast extract: 10g
Peptone: 20g
Glucose: 20g
Agar (for solid medium): 20g (optional)
Adjust pH to 5.8
Sterilize and cool to room temperature for use
YPDA Medium:
Add adenine sulfate (Ade): 0.12g to the YPD medium
Used to prevent yeast strains containing ADE1 and ADE2 mutant alleles from turning pink or red after long-term culture
SD Dropout Medium (Synthetic Dropout Medium):
SD double, triple, quadruple, and quintuple dropout media are compared with yeast SD complete medium, lacking the corresponding amino acids (or nucleic acids), resulting in yeast strains that cannot synthesize the corresponding components and cannot grow in the corresponding dropout medium.
2x YPAD Medium:
Yeast extract: 5g
Peptone: 10g
Glucose: 11g
Adenine hemisulfate: 25 mg
Add ddH2O to 250 mL
Agar (for solid medium): 5g/250mL
SD/-Leu Medium (for Y1HGold[p53-AbAi+pGADT7-p53] and Y1HGold[p53-AbAi+pGADT7]):
Yeast nitrogen base: 1.675g
-leu DO: 0.17g
Glucose: 5.5g
Add ddH2O to 250 mL
Agar (for solid medium): 5g/250mL
SD/-Ura Medium (for Y1HGold[p53-AbAi]):
Yeast nitrogen base: 1.675g
-Ura DO: 0.17g
Glucose: 5.5g
Add ddH2O to 250 mL
Agar (for solid medium): 5g/250mL
SD/-Leu/-Trp Medium:
Yeast nitrogen base: 1.675g
-leu/-trp DO: 0.17g
Glucose: 5.5g
Add ddH2O to 250 mL
Agar (for solid medium): 5g/250mL
SD/—Ade/—His/—Leu/—Trp (SC-4) Medium:
Yeast nitrogen base: 1.675g
—Ade/—His/—Leu/—Trp DO: 0.17g
Glucose: 5.5g
Add ddH2O to 250 mL
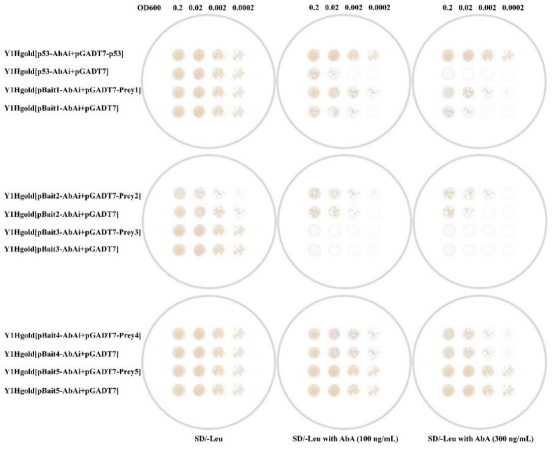
(Figure 2 from Y1HGold-pAbAi Yeast One-Hybrid Interaction Proving Kit, Coolaber: DE240914)
References
1. Cliften, P. F., et al. "The SH2 Domain-Mediated Interactome of the Yeast Kinome and Human Cancer." PLoS biology 4.7 (2006): e277.
2. Simon, J. A., et al. "Use of the yeast one-hybrid system to identify connections between DNA-binding proteins and cis-elements in the yeast genome." Methods in enzymology 350 (2002): 523-537.
3. Wang, S, Huang, Z, Liu, Y, Shao, S, Li, L, and Ma, M. "Application of the Nicotiana Allergic Necrosis Assay for the Validation of Protein-Protein Interactions between Fungal Effectors and Plant Receptor Kinases." Bio-protocol Preprint. bio-protocol.org/prep2729.
Appendix: Vectors used in yeast one hybrid(Y1H) experiments
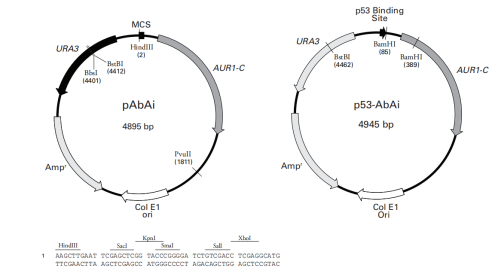
1. pAbAi Vector
Origin of Replication: ColE1
Promoter: URA3
Vector Size: 4895 base pairs (bp)
5' Sequencing Primer and Sequence: pABAI-F: GTTCCTTATATGTAGCTTTCGACA
3' Sequencing Primer and Sequence: pABAI-R: CCATCTCGAAAAAGGGTTTGCC
Vector Resistance: Ampicillin
Selection Markers: URA3, ABA (aureobasidin A resistance gene)
BstbI
![]()
BbsI
![]()
>pAbAi vector sequence
AAGCTTGAATTCGAGCTCGGTACCCGGGGATCTGTCGACCTCGAGGCATGTGCTCTGTATGTATATAAAACTCTTGTTTTCTTCTTTTCTCTAAATATTCTTTCCTTATACATTAGGTCCTTTGTAGCATAAATTACTATACTTCTATAGACACGCAAACACAAATACACACACTAAATTAATAATGGCAAACCCTTTTTCGAGATGGTTTCTATCAGAGAGACCTCCAAACTGCCATGTAGCCGATTTAGAAACAAGTTTAGATCCCCATCAAACGTTGTTGAAGGTGCAAAAATACAAACCCGCTTTAAGCGACTGGGTGCATTACATCTTCTTGGGATCCATCATGCTGTTTGTGTTCATTACTAATCCCGCACCTTGGATCTTCAAGATCCTTTTTTATTGTTTCTTGGGCACTTTATTCATCATTCCAGCTACGTCACAGTTTTTCTTCAATGCCTTGCCCATCCTAACATGGGTGGCGCTGTATTTCACTTCATCGTACTTTCCAGATGACCGCAGGCCTCCTATTACTGTCAAAGTGTTACCAGCGGTGGAAACAATTTTATACGGCGACAATTTAAGTGATATTCTTGCAACATCGACGAATTCCTTTTTGGACATTTTAGCATGGTTACCGTACGGACTATTTCATTATGGGGCCCCATTTGTCGTTGCTGCCATCTTATTCGTATTTGGTCCACCAACTGTTTTGCAAGGTTATGCTTTTGCATTTGGTTATATGAACCTGTTTGGTGTTATCATGCAAAATGTCTTTCCAGCCGCTCCCCCATGGTATAAAATTCTCTATGGATTGCAATCAGCCAACTATGATATGCATGGCTCGCCTGGTGGATTAGCTAGAATTGATAAGCTACTCGGTATTAATATGTATACTACATGTTTTTCAAATTCCTCCGTCATTTTCGGTGCTTTTCCTTCACTGCATTCCGGGTGTGCTACTATGGAAGCCCTGTTTTTCTGTTATTGTTTTCCAAAATTGAAGCCCTTGTTTATTGCTTATGTTTGCTGGTTATGGTGGTCAACTATGTATCTGACACACCATTATTTTGTAGACCTTATGGCAGGTTCTGTGCTGTCATACGTTATTTTCCAGTACACAAAGTACACACATTTACCAATTGTAGATACATCTCTTTTTTGCAGATGGTCATACACTTCAATTGAGAAATACGATATATCAAAGAGTGATCCATTGGCTGCAGATTCAAACGATATCGAAAGTGTCCCTTTGTCCAACTTGGAACTTGACTTTGATCTTAATATGACTGATGAACCCAGTGTAAGCCCTTCGTTATTTGATGGATCTACTTCTGTTTCTCGTTCGTCCGCCACGTCTATAACGTCACTAGGTGTAAAGAGGGCTTAAGGAAATCCATTATGTACTATTTAAAAAACACAAACTTTTGGATGTTCGGTTTATTCTTTTTCTTTTACTTTTTTATCATGGGAGCCTACTTCCCGTTTTTCCCGATTTGGCTACATGACATCAACCATATCAGCAAAAGTGATACGGGTATTATTTTTGCCGCTATTTCTCTGTTCTCGCTATTATTCCAACCGCTGTTTGGTCTGCTTTCTGACAAACTCGGCCTCGACTCTAGCGAATTAATTCGTAATCATGTCATAGCTGTTTCCTGTGTGAAATTGTTATCCGCTCACAATTCCACACAACATACGAGCCGGAAGCATAAAGTGTAAAGCCTGGGGTGCCTAATGAGTGAGCTAACTCACATTAATTGCGTTGCGCTCACTGCCCGCTTTCCAGTCGGGAAACCTGTCGTGCCAGCTGCATTAATGAATCGGCCAACGCGCGGGGAGAGGCGGTTTGCGTATTGGGCGCTCTTCCGCTTCCTCGCTCACTGACTCGCTGCGCTCGGTCGTTCGGCTGCGGCGAGCGGTATCAGCTCACTCAAAGGCGGTAATACGGTTATCCACAGAATCAGGGGATAACGCAGGAAAGAACATGTGAGCAAAAGGCCAGCAAAAGGCCAGGAACCGTAAAAAGGCCGCGTTGCTGGCGTTTTTCCATAGGCTCCGCCCCCCTGACGAGCATCACAAAAATCGACGCTCAAGTCAGAGGTGGCGAAACCCGACAGGACTATAAAGATACCAGGCGTTTCCCCCTGGAAGCTCCCTCGTGCGCTCTCCTGTTCCGACCCTGCCGCTTACCGGATACCTGTCCGCCTTTCTCCCTTCGGGAAGCGTGGCGCTTTCTCATAGCTCACGCTGTAGGTATCTCAGTTCGGTGTAGGTCGTTCGCTCCAAGCTGGGCTGTGTGCACGAACCCCCCGTTCAGCCCGACCGCTGCGCCTTATCCGGTAACTATCGTCTTGAGTCCAACCCGGTAAGACACGACTTATCGCCACTGGCAGCAGCCACTGGTAACAGGATTAGCAGAGCGAGGTATGTAGGCGGTGCTACAGAGTTCTTGAAGTGGTGGCCTAACTACGGCTACACTAGAAGAACAGTATTTGGTATCTGCGCTCTGCTGAAGCCAGTTACCTTCGGAAAAAGAGTTGGTAGCTCTTGATCCGGCAAACAAACCACCGCTGGTAGCGGTGGTTTTTTTGTTTGCAAGCAGCAGATTACGCGCAGAAAAAAAGGATCTCAAGAAGATCCTTTGATCTTTTCTACGGGGTCTGACGCTCAGTGGAACGAAAACTCACGTTAAGGGATTTTGGTCATGAGATTATCAAAAAGGATCTTCACCTAGATCCTTTTAAATTAAAAATGAAGTTTTAAATCAATCTAAAGTATATATGAGTAAACTTGGTCTGACAGTTACCAATGCTTAATCAGTGAGGCACCTATCTCAGCGATCTGTCTATTTCGTTCATCCATAGTTGCCTGACTCCCCGTCGTGTAGATAACTACGATACGGGAGGGCTTACCATCTGGCCCCAGTGCTGCAATGATACCGCGAGACCCACGCTCACCGGCTCCAGATTTATCAGCAATAAACCAGCCAGCCGGAAGGGCCGAGCGCAGAAGTGGTCCTGCAACTTTATCCGCCTCCATCCAGTCTATTAATTGTTGCCGGGAAGCTAGAGTAAGTAGTTCGCCAGTTAATAGTTTGCGCAACGTTGTTGCCATTGCTACAGGCATCGTGGTGTCACGCTCGTCGTTTGGTATGGCTTCATTCAGCTCCGGTTCCCAACGATCAAGGCGAGTTACATGATCCCCCATGTTGTGCAAAAAAGCGGTTAGCTCCTTCGGTCCTCCGATCGTTGTCAGAAGTAAGTTGGCCGCAGTGTTATCACTCATGGTTATGGCAGCACTGCATAATTCTCTTACTGTCATGCCATCCGTAAGATGCTTTTCTGTGACTGGTGAGTACTCAACCAAGTCATTCTGAGAATAGTGTATGCGGCGACCGAGTTGCTCTTGCCCGGCGTCAACACGGGATAATACCGCGCCACATAGCAGAACTTTAAAAGTGCTCATCATTGGAAAACGTTCTTCGGGGCGAAAACTCTCAAGGATCTTACCGCTGTTGAGATCCAGTTCGATGTAACCCACTCGTGCACCCAACTGATCTTCAGCATCTTTTACTTTCACCAGCGTTTCTGGGTGAGCAAAAACAGGAAGGCAAAATGCCGCAAAAAAGGGAATAAGGGCGACACGGAAATGTTGAATACTCATACTCTTCCTTTTTCAATATTATTGAAGCATTTATCAGGGTTATTGTCTCATGAGCGGATACATATTTGAATGTATTTAGAAAAATAAACAAATAGGGGTTCCGCGCACATTTCCCCGAAAAGTGCCACCTGGGTAATAACTGATATAATTAAATTGAAGCTCTAATTTGTGAGTTTAGTATACATGCATTTACTTATAATACAGTTTTTTAGTTTTGCTGGCCGCATCTTCTCAAATATGCTTCCCAGCCTGCTTTTCTGTAACGTTCACCCTCTACCTTAGCATCCCTTCCCTTTGCAAATAGTCCTCTTCCAACAATAATAATGTCAGATCCTGTAGAGACCACATCATCCACGGTTCTATACTGTTGACCCAATGCGTCTCCCTTGTCATCTAAACCCACACCGGGTGTCATAATCAACCAATCGTAACCTTCATCTCTTCCACCCATGTCTCTTTGAGCAATAAAGCCGATAACAAAATCTTTGTCGCTCTTCGCAATGTCAACAGTACCCTTAGTATATTCTCCAGTAGATAGGGAGCCCTTGCATGACAATTCTGCTAACATCAAAAGGCCTCTAGGTTCCTTTGTTACTTCTTCTGCCGCCTGCTTCAAACCGCTAACAATACCTGGGCCCACCACACCGTGTGCATTCGTAATGTCTGCCCATTCTGCTATTCTGTATACACCCGCAGAGTACTGCAATTTGACTGTATTACCAATGTCAGCAAATTTTCTGTCTTCGAAGAGTAAAAAATTGTACTTGGCGGATAATGCCTTTAGCGGCTTAACTGTGCCCTCCATGGAAAAATCAGTCAAGATATCCACATGTGTTTTTAGTAAACAAATTTTGGGACCTAATGCTTCAACTAACTCCAGTAATTCCTTGGTGGTACGAACATCCAATGAAGCACACAAGTTTGTTTGCTTTTCGTGCATGATATTAAATAGCTTGGCAGCAACAGGACTAGGATGAGTAGCAGCACGTTCCTTATATGTAGCTTTCGACATGATTTATCTTCGTTTCCTGCAGGTTTTTGTTCTGTGCAGTTGGGTTAAGAATACTGGGCAATTTCATGTTTCTTCAACACTACATATGCGTATATATACCAATCTAAGTCTGTGCTCCTTCCTTCGTTCTTCCTTCTGTTCGGAGATTACCGAATCAAAAAAATTTCAAGGAAACCGAAATCAAAAAAAAGAATAAAAAAAAAATGATGAATTGAA
2.Vector Name: p53-AbAi
Origin of Replication: ColE1
Promoter: URA3
Vector Size: 4945 base pairs (bp)
5' Sequencing Primer and Sequence: pABAI-F: GTTCCTTATATGTAGCTTTCGACA
3' Sequencing Primer and Sequence: pABAI-R: CCATCTCGAAAAAGGGTTTGCC
Vector Resistance: Ampicillin
Selection Markers: URA3, ABA (aureobasidin A resistance gene)
>p53-AbAi vector sequence
AAGCTTGAATTCGAGCTCGGTACCAGGCATGCCTAGCATGCCTAGGCATGCCTAGGCATGCCTAGGCATGCCTAGGCATGCCTGGATCCCTCGAGGCATGTGCTCTGTATGTATATAAAACTCTTGTTTTCTTCTTTTCTCTAAATATTCTTTCCTTATACATTAGGTCCTTTGTAGCATAAATTACTATACTTCTATAGACACGCAAACACAAATACACACACTAAATTAATAATGGCAAACCCTTTTTCGAGATGGTTTCTATCAGAGAGACCTCCAAACTGCCATGTAGCCGATTTAGAAACAAGTTTAGATCCCCATCAAACGTTGTTGAAGGTGCAAAAATACAAACCCGCTTTAAGCGACTGGGTGCATTACATCTTCTTGGGATCCATCATGCTGTTTGTGTTCATTACTAATCCCGCACCTTGGATCTTCAAGATCCTTTTTTATTGTTTCTTGGGCACTTTATTCATCATTCCAGCTACGTCACAGTTTTTCTTCAATGCCTTGCCCATCCTAACATGGGTGGCGCTGTATTTCACTTCATCGTACTTTCCAGATGACCGCAGGCCTCCTATTACTGTCAAAGTGTTACCAGCGGTGGAAACAATTTTATACGGCGACAATTTAAGTGATATTCTTGCAACATCGACGAATTCCTTTTTGGACATTTTAGCATGGTTACCGTACGGACTATTTCATTATGGGGCCCCATTTGTCGTTGCTGCCATCTTATTCGTATTTGGTCCACCAACTGTTTTGCAAGGTTATGCTTTTGCATTTGGTTATATGAACCTGTTTGGTGTTATCATGCAAAATGTCTTTCCAGCCGCTCCCCCATGGTATAAAATTCTCTATGGATTGCAATCAGCCAACTATGATATGCATGGCTCGCCTGGTGGATTAGCTAGAATTGATAAGCTACTCGGTATTAATATGTATACTACATGTTTTTCAAATTCCTCCGTCATTTTCGGTGCTTTTCCTTCACTGCATTCCGGGTGTGCTACTATGGAAGCCCTGTTTTTCTGTTATTGTTTTCCAAAATTGAAGCCCTTGTTTATTGCTTATGTTTGCTGGTTATGGTGGTCAACTATGTATCTGACACACCATTATTTTGTAGACCTTATGGCAGGTTCTGTGCTGTCATACGTTATTTTCCAGTACACAAAGTACACACATTTACCAATTGTAGATACATCTCTTTTTTGCAGATGGTCATACACTTCAATTGAGAAATACGATATATCAAAGAGTGATCCATTGGCTGCAGATTCAAACGATATCGAAAGTGTCCCTTTGTCCAACTTGGAACTTGACTTTGATCTTAATATGACTGATGAACCCAGTGTAAGCCCTTCGTTATTTGATGGATCTACTTCTGTTTCTCGTTCGTCCGCCACGTCTATAACGTCACTAGGTGTAAAGAGGGCTTAAGGAAATCCATTATGTACTATTTAAAAAACACAAACTTTTGGATGTTCGGTTTATTCTTTTTCTTTTACTTTTTTATCATGGGAGCCTACTTCCCGTTTTTCCCGATTTGGCTACATGACATCAACCATATCAGCAAAAGTGATACGGGTATTATTTTTGCCGCTATTTCTCTGTTCTCGCTATTATTCCAACCGCTGTTTGGTCTGCTTTCTGACAAACTCGGCCTCGACTCTAGCGAATTAATTCGTAATCATGTCATAGCTGTTTCCTGTGTGAAATTGTTATCCGCTCACAATTCCACACAACATACGAGCCGGAAGCATAAAGTGTAAAGCCTGGGGTGCCTAATGAGTGAGCTAACTCACATTAATTGCGTTGCGCTCACTGCCCGCTTTCCAGTCGGGAAACCTGTCGTGCCAGCTGCATTAATGAATCGGCCAACGCGCGGGGAGAGGCGGTTTGCGTATTGGGCGCTCTTCCGCTTCCTCGCTCACTGACTCGCTGCGCTCGGTCGTTCGGCTGCGGCGAGCGGTATCAGCTCACTCAAAGGCGGTAATACGGTTATCCACAGAATCAGGGGATAACGCAGGAAAGAACATGTGAGCAAAAGGCCAGCAAAAGGCCAGGAACCGTAAAAAGGCCGCGTTGCTGGCGTTTTTCCATAGGCTCCGCCCCCCTGACGAGCATCACAAAAATCGACGCTCAAGTCAGAGGTGGCGAAACCCGACAGGACTATAAAGATACCAGGCGTTTCCCCCTGGAAGCTCCCTCGTGCGCTCTCCTGTTCCGACCCTGCCGCTTACCGGATACCTGTCCGCCTTTCTCCCTTCGGGAAGCGTGGCGCTTTCTCATAGCTCACGCTGTAGGTATCTCAGTTCGGTGTAGGTCGTTCGCTCCAAGCTGGGCTGTGTGCACGAACCCCCCGTTCAGCCCGACCGCTGCGCCTTATCCGGTAACTATCGTCTTGAGTCCAACCCGGTAAGACACGACTTATCGCCACTGGCAGCAGCCACTGGTAACAGGATTAGCAGAGCGAGGTATGTAGGCGGTGCTACAGAGTTCTTGAAGTGGTGGCCTAACTACGGCTACACTAGAAGAACAGTATTTGGTATCTGCGCTCTGCTGAAGCCAGTTACCTTCGGAAAAAGAGTTGGTAGCTCTTGATCCGGCAAACAAACCACCGCTGGTAGCGGTGGTTTTTTTGTTTGCAAGCAGCAGATTACGCGCAGAAAAAAAGGATCTCAAGAAGATCCTTTGATCTTTTCTACGGGGTCTGACGCTCAGTGGAACGAAAACTCACGTTAAGGGATTTTGGTCATGAGATTATCAAAAAGGATCTTCACCTAGATCCTTTTAAATTAAAAATGAAGTTTTAAATCAATCTAAAGTATATATGAGTAAACTTGGTCTGACAGTTACCAATGCTTAATCAGTGAGGCACCTATCTCAGCGATCTGTCTATTTCGTTCATCCATAGTTGCCTGACTCCCCGTCGTGTAGATAACTACGATACGGGAGGGCTTACCATCTGGCCCCAGTGCTGCAATGATACCGCGAGACCCACGCTCACCGGCTCCAGATTTATCAGCAATAAACCAGCCAGCCGGAAGGGCCGAGCGCAGAAGTGGTCCTGCAACTTTATCCGCCTCCATCCAGTCTATTAATTGTTGCCGGGAAGCTAGAGTAAGTAGTTCGCCAGTTAATAGTTTGCGCAACGTTGTTGCCATTGCTACAGGCATCGTGGTGTCACGCTCGTCGTTTGGTATGGCTTCATTCAGCTCCGGTTCCCAACGATCAAGGCGAGTTACATGATCCCCCATGTTGTGCAAAAAAGCGGTTAGCTCCTTCGGTCCTCCGATCGTTGTCAGAAGTAAGTTGGCCGCAGTGTTATCACTCATGGTTATGGCAGCACTGCATAATTCTCTTACTGTCATGCCATCCGTAAGATGCTTTTCTGTGACTGGTGAGTACTCAACCAAGTCATTCTGAGAATAGTGTATGCGGCGACCGAGTTGCTCTTGCCCGGCGTCAACACGGGATAATACCGCGCCACATAGCAGAACTTTAAAAGTGCTCATCATTGGAAAACGTTCTTCGGGGCGAAAACTCTCAAGGATCTTACCGCTGTTGAGATCCAGTTCGATGTAACCCACTCGTGCACCCAACTGATCTTCAGCATCTTTTACTTTCACCAGCGTTTCTGGGTGAGCAAAAACAGGAAGGCAAAATGCCGCAAAAAAGGGAATAAGGGCGACACGGAAATGTTGAATACTCATACTCTTCCTTTTTCAATATTATTGAAGCATTTATCAGGGTTATTGTCTCATGAGCGGATACATATTTGAATGTATTTAGAAAAATAAACAAATAGGGGTTCCGCGCACATTTCCCCGAAAAGTGCCACCTGGGTAATAACTGATATAATTAAATTGAAGCTCTAATTTGTGAGTTTAGTATACATGCATTTACTTATAATACAGTTTTTTAGTTTTGCTGGCCGCATCTTCTCAAATATGCTTCCCAGCCTGCTTTTCTGTAACGTTCACCCTCTACCTTAGCATCCCTTCCCTTTGCAAATAGTCCTCTTCCAACAATAATAATGTCAGATCCTGTAGAGACCACATCATCCACGGTTCTATACTGTTGACCCAATGCGTCTCCCTTGTCATCTAAACCCACACCGGGTGTCATAATCAACCAATCGTAACCTTCATCTCTTCCACCCATGTCTCTTTGAGCAATAAAGCCGATAACAAAATCTTTGTCGCTCTTCGCAATGTCAACAGTACCCTTAGTATATTCTCCAGTAGATAGGGAGCCCTTGCATGACAATTCTGCTAACATCAAAAGGCCTCTAGGTTCCTTTGTTACTTCTTCTGCCGCCTGCTTCAAACCGCTAACAATACCTGGGCCCACCACACCGTGTGCATTCGTAATGTCTGCCCATTCTGCTATTCTGTATACACCCGCAGAGTACTGCAATTTGACTGTATTACCAATGTCAGCAAATTTTCTGTCTTCGAAGAGTAAAAAATTGTACTTGGCGGATAATGCCTTTAGCGGCTTAACTGTGCCCTCCATGGAAAAATCAGTCAAGATATCCACATGTGTTTTTAGTAAACAAATTTTGGGACCTAATGCTTCAACTAACTCCAGTAATTCCTTGGTGGTACGAACATCCAATGAAGCACACAAGTTTGTTTGCTTTTCGTGCATGATATTAAATAGCTTGGCAGCAACAGGACTAGGATGAGTAGCAGCACGTTCCTTATATGTAGCTTTCGACATGATTTATCTTCGTTTCCTGCAGGTTTTTGTTCTGTGCAGTTGGGTTAAGAATACTGGGCAATTTCATGTTTCTTCAACACTACATATGCGTATATATACCAATCTAAGTCTGTGCTCCTTCCTTCGTTCTTCCTTCTGTTCGGAGATTACCGAATCAAAAAAATTTCAAGGAAACCGAAATCAAAAAAAAGAATAAAAAAAAAATGATGAATTGAA
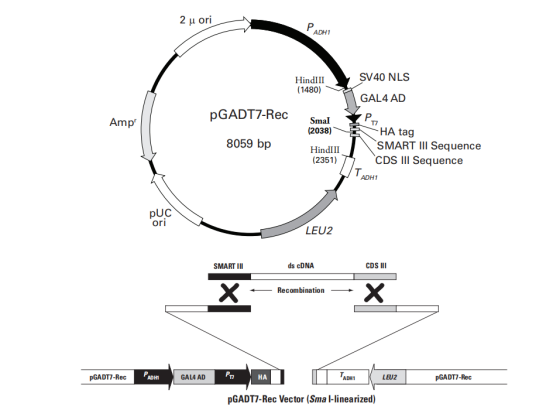
3.Vector Name: pGADT7-Rec
Origin of Replication: pUC
Promoter: ADH1
Vector Size: 8059 base pairs (bp)
5' Sequencing Primer and Sequence: 5'-CTATTCGATGATGAAGATACCCC-3'
3' Sequencing Primer and Sequence: 5'-GTGAACTTGCGGGGTTTTTCAG-3'
Vector Resistance: Ampicillin
Selection Markers: LEU2
>pGADT7-Rec vector sequence
TGCATGCCTGCAGGTCGAGATCCGGGATCGAAGAAATGATGGTAAATGAAATAGGAAATCAAGGAGCATGAAGGCAAAAGACAAATATAAGGGTCGAACGAAAAATAAAGTGAAAAGTGTTGATATGATGTATTTGGCTTTGCGGCGCCGAAAAAACGAGTTTACGCAATTGCACAATCATGCTGACTCTGTGGCGGACCCGCGCTCTTGCCGGCCCGGCGATAACGCTGGGCGTGAGGCTGTGCCCGGCGGAGTTTTTTGCGCCTGCATTTTCCAAGGTTTACCCTGCGCTAAGGGGCGAGATTGGAGAAGCAATAAGAATGCCGGTTGGGGTTGCGATGATGACGACCACGACAACTGGTGTCATTATTTAAGTTGCCGAAAGAACCTGAGTGCATTTGCAACATGAGTATACTAGAAGAATGAGCCAAGACTTGCGAGACGCGAGTTTGCCGGTGGTGCGAACAATAGAGCGACCATGACCTTGAAGGTGAGACGCGCATAACCGCTAGAGTACTTTGAAGAGGAAACAGCAATAGGGTTGCTACCAGTATAAATAGACAGGTACATACAACACTGGAAATGGTTGTCTGTTTGAGTACGCTTTCAATTCATTTGGGTGTGCACTTTATTATGTTACAATATGGAAGGGAACTTTACACTTCTCCTATGCACATATATTAATTAAAGTCCAATGCTAGTAGAGAAGGGGGGTAACACCCCTCCGCGCTCTTTTCCGATTTTTTTCTAAACCGTGGAATATTTCGGATATCCTTTTGTTGTTTCCGGGTGTACAATATGGACTTCCTCTTTTCTGGCAACCAAACCCATACATCGGGATTCCTATAATACCTTCGTTGGTCTCCCTAACATGTAGGTGGCGGAGGGGAGATATACAATAGAACAGATACCAGACAAGACATAATGGGCTAAACAAGACTACACCAATTACACTGCCTCATTGATGGTGGTACATAACGAACTAATACTGTAGCCCTAGACTTGATAGCCATCATCATATCGAAGTTTCACTACCCTTTTTCCATTTGCCATCTATTGAAGTAATAATAGGCGCATGCAACTTCTTTTCTTTTTTTTTCTTTTCTCTCTCCCCCGTTGTTGTCTCACCATATCCGCAATGACAAAAAAATGATGGAAGACACTAAAGGAAAAAATTAACGACAAAGACAGCACCAACAGATGTCGTTGTTCCAGAGCTGATGAGGGGTATCTCGAAGCACACGAAACTTTTTCCTTCCTTCATTCACGCACACTACTCTCTAATGAGCAACGGTATACGGCCTTCCTTCCAGTTACTTGAATTTGAAATAAAAAAAAGTTTGCTGTCTTGCTATCAAGTATAAATAGACCTGCAATTATTAATCTTTTGTTTCCTCGTCATTGTTCTCGTTCCCTTTCTTCCTTGTTTCTTTTTCTGCACAATATTTCAAGCTATACCAAGCATACAATCAACTCCAAGCTTTGCAAAGATGGATAAAGCGGAATTAATTCCCGAGCCTCCAAAAAAGAAGAGAAAGGTCGAATTGGGTACCGCCGCCAATTTTAATCAAAGTGGGAATATTGCTGATAGCTCATTGTCCTTCACTTTCACTAACAGTAGCAACGGTCCGAACCTCATAACAACTCAAACAAATTCTCAAGCGCTTTCACAACCAATTGCCTCCTCTAACGTTCATGATAACTTCATGAATAATGAAATCACGGCTAGTAAAATTGATGATGGTAATAATTCAAAACCACTGTCACCTGGTTGGACGGACCAAACTGCGTATAACGCGTTTGGAATCACTACAGGGATGTTTAATACCACTACAATGGATGATGTATATAACTATCTATTCGATGATGAAGATACCCCACCAAACCCAAAAAAAGAGATCTTTAATACGACTCACTATAGGGCGAGCGCCGCCATGGAGTACCCATACGACGTACCAGATTACGCTCATATGGCCATGGAGGCCAGTGAATTCCACCCAAGCAGTGGTATCAACGCAGAGTGGCCATTATGGCCCGGGAAAAAACATGTCGGCCGCCTCGGCCTCTAGAGGGTGGGCATCGATACGGGATCCATCGAGCTCGAGCTGCAGATGAATCGTAGATACTGAAAAACCCCGCAAGTTCACTTCAACTGTGCATCGTGCACCATCTCAATTTCTTTCATTTATACATCGTTTTGCCTTCTTTTATGTAACTATACTCCTCTAAGTTTCAATCTTGGCCATGTAACCTCTGATCTATAGAATTTTTTAAATGACTAGAATTAATGCCCATCTTTTTTTTGGACCTAAATTCTTCATGAAAATATATTACGAGGGCTTATTCAGAAGCTTTGGACTTCTTCGCCAGAGGTTTGGTCAAGTCTCCAATCAAGGTTGTCGGCTTGTCTACCTTGCCAGAAATTTACGAAAAGATGGAAAAGGGTCAAATCGTTGGTAGATACGTTGTTGACACTTCTAAATAAGCGAATTTCTTATGATTTATGATTTTTATTATTAAATAAGTTATAAAAAAAATAAGTGTATACAAATTTTAAAGTGACTCTTAGGTTTTAAAACGAAAATTCTTATTCTTGAGTAACTCTTTCCTGTAGGTCAGGTTGCTTTCTCAGGTATAGCATGAGGTCGCTCTTATTGACCACACCTCTACCGGCCGGTCGAAATTCCCCTACCCTATGAACATATTCCATTTTGTAATTTCGTGTCGTTTCTATTATGAATTTCATTTATAAAGTTTATGTACAAATATCATAAAAAAAGAGAATCTTTTTAAGCAAGGATTTTCTTAACTTCTTCGGCGACAGCATCACCGACTTCGGTGGTACTGTTGGAACCACCTAAATCACCAGTTCTGATACCTGCATCCAAAACCTTTTTAACTGCATCTTCAATGGCCTTACCTTCTTCAGGCAAGTTCAATGACAATTTCAACATCATTGCAGCAGACAAGATAGTGGCGATAGGGTTGACCTTATTCTTTGGCAAATCTGGAGCAGAACCGTGGCATGGTTCGTACAAACCAAATGCGGTGTTCTTGTCTGGCAAAGAGGCCAAGGACGCAGATGGCAACAAACCCAAGGAACCTGGGATAACGGAGGCTTCATCGGAGATGATATCACCAAACATGTTGCTGGTGATTATAATACCATTTAGGTGGGTTGGGTTCTTAACTAGGATCATGGCGGCAGAATCAATCAATTGATGTTGAACCTTCAATGTAGGAAATTCGTTCTTGATGGTTTCCTCCACAGTTTTTCTCCATAATCTTGAAGAGGCCAAAACATTAGCTTTATCCAAGGACCAAATAGGCAATGGTGGCTCATGTTGTAGGGCCATGAAAGCGGCCATTCTTGTGATTCTTTGCACTTCTGGAACGGTGTATTGTTCACTATCCCAAGCGACACCATCACCATCGTCTTCCTTTCTCTTACCAAAGTAAATACCTCCCACTAATTCTCTGACAACAACGAAGTCAGTACCTTTAGCAAATTGTGGCTTGATTGGAGATAAGTCTAAAAGAGAGTCGGATGCAAAGTTACATGGTCTTAAGTTGGCGTACAATTGAAGTTCTTTACGGATTTTTAGTAAACCTTGTTCAGGTCTAACACTACCTGTACCCCATTTAGGACCACCCACAGCACCTAACAAAACGGCATCAGCCTTCTTGGAGGCTTCCAGCGCCTCATCTGGAAGTGGGACACCTGTAGCTTCGATAGCAGCACCACCAATTAAATGATTTTCGAAATCGAACTTGACATTGGAACGAACATCAGAAATAGCTTTAAGAACCTTAATGGCTTCGGCTGTGATTTCTTGACCAACGTGGTCACCTGGCAAAACGACGATCTTCTTAGGGGCAGACATTAGAATGGTATATCCTTGAAATATATATATATATTGCTGAAATGTAAAAGGTAAGAAAAGTTAGAAAGTAAGACGATTGCTAACCACCTATTGGAAAAAACAATAGGTCCTTAAATAATATTGTCAACTTCAAGTATTGTGATGCAAGCATTTAGTCATGAACGCTTCTCTATTCTATATGAAAAGCCGGTTCCGGCGCTCTCACCTTTCCTTTTTCTCCCAATTTTTCAGTTGAAAAAGGTATATGCGTCAGGCGACCTCTGAAATTAACAAAAAATTTCCAGTCATCGAATTTGATTCTGTGCGATAGCGCCCCTGTGTGTTCTCGTTATGTTGAGGAAAAAAATAATGGTTGCTAAGAGATTCGAACTCTTGCATCTTACGATACCTGAGTATTCCCACAGTTGGGGGATCTCGACTCTAGCTAGAGGATCAATTCGTAATCATGTCATAGCTGTTTCCTGTGTGAAATTGTTATCCGCTCACAATTCCACACAACATACGAGCCGGAAGCATAAAGTGTAAAGCCTGGGGTGCCTAATGAGTGAGCTAACTCACATTAATTGCGTTGCGCTCACTGCCCGCTTTCCAGTCGGGAAACCTGTCGTGCCAGCTGATAACTTCGTATAATGTATGCTATACGAAGTTATTAGGTCTGAAGAGGAGTTTACGTCCAGCCAAGCTAGCTTGGCTGCAGGTCGAGCGGCCGCGATCCGGAACCCTTAATATAACTTCGTATAATGTATGCTATACGAAGTTATCAGCTGCATTAATGAATCGGCCAACGCGCGGGGAGAGGCGGTTTGCGTATTGGGCGCTCTTCCGCTTCCTCGCTCACTGACTCGCTGCGCTCGGTCGTTCGGCTGCGGCGAGCGGTATCAGCTCACTCAAAGGCGGTAATACGGTTATCCACAGAATCAGGGGATAACGCAGGAAAGAACATGTGAGCAAAAGGCCAGCAAAAGGCCAGGAACCGTAAAAAGGCCGCGTTGCTGGCGTTTTTCCATAGGCTCCGCCCCCCTGACGAGCATCACAAAAATCGACGCTCAAGTCAGAGGTGGCGAAACCCGACAGGACTATAAAGATACCAGGCGTTTCCCCCTGGAAGCTCCCTCGTGCGCTCTCCTGTTCCGACCCTGCCGCTTACCGGATACCTGTCCGCCTTTCTCCCTTCGGGAAGCGTGGCGCTTTCTCATAGCTCACGCTGTAGGTATCTCAGTTCGGTGTAGGTCGTTCGCTCCAAGCTGGGCTGTGTGCACGAACCCCCCGTTCAGCCCGACCGCTGCGCCTTATCCGGTAACTATCGTCTTGAGTCCAACCCGGTAAGACACGACTTATCGCCACTGGCAGCAGCCACTGGTAACAGGATTAGCAGAGCGAGGTATGTAGGCGGTGCTACAGAGTTCTTGAAGTGGTGGCCTAACTACGGCTACACTAGAAGAACAGTATTTGGTATCTGCGCTCTGCTGAAGCCAGTTACCTTCGGAAAAAGAGTTGGTAGCTCTTGATCCGGCAAACAAACCACCGCTGGTAGCGGTGGTTTTTTTGTTTGCAAGCAGCAGATTACGCGCAGAAAAAAAGGATCTCAAGAAGATCCTTTGATCTTTTCTACGGGGTCTGACGCTCAGTGGAACGAAAACTCACGTTAAGGGATTTTGGTCATGAGATTATCAAAAAGGATCTTCACCTAGATCCTTTTAAATTAAAAATGAAGTTTTAAATCAATCTAAAGTATATATGAGTAAACTTGGTCTGACAGTTACCAATGCTTAATCAGTGAGGCACCTATCTCAGCGATCTGTCTATTTCGTTCATCCATAGTTGCCTGACTCCCCGTCGTGTAGATAACTACGATACGGGAGGGCTTACCATCTGGCCCCAGTGCTGCAATGATACCGCGAGACCCACGCTCACCGGCTCCAGATTTATCAGCAATAAACCAGCCAGCCGGAAGGGCCGAGCGCAGAAGTGGTCCTGCAACTTTATCCGCCTCCATCCAGTCTATTAATTGTTGCCGGGAAGCTAGAGTAAGTAGTTCGCCAGTTAATAGTTTGCGCAACGTTGTTGCCATTGCTACAGGCATCGTGGTGTCACGCTCGTCGTTTGGTATGGCTTCATTCAGCTCCGGTTCCCAACGATCAAGGCGAGTTACATGATCCCCCATGTTGTGCAAAAAAGCGGTTAGCTCCTTCGGTCCTCCGATCGTTGTCAGAAGTAAGTTGGCCGCAGTGTTATCACTCATGGTTATGGCAGCACTGCATAATTCTCTTACTGTCATGCCATCCGTAAGATGCTTTTCTGTGACTGGTGAGTACTCAACCAAGTCATTCTGAGAATAGTGTATGCGGCGACCGAGTTGCTCTTGCCCGGCGTCAATACGGGATAATACCGCGCCACATAGCAGAACTTTAAAAGTGCTCATCATTGGAAAACGTTCTTCGGGGCGAAAACTCTCAAGGATCTTACCGCTGTTGAGATCCAGTTCGATGTAACCCACTCGTGCACCCAACTGATCTTCAGCATCTTTTACTTTCACCAGCGTTTCTGGGTGAGCAAAAACAGGAAGGCAAAATGCCGCAAAAAAGGGAATAAGGGCGACACGGAAATGTTGAATACTCATACTCTTCCTTTTTCAATATTATTGAAGCATTTATCAGGGTTATTGTCTCATGAGCGGATACATATTTGAATGTATTTAGAAAAATAAACAAATAGGGGTTCCGCGCACATTTCCCCGAAAAGTGCCACCTGACGTCTAAGAAACCATTATTATCATGACATTAACCTATAAAAATAGGCGTATCACGAGGCCCTTTCGTCTCGCGCGTTTCGGTGATGACGGTGAAAACCTCTGACACATGCAGCTCCCGGAGACGGTCACAGCTTGTCTGTAAGCGGATGCCGGGAGCAGACAAGCCCGTCAGGGCGCGTCAGCGGGTGTTGGCGGGTGTCGGGGCTGGCTTAACTATGCGGCATCAGAGCAGATTGTACTGAGAGTGCACCATAACGCATTTAAGCATAAACACGCACTATGCCGTTCTTCTCATGTATATATATATACAGGCAACACGCAGATATAGGTGCGACGTGAACAGTGAGCTGTATGTGCGCAGCTCGCGTTGCATTTTCGGAAGCGCTCGTTTTCGGAAACGCTTTGAAGTTCCTATTCCGAAGTTCCTATTCTCTAGCTAGAAAGTATAGGAACTTCAGAGCGCTTTTGAAAACCAAAAGCGCTCTGAAGACGCACTTTCAAAAAACCAAAAACGCACCGGACTGTAACGAGCTACTAAAATATTGCGAATACCGCTTCCACAAACATTGCTCAAAAGTATCTCTTTGCTATATATCTCTGTGCTATATCCCTATATAACCTACCCATCCACCTTTCGCTCCTTGAACTTGCATCTAAACTCGACCTCTACATTTTTTATGTTTATCTCTAGTATTACTCTTTAGACAAAAAAATTGTAGTAAGAACTATTCATAGAGTGAATCGAAAACAATACGAAAATGTAAACATTTCCTATACGTAGTATATAGAGACAAAATAGAAGAAACCGTTCATAATTTTCTGACCAATGAAGAATCATCAACGCTATCACTTTCTGTTCACAAAGTATGCGCAATCCACATCGGTATAGAATATAATCGGGGATGCCTTTATCTTGAAAAAATGCACCCGCAGCTTCGCTAGTAATCAGTAAACGCGGGAAGTGGAGTCAGGCTTTTTTTATGGAAGAGAAAATAGACACCAAAGTAGCCTTCTTCTAACCTTAACGGACCTACAGTGCAAAAAGTTATCAAGAGACTGCATTATAGAGCGCACAAAGGAGAAAAAAAGTAATCTAAGATGCTTTGTTAGAAAAATAGCGCTCTCGGGATGCATTTTTGTAGAACAAAAAAGAAGTATAGATTCTTTGTTGGTAAAATAGCGCTCTCGCGTTGCATTTCTGTTCTGTAAAAATGCAGCTCAGATTCTTTGTTTGAAAAATTAGCGCTCTCGCGTTGCATTTTTGTTTTACAAAAATGAAGCACAGATTCTTCGTTGGTAAAATAGCGCTTTCGCGTTGCATTTCTGTTCTGTAAAAATGCAGCTCAGATTCTTTGTTTGAAAAATTAGCGCTCTCGCGTTGCATTTTTGTTCTACAAAATGAAGCACAGATGCTTCGTTGCT
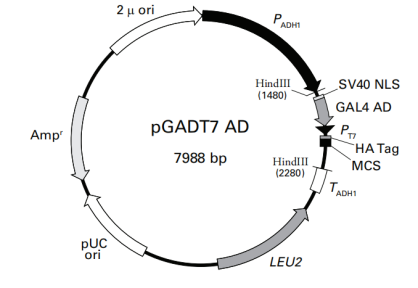
>pGADT7 vector sequence
TGCATGCCTGCAGGTCGAGATCCGGGATCGAAGAAATGATGGTAAATGAAATAGGAAATCAAGGAGCATGAAGGCAAAAGACAAATATAAGGGTCGAACGAAAAATAAAGTGAAAAGTGTTGATATGATGTATTTGGCTTTGCGGCGCCGAAAAAACGAGTTTACGCAATTGCACAATCATGCTGACTCTGTGGCGGACCCGCGCTCTTGCCGGCCCGGCGATAACGCTGGGCGTGAGGCTGTGCCCGGCGGAGTTTTTTGCGCCTGCATTTTCCAAGGTTTACCCTGCGCTAAGGGGCGAGATTGGAGAAGCAATAAGAATGCCGGTTGGGGTTGCGATGATGACGACCACGACAACTGGTGTCATTATTTAAGTTGCCGAAAGAACCTGAGTGCATTTGCAACATGAGTATACTAGAAGAATGAGCCAAGACTTGCGAGACGCGAGTTTGCCGGTGGTGCGAACAATAGAGCGACCATGACCTTGAAGGTGAGACGCGCATAACCGCTAGAGTACTTTGAAGAGGAAACAGCAATAGGGTTGCTACCAGTATAAATAGACAGGTACATACAACACTGGAAATGGTTGTCTGTTTGAGTACGCTTTCAATTCATTTGGGTGTGCACTTTATTATGTTACAATATGGAAGGGAACTTTACACTTCTCCTATGCACATATATTAATTAAAGTCCAATGCTAGTAGAGAAGGGGGGTAACACCCCTCCGCGCTCTTTTCCGATTTTTTTCTAAACCGTGGAATATTTCGGATATCCTTTTGTTGTTTCCGGGTGTACAATATGGACTTCCTCTTTTCTGGCAACCAAACCCATACATCGGGATTCCTATAATACCTTCGTTGGTCTCCCTAACATGTAGGTGGCGGAGGGGAGATATACAATAGAACAGATACCAGACAAGACATAATGGGCTAAACAAGACTACACCAATTACACTGCCTCATTGATGGTGGTACATAACGAACTAATACTGTAGCCCTAGACTTGATAGCCATCATCATATCGAAGTTTCACTACCCTTTTTCCATTTGCCATCTATTGAAGTAATAATAGGCGCATGCAACTTCTTTTCTTTTTTTTTCTTTTCTCTCTCCCCCGTTGTTGTCTCACCATATCCGCAATGACAAAAAAATGATGGAAGACACTAAAGGAAAAAATTAACGACAAAGACAGCACCAACAGATGTCGTTGTTCCAGAGCTGATGAGGGGTATCTCGAAGCACACGAAACTTTTTCCTTCCTTCATTCACGCACACTACTCTCTAATGAGCAACGGTATACGGCCTTCCTTCCAGTTACTTGAATTTGAAATAAAAAAAAGTTTGCTGTCTTGCTATCAAGTATAAATAGACCTGCAATTATTAATCTTTTGTTTCCTCGTCATTGTTCTCGTTCCCTTTCTTCCTTGTTTCTTTTTCTGCACAATATTTCAAGCTATACCAAGCATACAATCAACTCCAAGCTTTGCAAAGATGGATAAAGCGGAATTAATTCCCGAGCCTCCAAAAAAGAAGAGAAAGGTCGAATTGGGTACCGCCGCCAATTTTAATCAAAGTGGGAATATTGCTGATAGCTCATTGTCCTTCACTTTCACTAACAGTAGCAACGGTCCGAACCTCATAACAACTCAAACAAATTCTCAAGCGCTTTCACAACCAATTGCCTCCTCTAACGTTCATGATAACTTCATGAATAATGAAATCACGGCTAGTAAAATTGATGATGGTAATAATTCAAAACCACTGTCACCTGGTTGGACGGACCAAACTGCGTATAACGCGTTTGGAATCACTACAGGGATGTTTAATACCACTACAATGGATGATGTATATAACTATCTATTCGATGATGAAGATACCCCACCAAACCCAAAAAAAGAGATCTTTAATACGACTCACTATAGGGCGAGCGCCGCCATGGAGTACCCATACGACGTACCAGATTACGCTCATATGGCCATGGAGGCCAGTGAATTCCACCCGGGTGGGCATCGATACGGGATCCATCGAGCTCGAGCTGCAGATGAATCGTAGATACTGAAAAACCCCGCAAGTTCACTTCAACTGTGCATCGTGCACCATCTCAATTTCTTTCATTTATACATCGTTTTGCCTTCTTTTATGTAACTATACTCCTCTAAGTTTCAATCTTGGCCATGTAACCTCTGATCTATAGAATTTTTTAAATGACTAGAATTAATGCCCATCTTTTTTTTGGACCTAAATTCTTCATGAAAATATATTACGAGGGCTTATTCAGAAGCTTTGGACTTCTTCGCCAGAGGTTTGGTCAAGTCTCCAATCAAGGTTGTCGGCTTGTCTACCTTGCCAGAAATTTACGAAAAGATGGAAAAGGGTCAAATCGTTGGTAGATACGTTGTTGACACTTCTAAATAAGCGAATTTCTTATGATTTATGATTTTTATTATTAAATAAGTTATAAAAAAAATAAGTGTATACAAATTTTAAAGTGACTCTTAGGTTTTAAAACGAAAATTCTTATTCTTGAGTAACTCTTTCCTGTAGGTCAGGTTGCTTTCTCAGGTATAGCATGAGGTCGCTCTTATTGACCACACCTCTACCGGCCGGTCGAAATTCCCCTACCCTATGAACATATTCCATTTTGTAATTTCGTGTCGTTTCTATTATGAATTTCATTTATAAAGTTTATGTACAAATATCATAAAAAAAGAGAATCTTTTTAAGCAAGGATTTTCTTAACTTCTTCGGCGACAGCATCACCGACTTCGGTGGTACTGTTGGAACCACCTAAATCACCAGTTCTGATACCTGCATCCAAAACCTTTTTAACTGCATCTTCAATGGCCTTACCTTCTTCAGGCAAGTTCAATGACAATTTCAACATCATTGCAGCAGACAAGATAGTGGCGATAGGGTTGACCTTATTCTTTGGCAAATCTGGAGCAGAACCGTGGCATGGTTCGTACAAACCAAATGCGGTGTTCTTGTCTGGCAAAGAGGCCAAGGACGCAGATGGCAACAAACCCAAGGAACCTGGGATAACGGAGGCTTCATCGGAGATGATATCACCAAACATGTTGCTGGTGATTATAATACCATTTAGGTGGGTTGGGTTCTTAACTAGGATCATGGCGGCAGAATCAATCAATTGATGTTGAACCTTCAATGTAGGAAATTCGTTCTTGATGGTTTCCTCCACAGTTTTTCTCCATAATCTTGAAGAGGCCAAAACATTAGCTTTATCCAAGGACCAAATAGGCAATGGTGGCTCATGTTGTAGGGCCATGAAAGCGGCCATTCTTGTGATTCTTTGCACTTCTGGAACGGTGTATTGTTCACTATCCCAAGCGACACCATCACCATCGTCTTCCTTTCTCTTACCAAAGTAAATACCTCCCACTAATTCTCTGACAACAACGAAGTCAGTACCTTTAGCAAATTGTGGCTTGATTGGAGATAAGTCTAAAAGAGAGTCGGATGCAAAGTTACATGGTCTTAAGTTGGCGTACAATTGAAGTTCTTTACGGATTTTTAGTAAACCTTGTTCAGGTCTAACACTACCTGTACCCCATTTAGGACCACCCACAGCACCTAACAAAACGGCATCAGCCTTCTTGGAGGCTTCCAGCGCCTCATCTGGAAGTGGGACACCTGTAGCTTCGATAGCAGCACCACCAATTAAATGATTTTCGAAATCGAACTTGACATTGGAACGAACATCAGAAATAGCTTTAAGAACCTTAATGGCTTCGGCTGTGATTTCTTGACCAACGTGGTCACCTGGCAAAACGACGATCTTCTTAGGGGCAGACATTAGAATGGTATATCCTTGAAATATATATATATATTGCTGAAATGTAAAAGGTAAGAAAAGTTAGAAAGTAAGACGATTGCTAACCACCTATTGGAAAAAACAATAGGTCCTTAAATAATATTGTCAACTTCAAGTATTGTGATGCAAGCATTTAGTCATGAACGCTTCTCTATTCTATATGAAAAGCCGGTTCCGGCGCTCTCACCTTTCCTTTTTCTCCCAATTTTTCAGTTGAAAAAGGTATATGCGTCAGGCGACCTCTGAAATTAACAAAAAATTTCCAGTCATCGAATTTGATTCTGTGCGATAGCGCCCCTGTGTGTTCTCGTTATGTTGAGGAAAAAAATAATGGTTGCTAAGAGATTCGAACTCTTGCATCTTACGATACCTGAGTATTCCCACAGTTGGGGGATCTCGACTCTAGCTAGAGGATCAATTCGTAATCATGTCATAGCTGTTTCCTGTGTGAAATTGTTATCCGCTCACAATTCCACACAACATACGAGCCGGAAGCATAAAGTGTAAAGCCTGGGGTGCCTAATGAGTGAGCTAACTCACATTAATTGCGTTGCGCTCACTGCCCGCTTTCCAGTCGGGAAACCTGTCGTGCCAGCTGATAACTTCGTATAATGTATGCTATACGAAGTTATTAGGTCTGAAGAGGAGTTTACGTCCAGCCAAGCTAGCTTGGCTGCAGGTCGAGCGGCCGCGATCCGGAACCCTTAATATAACTTCGTATAATGTATGCTATACGAAGTTATCAGCTGCATTAATGAATCGGCCAACGCGCGGGGAGAGGCGGTTTGCGTATTGGGCGCTCTTCCGCTTCCTCGCTCACTGACTCGCTGCGCTCGGTCGTTCGGCTGCGGCGAGCGGTATCAGCTCACTCAAAGGCGGTAATACGGTTATCCACAGAATCAGGGGATAACGCAGGAAAGAACATGTGAGCAAAAGGCCAGCAAAAGGCCAGGAACCGTAAAAAGGCCGCGTTGCTGGCGTTTTTCCATAGGCTCCGCCCCCCTGACGAGCATCACAAAAATCGACGCTCAAGTCAGAGGTGGCGAAACCCGACAGGACTATAAAGATACCAGGCGTTTCCCCCTGGAAGCTCCCTCGTGCGCTCTCCTGTTCCGACCCTGCCGCTTACCGGATACCTGTCCGCCTTTCTCCCTTCGGGAAGCGTGGCGCTTTCTCATAGCTCACGCTGTAGGTATCTCAGTTCGGTGTAGGTCGTTCGCTCCAAGCTGGGCTGTGTGCACGAACCCCCCGTTCAGCCCGACCGCTGCGCCTTATCCGGTAACTATCGTCTTGAGTCCAACCCGGTAAGACACGACTTATCGCCACTGGCAGCAGCCACTGGTAACAGGATTAGCAGAGCGAGGTATGTAGGCGGTGCTACAGAGTTCTTGAAGTGGTGGCCTAACTACGGCTACACTAGAAGAACAGTATTTGGTATCTGCGCTCTGCTGAAGCCAGTTACCTTCGGAAAAAGAGTTGGTAGCTCTTGATCCGGCAAACAAACCACCGCTGGTAGCGGTGGTTTTTTTGTTTGCAAGCAGCAGATTACGCGCAGAAAAAAAGGATCTCAAGAAGATCCTTTGATCTTTTCTACGGGGTCTGACGCTCAGTGGAACGAAAACTCACGTTAAGGGATTTTGGTCATGAGATTATCAAAAAGGATCTTCACCTAGATCCTTTTAAATTAAAAATGAAGTTTTAAATCAATCTAAAGTATATATGAGTAAACTTGGTCTGACAGTTACCAATGCTTAATCAGTGAGGCACCTATCTCAGCGATCTGTCTATTTCGTTCATCCATAGTTGCCTGACTCCCCGTCGTGTAGATAACTACGATACGGGAGGGCTTACCATCTGGCCCCAGTGCTGCAATGATACCGCGAGACCCACGCTCACCGGCTCCAGATTTATCAGCAATAAACCAGCCAGCCGGAAGGGCCGAGCGCAGAAGTGGTCCTGCAACTTTATCCGCCTCCATCCAGTCTATTAATTGTTGCCGGGAAGCTAGAGTAAGTAGTTCGCCAGTTAATAGTTTGCGCAACGTTGTTGCCATTGCTACAGGCATCGTGGTGTCACGCTCGTCGTTTGGTATGGCTTCATTCAGCTCCGGTTCCCAACGATCAAGGCGAGTTACATGATCCCCCATGTTGTGCAAAAAAGCGGTTAGCTCCTTCGGTCCTCCGATCGTTGTCAGAAGTAAGTTGGCCGCAGTGTTATCACTCATGGTTATGGCAGCACTGCATAATTCTCTTACTGTCATGCCATCCGTAAGATGCTTTTCTGTGACTGGTGAGTACTCAACCAAGTCATTCTGAGAATAGTGTATGCGGCGACCGAGTTGCTCTTGCCCGGCGTCAATACGGGATAATACCGCGCCACATAGCAGAACTTTAAAAGTGCTCATCATTGGAAAACGTTCTTCGGGGCGAAAACTCTCAAGGATCTTACCGCTGTTGAGATCCAGTTCGATGTAACCCACTCGTGCACCCAACTGATCTTCAGCATCTTTTACTTTCACCAGCGTTTCTGGGTGAGCAAAAACAGGAAGGCAAAATGCCGCAAAAAAGGGAATAAGGGCGACACGGAAATGTTGAATACTCATACTCTTCCTTTTTCAATATTATTGAAGCATTTATCAGGGTTATTGTCTCATGAGCGGATACATATTTGAATGTATTTAGAAAAATAAACAAATAGGGGTTCCGCGCACATTTCCCCGAAAAGTGCCACCTGACGTCTAAGAAACCATTATTATCATGACATTAACCTATAAAAATAGGCGTATCACGAGGCCCTTTCGTCTCGCGCGTTTCGGTGATGACGGTGAAAACCTCTGACACATGCAGCTCCCGGAGACGGTCACAGCTTGTCTGTAAGCGGATGCCGGGAGCAGACAAGCCCGTCAGGGCGCGTCAGCGGGTGTTGGCGGGTGTCGGGGCTGGCTTAACTATGCGGCATCAGAGCAGATTGTACTGAGAGTGCACCATAACGCATTTAAGCATAAACACGCACTATGCCGTTCTTCTCATGTATATATATATACAGGCAACACGCAGATATAGGTGCGACGTGAACAGTGAGCTGTATGTGCGCAGCTCGCGTTGCATTTTCGGAAGCGCTCGTTTTCGGAAACGCTTTGAAGTTCCTATTCCGAAGTTCCTATTCTCTAGCTAGAAAGTATAGGAACTTCAGAGCGCTTTTGAAAACCAAAAGCGCTCTGAAGACGCACTTTCAAAAAACCAAAAACGCACCGGACTGTAACGAGCTACTAAAATATTGCGAATACCGCTTCCACAAACATTGCTCAAAAGTATCTCTTTGCTATATATCTCTGTGCTATATCCCTATATAACCTACCCATCCACCTTTCGCTCCTTGAACTTGCATCTAAACTCGACCTCTACATTTTTTATGTTTATCTCTAGTATTACTCTTTAGACAAAAAAATTGTAGTAAGAACTATTCATAGAGTGAATCGAAAACAATACGAAAATGTAAACATTTCCTATACGTAGTATATAGAGACAAAATAGAAGAAACCGTTCATAATTTTCTGACCAATGAAGAATCATCAACGCTATCACTTTCTGTTCACAAAGTATGCGCAATCCACATCGGTATAGAATATAATCGGGGATGCCTTTATCTTGAAAAAATGCACCCGCAGCTTCGCTAGTAATCAGTAAACGCGGGAAGTGGAGTCAGGCTTTTTTTATGGAAGAGAAAATAGACACCAAAGTAGCCTTCTTCTAACCTTAACGGACCTACAGTGCAAAAAGTTATCAAGAGACTGCATTATAGAGCGCACAAAGGAGAAAAAAAGTAATCTAAGATGCTTTGTTAGAAAAATAGCGCTCTCGGGATGCATTTTTGTAGAACAAAAAAGAAGTATAGATTCTTTGTTGGTAAAATAGCGCTCTCGCGTTGCATTTCTGTTCTGTAAAAATGCAGCTCAGATTCTTTGTTTGAAAAATTAGCGCTCTCGCGTTGCATTTTTGTTTTACAAAAATGAAGCACAGATTCTTCGTTGGTAAAATAGCGCTTTCGCGTTGCATTTCTGTTCTGTAAAAATGCAGCTCAGATTCTTTGTTTGAAAAATTAGCGCTCTCGCGTTGCATTTTTGTTCTACAAAATGAAGCACAGATGCTTCGTTGCT
- Wang, S(2024). Y1HGold Yeast One-Hybrid Screening and Validation Experiment Protocol. Bio-protocol Preprint. bio-protocol.org/prep2732.
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
