Advanced Search
Influenza A genome sequencing
Last updated date: Nov 20, 2019 Views: 1737 Forks: 0
RNA extraction
Requirements
- QIAamp Viral RNA Mini Kit (Qiagen, 52904)
- 96%-100% ethanol
- 1.5 mL microcentrifuge tubes
Notes when opening a new kit
- Add 310 μl Buffer AVE to the tube containing 310 μg lyophilized carrier RNA to obtain a solution of 1 μg/μl. Dissolve the carrier RNA thoroughly, divide it into conveniently sized aliquots, and store it at –20°C. Do not freeze–thaw the aliquots of carrier RNA more than 3 times.
- Add ethanol to buffer AW1 according to instructions on bottle label
Protocol
Pipet 560 μl of prepared Buffer AVL and 5.6 μl dissolved carrier RNA (1 μg/ μl) into a 1.5 ml microcentrifuge tube. Prepare a master-mix when processing multiple samples.
Add 140 μl plasma, serum, urine, cell-culture supernatant, or cell-free body fluid to the Buffer AVL–carrier RNA in the microcentrifuge tube. Mix by pulse-vortexing for 15 s.
Incubate at room temperature (15–25°C) for 10 min.
Briefly centrifuge the tube to remove drops from the inside of the lid.
Add 560 μl of ethanol (96–100%) to the sample, and mix by pulse-vortexing for 15 s. After mixing, briefly centrifuge the tube to remove drops from inside the lid.
Carefully apply 630 μl of the solution from step 5 to the QIAamp Mini column (in a 2 ml collection tube) without wetting the rim. Close the cap, and centrifuge at 6000 x g (8000 rpm) for 1 min. Place the QIAamp Mini column into a clean 2 ml collection tube, and discard the tube containing the filtrate.
Carefully open the QIAamp Mini column, and repeat step 6.
Carefully open the QIAamp Mini column, and add 500 μl of Buffer AW1. Close the cap, and centrifuge at 6000 x g (8000 rpm) for 1 min. Place the QIAamp Mini column in a clean 2 ml collection tube (provided), and discard the tube containing the filtrate.
Carefully open the QIAamp Mini column, and add 500 μl of Buffer AW2. Close the cap and centrifuge at full speed (20,000 x g; 14,000 rpm) for 3 min.
Place the QIAamp Mini column in a new 2 ml collection tube (not provided), and discard the old collection tube with the filtrate. Centrifuge at full speed for 1 min.
Place the QIAamp Mini column in a clean 1.5 ml microcentrifuge tube (not provided). Discard the old collection tube containing the filtrate. Carefully open the QIAamp Mini column and add 60 μl of Buffer AVE equilibrated to room temperature. Close the cap, and incubate at room temperature for 1 min. Centrifuge at 6000 x g (8000 rpm) for 1 min to elute.
Store RNA samples at −80°C, or keep samples on ice and proceed directly with MS-RTPCR.
MS-RTPCR amplification
Requirements
- Superscript III high-fidelity RT-PCR kit (Invitrogen, 12574-035)
- Opti1 primer set, 10 μM stocks (HPLC purified; influenza-complementary sequences underlined)
Opti1-F1 5’ GTTACGCGCCAGCAAAAGCAGG
Opti1-F2 5’ GTTACGCGCCAGCGAAAGCAGG (predominant in PB segments)
Opti1-R1 5’ GTTACGCGCCAGTAGAAACAAGG
- Nuclease-free water
- RNase-free PCR tubes
Protocol
Thaw RNA and the Opti1 primer set and place on ice.
Thaw Superscript III HF RT-PCR reagents and mix by vortexing (except for the enzyme). Keep all components on ice while preparing the reaction to help prevent non-specific annealing.
For each sample, mix the following in order on ice. Make a master mix when preparing multiple samples and mix by pipetting.
Nuclease-free water 17 μL
2x RT-PCR buffer 25 μL
10 μM Opti1-F1 0.5 μL
10 μM Opti1-F2 0.5 μL
10 μM Opti1-R1 1 μL
RT/Taq enzyme mix 1 μL
Total 45 μL
Distribute 45 μL of the Master Mix to each PCR tube (0.2 ml) on ice.
Add 5 μL of RNA template (optionally including negative RNA purification control) or ddH2O (PCR negative control) to tubes.
Place reaction tubes into a thermo cycler that is paused at 55°C (the warm start helps to reduce nonspecific amplification products).
Select the following cycling parameters:
- 55°C/2 min
- 42°C/60 min
- 94°C/2 min
- 5 cycles of (94°C/30 s; 44°C/30 s; 68°C/3.5 min)
- 26 cycles of (94°C/30 s; 57°C/30 s; 68°C/3.5 min)
- 68°C/10 min
- hold at 4°CAnalyze 5 μL of the M-RTPCR reactions by 0.8% agarose gel electrophoresis and verify that all genomic segments are present (PB1 and PB2 migrate together at 2.3 Kb), with a minimal amount of non-specific amplification (i.e. bands migrating lower than 800 bp).
Proceed with purification of amplicons, or store at −20°C.
Purification of DNA amplicons
Requirements
- Agencourt AMPure XP 5 mL Kit (Beckman Coulter, A63880)
- Magnetic stand (e.g. Invitrogen, DynaMag-2, 12321D)
- 70% Ethanol
- 1x TE (elution buffer)
- 1.5 mL microcentrifuge tubes
Notes
- The volume of AMPure XP beads added to the PCR reaction is critical and the ratio chosen here will deplete non-specific amplification products <700 bp.
Protocol
Gently shake the Agencourt AMPure XP bottle to resuspend any magnetic beads that may have settled.
Transfer 40 μL of the PCR reaction to a new 1.5 mL tube and add 18 μL (0.45x volume) of resuspended AMPure XP beads.
Mix reagent and PCR reaction thoroughly by pipetting at least 10 times. Let the mixed sample incubate for 5 minutes at room temperature.
Place the microcentrifuge tube containing the beads on a magnetic stand for 2 minutes. Wait for the solution to clear before proceeding to the next step.
Aspirate the cleared solution from the tube and discard.
Keep the tube on the magnetic stand, dispense 200 μL of 70% ethanol and incubate for 30 seconds at room temperature. Aspirate out the ethanol and discard. Repeat for a total of two washes. Take care not to disturb the beads while washing.
Air-dry beads for 10 minutes while the tube is on the magnetic stand with the lid open.
Take the tube off the magnetic stand, add 43 μL elution buffer (1x TE) and mix by pipetting 10 times.
Place the tube on the magnetic stand for 2 minutes to separate the beads, and transfer 40 μL to a new 1.5 mL tube. Avoid transferring any beads.
Measure the concentration of the purified DNA with a Nanodrop or Qubit (preferred) and store at −20°C. This procedure typically yields 50–80 ng/ μL of DNA, depending on the amount and quality of the template RNA.
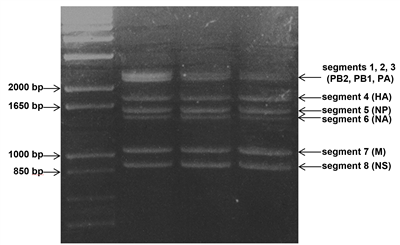
Example of RT-PCR amplification of the 8 viral RNA segments of influenza A genome.
5 μl of a 50 μl RT-PCR reaction were loaded in a 1% agarose gel.
Related files
 Influenza MS-RT PCR figure.pptx
Influenza MS-RT PCR figure.pptx  Influenza-MS-RTPCR.pdf
Influenza-MS-RTPCR.pdf - Mena, N(2019). Influenza A genome sequencing. Bio-protocol Preprint. bio-protocol.org/prep27.
- Mena, I., Nelson, M. I., Quezada-Monroy, F., Dutta, J., Cortes-Fernández, R., Lara-Puente, J. H., Castro-Peralta, F., Cunha, L. F., Trovão, N. S., Lozano-Dubernard, B., Rambaut, A., van Bakel, H. and García-Sastre, A.(2016). Origins of the 2009 H1N1 influenza pandemic in swine in Mexico. eLife. DOI: 10.7554/eLife.16777
Category
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
