Advanced Search
DIY Intervention Testing in Daphnia
Last updated date: Dec 28, 2023 Views: 2639 Forks: 0
DIY Intervention Testing in Daphnia
A simplified way to test lifespan interventions at home (v0.2, Dec 2023)
Cora Anderson1, Rachael A Jonas-Closs1, Benyamin Matei-Dediu2, Edouard Debonneuil2, Leonid Peshkin*1
1 Systems Biology, Harvard Medical School, Boston, MA 02115, USA
2 Association Longévité & Santé, Sceaux 92330, France
* correspondence to: pesha@hms.harvard.edu
Abstract. This guide is for amateur science enthusiasts who are looking to conduct safe and easy animal experiments at home. This can be done with the aim of educating yourself and others about ecology, aquaculture, and pharmaco-biology. Additionally, by using a safe and accessible organism, you could contribute effort and data to the community science movement. We provide accessible ways for obtaining Daphnia, keeping daphnids at home, conducting experiments, troubleshooting, recording parameters of health and lifespan, and reporting the results. You will be able to begin immediately and build up gradually. A typical experiment might take 4-8 hours a week and last 1-2 months. The basic setup cost is around $50, depending on how food and equipment are sourced. We strongly encourage the reader to contact us with any suggestions and feedback to help further improve and develop this guide.
Contents
- introduction to Daphnia
- quick start: setting up a “grandmother culture”
- maintaining groups
- how to experiment
- your first experiment: collecting normal aging data
- start testing drugs
Consider skipping to section 2, which defines quick and dirty of the Daphnia experience – a self-sustaining culture in a bucket of rainwater. Once you have it going you can start enjoying simple interactions with the animals - observing the lifecycle, exposing the animals to bright light, trying to figure out whether Daphnia sleep and play with one another. Then gradually build your technique, learn about controlling the diet, obtaining and maintaining groups for control and experimental perturbation.
- Introduction to Daphnia?
Daphnia, a.k.a. the water flea, is a large genus – comprising over 200 species. The individual organisms are referred to as daphnids. These small freshwater crustaceans are commonly used as a model organism in research. Being extremely sensitive to small concentrations of substances in water, they are well suited for studying the effects of environmental stresses, toxicology, and more recently, longevity and aging [1]. Its short lifespan of a few weeks allows for efficient study of longevity interventions, while its transparency enables real-time observation of internal organs and physiological processes. Additionally, its simple and well-characterized genome makes it convenient for genetic manipulations and gene expression studies.
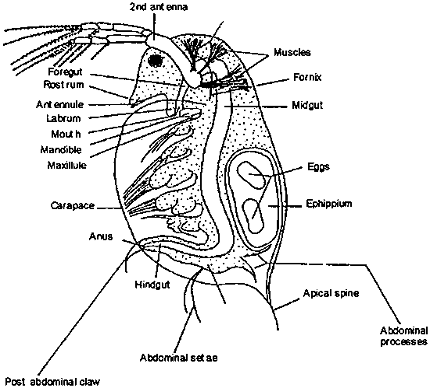
1a. Reproduction and offspring
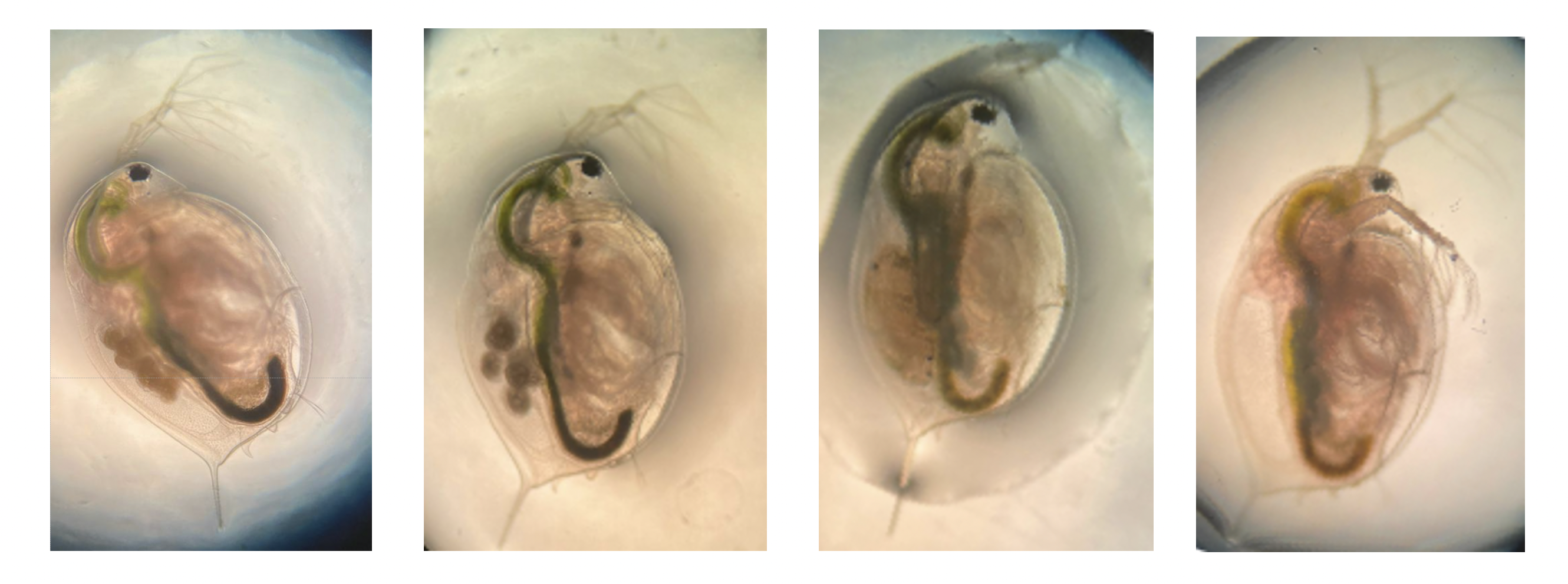
Female daphnids develop ovaries and lay eggs directly into their brood chamber located on the dorsal side of the gut tract. The eggs then hatch within the brood chamber and emerge as neonates. These are formed asexually (parthenogenesis) and thus are genetic clones of the mother. Females can also lay resting eggs (typically through sexual and rarely through asexual reproduction). Typical egg formation starts with the ripening/darkening of the ovaries before small, oblong eggs appear in the brood chamber (true for both asexual and resting eggs). As the eggs develop, they become more rounded. In resting eggs, an envelope known as ephippium forms. The stages of transition of asexual eggs to neonates are named A1-A9 [2]. The first image on the left is between A2-4 where eggs are round and begin to darken. The next image is approximately staged at A5-A7 when the embryonic bodies begin to elongate and develop eyespots. The next image is stage A8-A9 when the offspring are almost fully developed, have eyes, antennae, body shape, and organs developed. They will soon emerge as neonates leaving the empty brood chamber. A group of neonates (typically 5-10) from one mother obtained during the same molt cycle is called a clutch.
1b. Resting egg formation
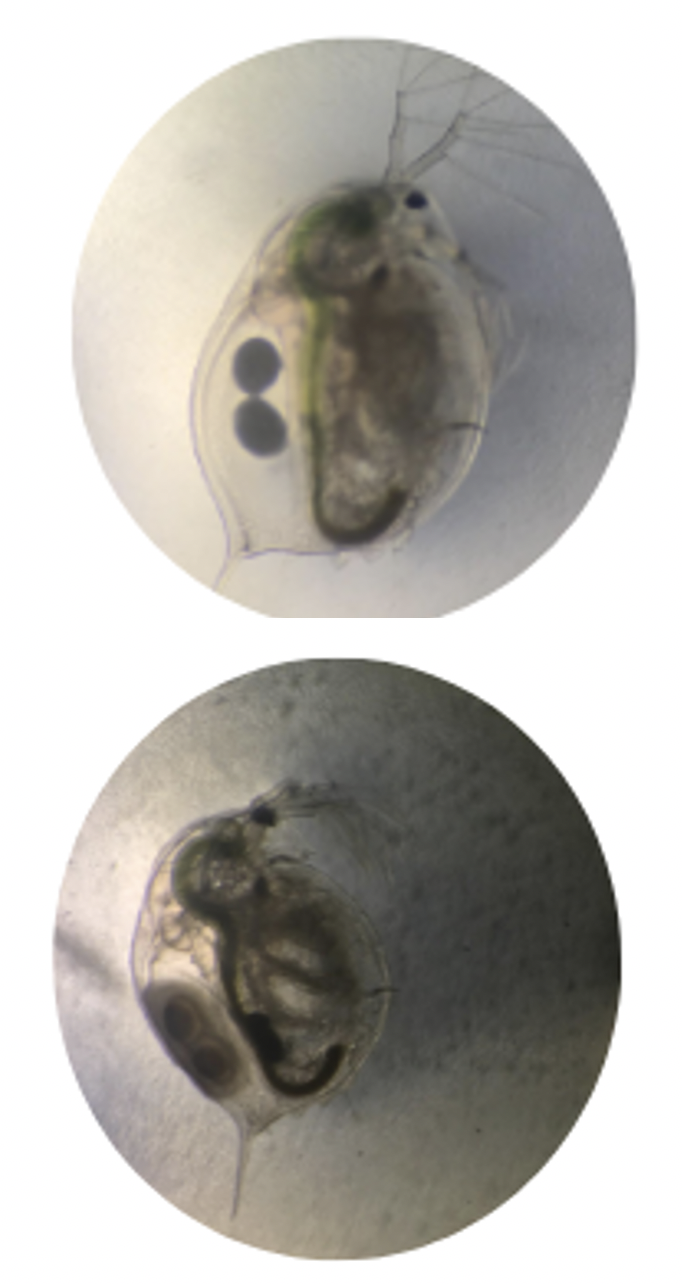
The formation of ephippium can be a sign of external stress from the environment. These eggs are laid in the brood chamber, without hatching. Resting or diapause eggs require stimuli for hatching such as chemical change or desiccation and rehydration. The intensity of stress required varies across populations within a given species. These stressors can be from overpopulation, low oxygen, higher temperature, increased salinity and other chemical build-up from sources such as rotting material or bacteria. These are all pressures that occur in nature if their habitat is drying up indicating it is time for them to reproduce sexually and make diapause eggs to rest in the silt, awaiting better conditions. In the next wet season, rain and runoff rehydrate the silt and gradually refill the body of water; this process stimulates the hatching of the resting eggs. Thus, an increased resting egg production in your population indicates animals are under one of the triggering stressors. Interestingly, not all ephippia or diapause eggs are fertilized, the envelope will also develop around unfertilized eggs (also called winter eggs) [3]. The top image shows the formation of the envelope that contains the eggs. The bottom envelope darkened and solidified.
1c. Males vs females
Females are typically larger than males and have ovaries and brood chambers. In contrast, males are smaller and have a caudal antenna, which is a lower, short projection below the rostrum. Although not a form of external genitalia, the caudal antenna is used to hold onto the female during mating. This feature can be observed under a dissecting microscope. Many things can trigger male production, specifically maternal age and environmental stress: male clutches can be a function of senescence, the drying body of water may also trigger male clutches for sexual reproduction. If you are sexing your Daphnia for an experiment without a dissection scope or magnification, here are some differences that you can see through your container:
- Males are smaller and swim differently than females. If you see that the offspring produced from your female are significantly smaller (after aging for 3-5 days, to adult size) and swim in especially jerky/twitchy bursts, you probably have males.
- If you are unsure about using size and swim pattern to sex the animals, just separate out your clutch and wait 5-10 days. If they have grown and not produced offspring by then, you have males. You can also experiment on males, but there is less data on lifespan and other components.
- Daphnia seldom produce mixed-sex clutches, so if you suspect some neonates are males, they are likely all males. In addition, once male production has started in your female, the chance of her producing a female clutch for the next cycle goes down. Best to start over with a fresh female if you want females for your experiment.
2. Quick Start: setting up a Grandmother Culture
Daphnia magna are the recommended species of Daphnia to culture at home, as they are larger and generally considered more robust. The first step is to get a Daphnia magna culture going in a robust setting – henceforth the “grandmother culture”. The logic is that individuals from this grandmother culture can be removed and propagated to generate the “mother culture” which in turn produces controlled healthy progeny for experimental use. Developing a grandmother culture without compromising or crashing it takes trial, error, and practice; eventually, it becomes a semi-self-sustaining way to keep animals available even when the time to invest in your Daphnia is limited.
2.a Getting started

The conditions for this culture should be established prior to introducing Daphnia. Take a large spring water jug and cut off the top in order to have a light transparent bucket with a side handle. Best practice is to collect some rainwater, mark the water level and let it stand in indirect sunlight. Add a drop/granule of any plant mineral fertilizer to generate “green water” which will serve both as food and provide autogenous biological filtration. Once the water begins to turn green Daphnia may be added. This process may be expedited by adding a few grains of soil. Once the grandmother culture is established and stabilized the only regular water quality maintenance required is to compensate for evaporation by adding rain or distilled water to the original level. Detritus and biofilm naturally accumulate in this culture. We do not recommend artificial oxygenation using aquarium pumps and sandstones. The widely open top surface is sufficient for the gas exchange. It is hard to maintain low air flow hurt the daphnids, and it would depend on size and shape of the container. Also oxygenated water leads to explosive algae growth which in turn destabilizes the culture.
2.b Density control
Maintaining a healthy density is critical to the health of the culture. Having too many Daphnia in your culture can cause a crash in your population from metabolic waste accumulation, or the overgrowth of saprophytic organisms from excess molts or dead animals. If the water loses its green tint it is because the Daphnia are eating algae faster than it is growing. This is a good indicator that your culture is exceeding a healthy density. Where the culture is at such high density, it is time to cull or separate some daphnids into a new container. If the water does not regain its green tint following the cull it is advised that the culture be moved to a location with more indirect sunlight or a small amount of fertilizer be dosed, again.
Do not over-reduce the culture density, there should always be enough healthy females present for the culture to regenerate, slowly. While you learn the ins and outs of Daphnia keeping, house some removed daphnids separately. Observe the remaining grandmother culture for a few days to ensure it is stable before culling the removed animals. To cull Daphnia, the recommended humane technique is bleaching the animals before disposing of them. Besides, non-native animals, including Daphnia, should never be introduced into the wild.
2.c Ways to get Daphnia
Many pet stores have Daphnia as they are raised and sold as live fish food. For this purpose, a few different species are frequently carried so it is important that the animals procured be specified as Daphnia magna. You can also order online from pet fish suppliers, biological suppliers, or Amazon. There may be other enthusiasts who culture Daphniamagna and are willing to share. Daphnia survive a few day shipment in a sealed container during a warmer temperate season. When you receive the animals, open the lid and keep a few in original container - it is common for adult Daphnia to die off a few days after they are transferred, due to acclimation stress. If conditions are acceptable, the neonates they lay in this time will develop into a thriving culture.
2.d Water quality
For the purposes of the grandmother culture, rainwater is recommended. If collecting a large volume of rainwater is not an option then well/spring water is the preferred alternative. Do not use untreated tap water as it contains chemicals that will kill Daphnia. Use plain non-mineral spring water or drinking water that has been dechlorinated (by either household water filters or aquarium dechlorination products). Please note that setting water out or aerating it is not an effective method of dechlorination if you have a modern municipal water system that utilizes chloramine. It is best to test your source of water by leaving a few animals in it for a day before using it on your whole colony. Culture water can be kept at 15-25°C but should not have drastic temperature fluctuations. For this reason, keep the culture out of direct sunlight.
2.e Troubleshooting
If your culture crashed, the container should be sanitized before an attempt to restart it. Do not use detergents when cleaning containers, this can stress or kill Daphnia or destabilize the healthy growth of algae. Melamine sponges are recommended for removing biofilms. Note that these factors could also affect the outcome of your experiment. If you are struggling to maintain a grandmother culture, try making multiple mini grandmother cultures in clear plastic bottles with the tops cut off. Try adjusting such parameters as source of water, temperature, how much indirect sunlight the container receives and how long the green water has been developing before Daphnia are introduced.
3. How to maintain groups for the mother and experimental generation
In order to experiment with Daphnia, it is important to have a solid methodology in place to ensure that your results are reputable, reliable and accurate. In this section, we will provide you with some essential information on how to choose the right container, calculate population density, feed your Daphnia during the experiment, and maintain healthy experimental population (as differs from a grandmother culture).
3a. Diet
In their natural environment, Daphnia feed on algae, bacteria and yeasts that are naturally present. This is replicated in the grandmother culture where the Daphnia lives in green water and coexist with other microbial life that they feed on. Though green water is an excellent food source for the grandmother culture, it is not stable or consistent enough to be fed to the mother generation or experimental groups. The ideal diet for these is live green algae (Scenedesmus, Chlorella vulgaris or Spirulina spp. are the most commonly used). Green algae culturing kits can be purchased from vendors (e.g. Algae Research Supply, Carolina Biological). Alternatively, please refer to DIY algae culturing manuals available elsewhere. Culturing green algae is its own skill to learn, requiring much trial and error.
Artificial algae have been found to lead to low fecundity while algae pellets and wafers frequently lead to significant overfeeding and poor water quality. For these reasons, we recommend that most individuals start by feeding a solution of freeze-dried microalgae: spirulina. Freeze-dried spirulina is a powder of preserved cyanobacteria (blue-green algae). Though there are other freeze-dried algae, none are as readily available as spirulina, which can be found through both health food and aquaculture vendors. The disadvantage of feeding nonliving algae is that any uneaten food will rot and foul the water. As regular water changes are performed for the mother and experimental generations the impact of this is manageable as long as animals are not being significantly overfed and containers are replaced and cleaned regularly.
When feeding experimental groups consistency is key. Create a mixture of 1 tsp (3g) freeze-dried spirulina in 1/3c (75mL) water and scale up proportionally as needed. Ensure that it is mixed thoroughly and then filter through a fine fish net or similar to remove any fragments that have not gone into the solution. Mix again and immediately pour into a silicone tray for making small ice cubes and then freeze. A few hours before feeding the required amount of cubes can be thawed and remixed to ensure a constant concentration of food is used for the duration of your experiments. It is recommended to feed 4 drops or approximately 40uL per animal (if you have many animals every 10 drops is approximately 1 mL) but this may need to be adjusted depending on other conditions. If uneaten food collects at the bottom, try feeding less, basically we want to feed daphnids so they clear up the water as they feed. Once you establish how much food gets cleared by daphnids in a few hours, keep careful measure and feed the same amount. Frequent (once a day or once every other day) feeding at regular intervals helps prevent stress from a lack of food that can interact with your experiment. Uniform feeding is required for accurately comparing across experimental conditions. It is not recommended that spirulina or any other nonliving food be fed to the grandmother culture, as the rotting of any uneaten food will destabilize it. The ill effects of rotting food are mitigated in the mother generation and experimental groups by performing regular water changes.
3b. Mother generation
Once your grandmother culture is stabilized, it is time to learn how to keep the routine husbandry and prepare for experimentation by culturing a mother generation. In order to have healthy mothers that produce large clutches they will need to be reared in controlled conditions, with regular feedings and water changes. For the purposes of static husbandry, we recommend using dechlorinated tap or well/spring water as the makeup water (this will also be used for your experimental controls). Before use, his water should be collected, treated as needed following the instructions in section 2 and tempered (set out to reach room temperature). As mothers will be housed in small groups or isolation this is also a chance to try various isolation containers that may be used for experimentation.
3c. How to choose containers
With Daphnia magna, containers can range in volume depending on use.
- Small, identically sized, clear containers (e.g. yogurt container or baby food jar). Ideally made of glass or a minimally porous plastic (acrylic, polycarbonate).
- The transparency of containers allows for photosynthesis of the algae and helps maintain a standard day/night cycle.
- The containers will need to be cleaned before and between uses and a porous or scratchable material will make it challenging to do this effectively.
- Do not use containers that may have an oily residue, this can inhibit algal growth and cause Daphnia to become trapped to the surface of the water.
- Be mindful of where these containers are placed during experiments, as they need to have equal and adequate sunlight and water temperature.
3d. Making mothers
Identify three large females from the grandmother culture, preferably the ones with neonates visible in the brood chamber. Isolate each of these females into their own container. Feed these females spirulina every other day and complete 100% water changes on the other days. Within a few days, the isolated grandmothers should be laying large healthy clutches.
N.B.: It is common for Daphnia to have poor survival when their conditions are altered drastically. This is often the case with the initial Daphnia import, as you move daphnids from the shipped container into a fresh one with fresh media. If you are unable to keep the isolated grandmothers alive for long, enough to produce a mother clutch it is recommended that you troubleshoot your conditions.
Any clutch size of Daphnia may become the experiment generation but if a large number of animals is required to complete your experiments, you should wait until a large clutch is laid. The longer the mothers are fed regularly and receive regular water changes, the more likely they are to produce large clutches. Note that removing offspring regularly is an essential part of static husbandry. If a neonate develops unnoticed in your experiment, it will skew your measurement of lifespan! There are two ways to do it depending on the specificities of your experiment.
3e. Water changes and removal of offspring
Pipette out the mother(s) and transfer them into a new container with fresh water. Check the container carefully to ensure that no neonates have been transferred accidentally. This technique leaves the offspring behind to mature into the mother generation.
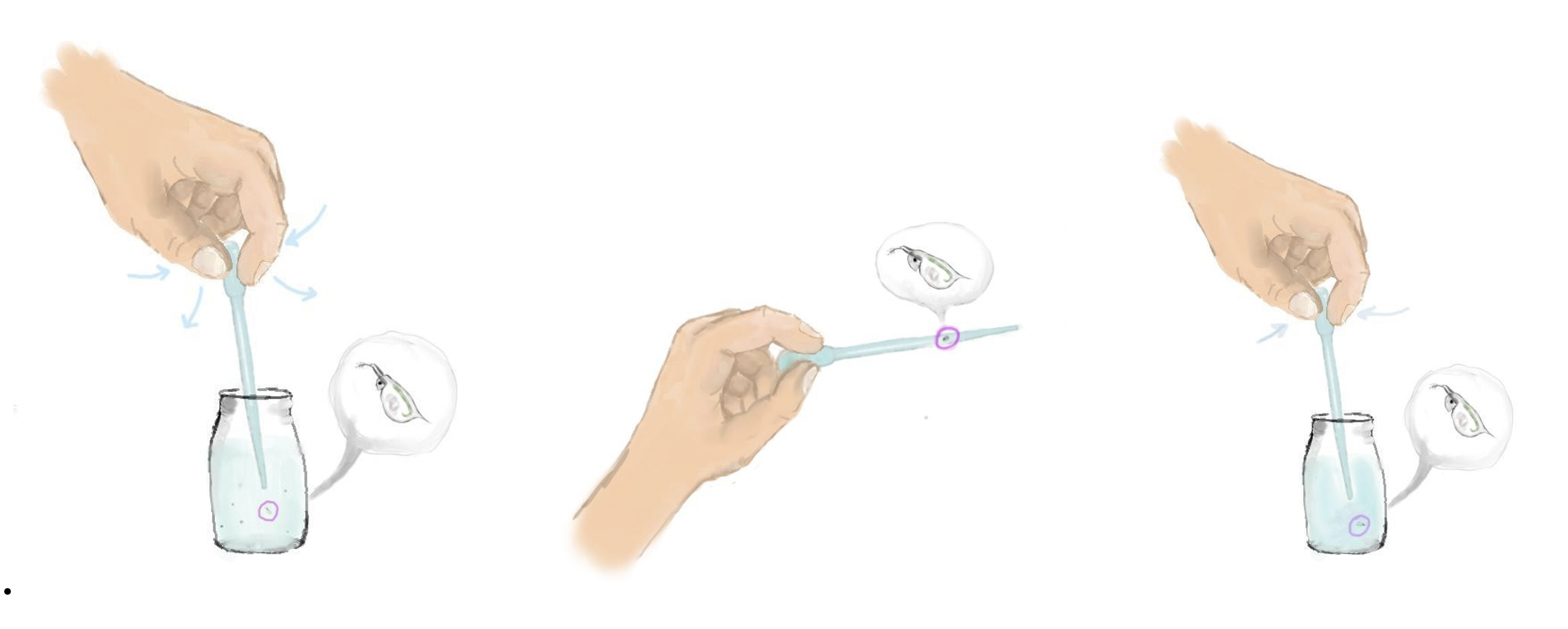
Once a suitable clutch has been collected, it may be raised as a group receiving regular feeds and water changes. Once individuals in this group begin to show signs of sexual maturity (5-7 days) they should be split and housed individually or in pairs.
Any neonates the mother generation produces may be used for experimentation. Alternatively, if your mother generation is not producing enough neonates for your required sample size, create additional mother groups. It is acceptable to use the progeny of the mother generation (instead of the grandmother culture) to make these groups. These younger mothers must be kept individually or paired with each other so that each clutch collected has a uniform maternal age.
Any animals that are not used should be disposed of using the instructions in section 2b.
4. Beginning Experimentation
4a. Are you ready to start an experiment?
Estimate the population size needed for a particular experiment – can you produce enough? Here we present a good way to determine the need for water, containers and animals.
When measuring lifespan in any experiment always keep control groups to have a baseline of lifespan for your cohort outside of the experimental conditions. Please be sure to have enough of a control (at the very minimum 30 individuals across 4 jars) so you characterize the lifespan of your Daphnia cohort outside of your experiments as well as specifically for each experiment to get the numbers to statistical significance.
There should be at least five replicates (containers with the same conditions) per experimental condition. The size of each of your replicates will be dependent on the scale of your experiment and the volume of water that the containers you select hold. The absolute minimum replicate population is ten animals.
- The “mother generation” containers have 1-2 mothers that have clutches of 5-30 neonates each. (see section 3.d)
- 10 mL of media per individual Daphnia so the volume of experimental containers will be the number of individuals multiplied by 10mL to make sure you have the correct number for your container or the correct container for the number (see section 3.b). We recommend only filling the volume of the container ⅔ of the way so that the media is not easily spoiled when the containers are being moved.
- In longevity experiments, adequate statistical power for conditions that may extend the life span by 10% or more typically require at least 50 individuals per group when followed throughout their lifespan (more is better, statistically). Consider this when setting up the mothers for your experiment.
4b. Synchronizing ages
Start with animals synchronized in age as follows, using the recommended technique for collecting the mother generation (4d). Single females (preferably from the same clutch and at the same egg stage) are moved to a new container and fed well, to favor progeny.
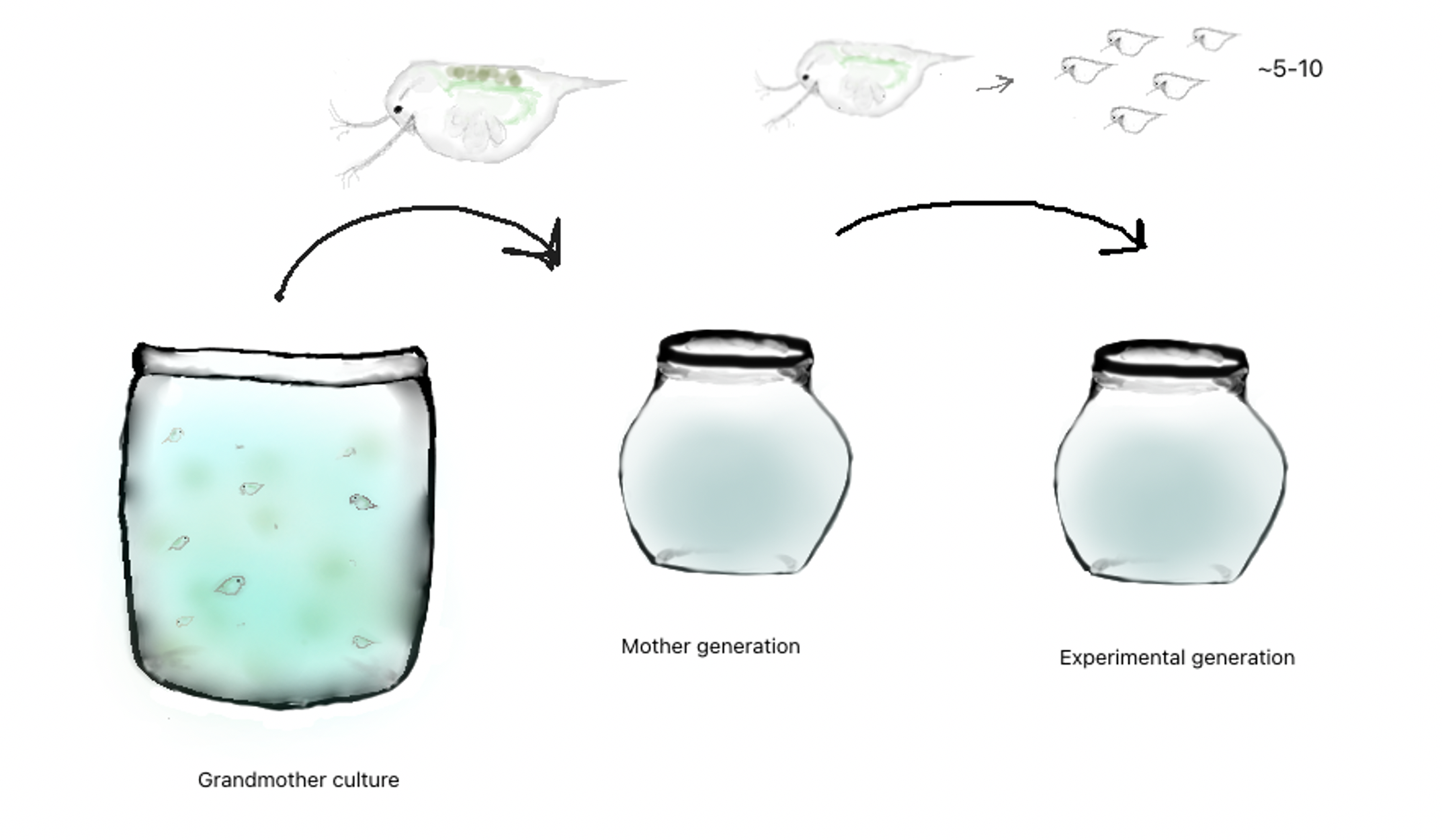
- Using the sexually mature progeny of the grandmothers Set up as many jars of isolated mothers as will meet the needs of your experiment. Try to pull females at the same egg stage (shown above) to have a better chance of synchronizing neonate emergence. Overfeed these mothers and perform water changes at least every 3 days (so waste wont build up from the increased feeding), this will lead to larger, more frequently produced and healthier clutches.
- Once female Daphnia reach sexual maturity they can produce a clutch every molt (approximately 5 days) and can produce up to 30 offspring depending on the conditions and maternal age.
- Mothers are significantly larger than the neonates are so the next few days is the ideal time to separate them!
- Let the clutches reach sexual maturity to confirm that they are all female.
- You will want to use clutches born on the same day (or close) to have a consistent age across your experiment. To manage variation between the clutches, combine these clutches, they are now considered a single age cohort that can be split into experimental groups.
- If the experimental design requires it males can be used, however this can be a little more difficult and can require a microscope or magnifying glass for the immediate identification. The “mother generation” of male clutches may also need a different treatment in order to stimulate the production of males such as controlled stress like increased salinity, alkalinity or alcohol juvenile growth hormone III.
- An ideal density in the experimental groups is 10mL of water per daphnid. The size of your experimental groups will therefore be dependent on the number of synchronized clutches you have as well as the volume of your containers.
4c. Removal of offspring without water changes
The technique used to make the mother generation is good for experiments where the animals have a 100% water change.
For experiments where expensive or hard to source chemicals are used, an alternative technique is preferable to remove the offspring and keep the group in the same experimental solution. This practice should be maintained for the experiment's control groups, as to not introduce variation in husbandry. It is recommended that Daphnia in these conditions be fed live algae either by culturing it at home or purchasing it live and storing it appropriately. Though metabolic waste will still accumulate in the unchanged water, feeding live food will reduce the negative impact of rotting food on water quality, over time.
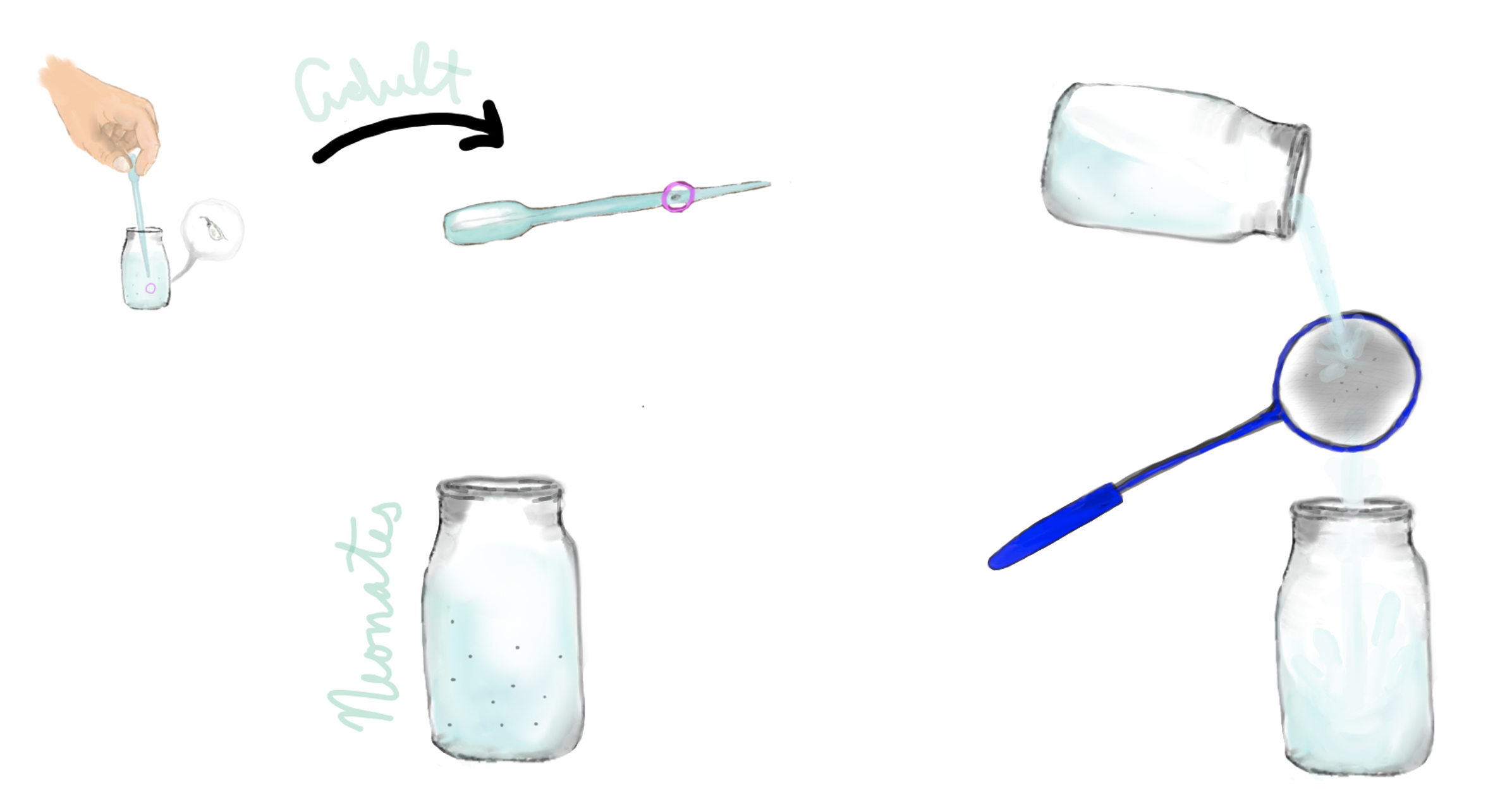
- Temporarily remove individuals in the experimental group with a pipette. For groups of only a few daphnids you may leave them in the water in the pipette. For larger groups you should transfer them into your makeup water.
- Filter the water through a mesh small enough to capture the offspring and animal molts while allowing the experimental solution to move into a separate, clean container.
- Reintroduce the experimental group into the water that is now free of the offspring.
- If the offspring are being kept for counting or any other reason, quickly rinse them from the mesh into a new container.
- Do not use offspring from experiments for stock culture or any other experiments unless you specifically want to observe the effect of the current experimental conditions on offspring!
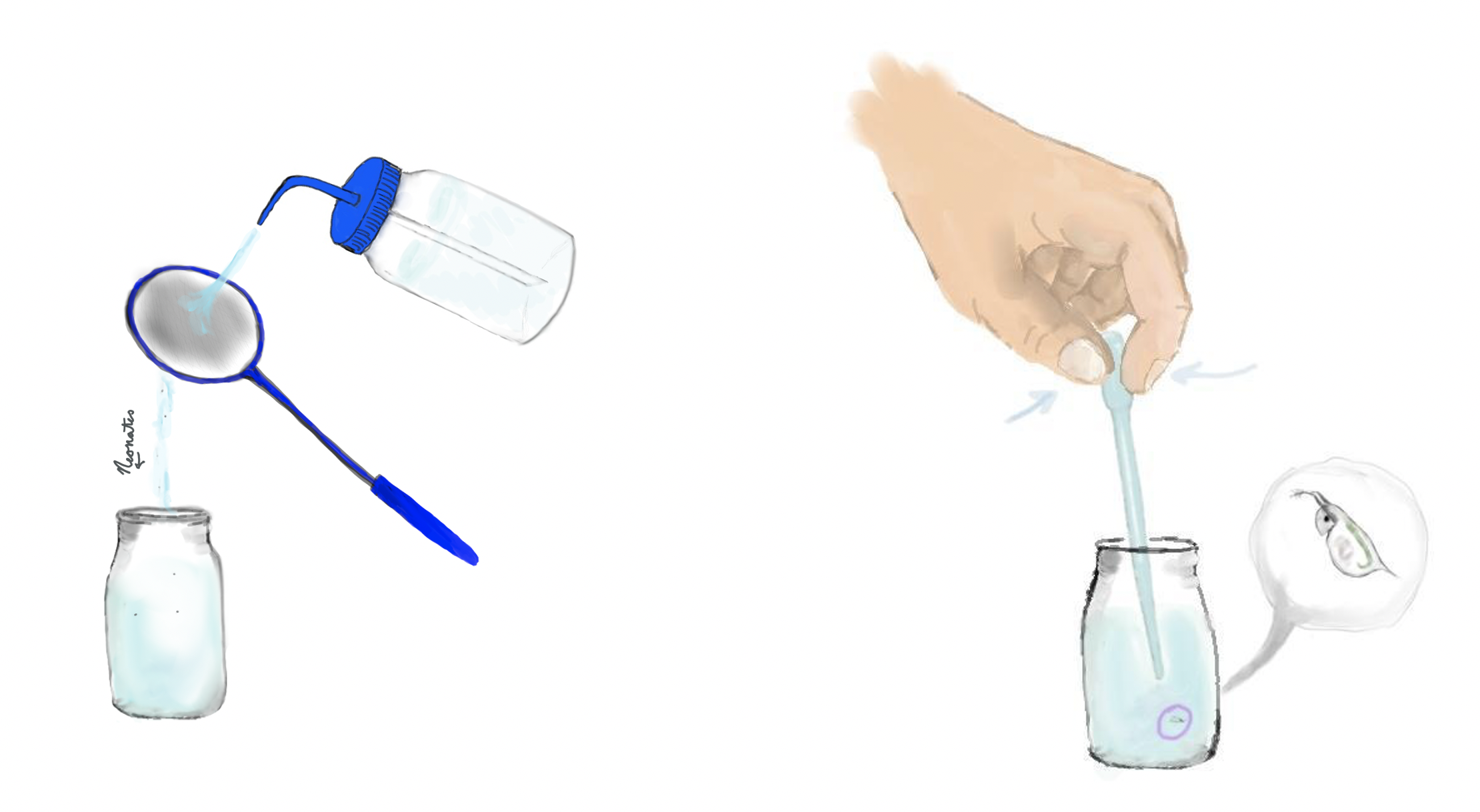
4d. Maintaining water volume and concentration
You will want to keep a constant water volume across your experiment and control groups. How this is done is dependent upon the experiment being run. The standard way to do this is a complete water change but if a drug is expensive or challenging to source you may choose to perform partial volume water changes at regular intervals to dilute metabolic waste and offset changes in concentration due to evaporative loss.
4e. Troubleshooting
As mentioned, we recommend starting the mother generation with at least three egg-baring grandmothers. This is because it is common for grandmothers transferred into the make-up water to live for 1-3 molt cycles. It is also not uncommon for a small portion of mother clutches to crash early in their development. If all of the progeny produced from the grandmothers in these cycles, (the intended mothers) fail to thrive then your setup may require troubleshooting. In these cases, it is recommended that you test your make up water to ensure that there is no total chlorine detectable (using dip tests capable of reading .5 mg/L total chlorine).
5. Your first experiment: collecting normal aging data – control studies
Keeping Daphnia under experimental conditions is challenging - it requires careful planning and execution. To ensure that your results are accurate and reliable, it is crucially important to be comfortable maintaining experimental groups in control conditions before you begin testing interventions. Start with varying some easy parameters to practice before trying drug testing or other complex interventions. Some examples of this are adjusting the feed volume or animal density. Later you may practice drug dosing with easily sourced compounds like table salt or baking soda. Below we detail how to collect the data to get started. Suggested measurements to record are: Lifespan, Number of offspring (a.k.a. fecundity), Physical changes, Behavioral changes.
5a. Lifespan
- Always start with Daphnia born on the same day and ensure that the neonates from each mother are split evenly between groups, refer to section 5b to see how to achieve this best. Note this day as day one for your cohort as well as the day of death for each individual along with their condition (experiment specifics, control etc.)
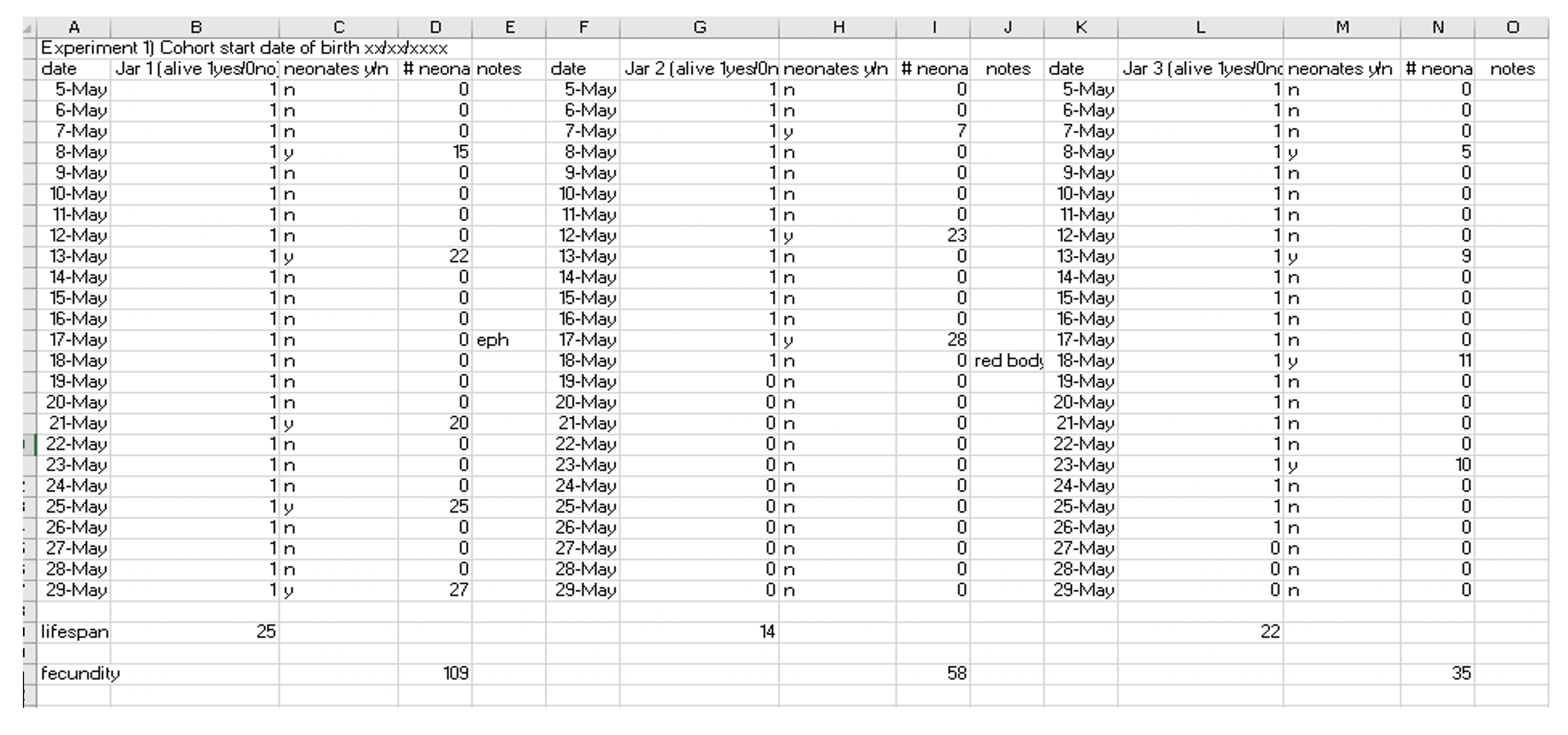
- Life span in Daphnia can be easily measured in days given that the average life span is around 20-30 days. However, it is normal for there to be significant deviation from this usually ranging between 10 and as many as 100 days (8). This is why it is very important to know the baseline for your particular culture and the conditions of your control groups (cohorts kept in identical conditions of experimental groups but without interventions like drugs).
5b. Fecundity
Fecundity is when and how many offspring are produced; here are some things to remember when measuring it:
- Daphnia can have offspring at every molt. For healthy animals, molting happens every 3-5 days and the neonates grow very quickly. You do not want to skip offspring removal for very long or they will grow and become indistinguishable from the group being studied. Offspring will also change the conditions in the container (food availability, waste accumulation, etc.). This creates additional variation between your experimental groups.
- Neonates are very small when they first emerge and are challenging to visualize and count. First, separate them from the mothers and allow them to grow for a couple of days when this is easier.
- Fecundity is likely to change over the lifespan of an individual as they age. The timing and rate of this change may be as interesting to study as the average total fecundity between groups.
Fecundity reflects the health, stress, age, and functionality of Daphnia. Changes in fecundity within replicates can reflect unseen variables that are much more difficult to control at home.
5c. Physical changes
- Color changes are the easiest parameter to observe in a meaningful way.
- Daphnia will turn red when in water with low dissolved oxygen as it triggers the production of hemoglobin. The extra hemoglobin makes them better able to absorb and use oxygen [5].
- Daphnia can also appear brownish or orange when they are building up lipid droplets as fat storage.
- The greenish color of the gut tract or if it is throughout the gut or not can be indicative of Daphnia health... If it is partially brown or completely clear this is a sign of health issues that could stem from low food availability, inability to eat properly, not digesting correctly, or other health issues, which could be useful to monitor and track.
- Daphnia can experience developmental problems or altered body shape under certain conditions. Body shape changes are easier to observe under magnification than with the naked eye.
- The production of male neonates can be indicative of stress or damage to the mother. See the above “Female vs. male” for more insight on what can cause this. If your experimental groups are making more males -- it is an interesting feature to track.
5d. Behavioral changes
- Many behavioral changes may happen. Here we described only a few, common behavioral changes. You may find others in your experiment. As always, differences from your control are of particular interest, even though behaviors in the control can also matter to judge if conditions are correct.
- Are my Daphnia swimming differently? Less? More?
- Has my experimental group increased or decreased feeding rate compared to the control?
- Are my Daphnia producing ephippia/resting eggs?
- Have my Daphnia started staying on the bottom of the jar? Are they occupying any specific vertical area in my jar?
- Are my Daphnia still responding to light?
6. Start testing drugs
Now that you have established your Daphnia culture, learned the methodology, and collected some data, it is time for you to begin to explore the effects of different drugs on Daphnia.
6a. Safety
Properly labeling chemicals is always important but at home, there are added safety risks. Ensure that all equipment (chemical and chemical measuring tools) are clearly labeled and kept completely separate from home goods. Items like measuring cups, tablespoons, and stirrers should never be shared. All drugs and drug solutions must be kept out of reach of children and pets. Try to promptly dispose of any leftover drugs.
6b. Selecting and storing drugs
Your bathroom cupboard probably has a few drugs you could use to start. Restrict your analysis to water-soluble drugs so that Daphnia are exposed uniformly in the intervention jars. To determine this, search online for a drug's water solubility. If your drug is in tablet form, thoroughly crush it before trying to get it into solution. Though it is not the case with simple compounds like table salt and baking soda, many medications will lose potency if they are not stored under conditions specific to their composition. Thus, it is recommended that drug solutions be mixed fresh before each water change.
6c. Find your drug's toxic dose in literature
The goal of this work is to study longevity (healthy lifespan) and not survival (ability to tolerate conditions). But what dose to use ? Converting doses across species is a convoluted subject. Our approach is to start with a acute toxic dose and step-wise dilute it down to explore the effects of chronic intervention. Thus knowing the drug dose that lead to the early deaths of animals are very useful as a starting point for determining the dosage for your study.
It is recommended that beginners start with drugs with known toxicity to Daphnia or other cladocerans such as EC50 or LC50. EC50 is the effective concentration, the dose at which a drug has an adverse effect on 50% of the population (such as immobility), within a given period. Correspondingly, the LC50 (sometimes called the LD50) is the concentration at which 50% of the population will die off within a given period (usually 24 hours).
A comprehensive list of pharmacological EC50’s currently available has been published [6]. Note that there are multiple studies for certain drugs with drastically different EC50’s. This is due to the different types and durations of toxicology tests as well as other kinds of variability in experimental conditions such as temperature regime, water chemistry, and genetic composition. It is because of this variability in the type of toxicity tests that your at-home EC50 must be found before a full-scale experiment is started.
If you wish to use a drug that has no available LC or EC50 for Daphnia then we recommend starting with the dosage reported for another invertebrate or aquatic model (i.e. C. elegans and zebrafish). It is likely that it will take you longer to fine-tune your dosage form here.
If no toxicity testing can be found for the drugs you wish to test and there is no other literature to base your doses on then it is recommended that you perform at-home toxicology bioassays to help find your starting dose [7].
6d. Short-term toxicity test: Find your EC048
Collect a large group of neonates and split them evenly between seven containers. As a dose that is lethal to 50% of animals is always going to be higher than a dose that has an effect on 50% of animals we recommend using different dilutions depending on if you are starting with an EC50 or LC50, for your drug.
EC 50: 0% (control), 0% (control), 100%, 50%, 20%, 10%, 5%
LC 50: 0% (control), 0% (control), 50%, 25%, 10%, 5%, 1%
If you are setting your dosage based on an LC50 then it is recommended that you use greater dilutions of that dose, to determine your at-home EC048 Make 2 of these containers the controls and have the other five contain the LC50. 25% the concentration of the LC50, 10% the concentration of the LC50, 5% the concentration of the Lc50. and 1% of the concentration of the LC50.
It will be easiest to make a stock solution of the LC50 and then dilute it for the other concentrations.
Lightly feed your neonates and leave them in their solutions for 48 hours. Count the number of neonates immobilized in each group. How do the different doses compare to the control?
Let us assume that each of your containers had 10 neonates in it and after 48 each had
Control 1: 9/10 daphnids swimming
Control 2: 10/10 daphnids swimming
100%: 5/10 daphnids swimming
50%: 7/10 daphnids swimming
20%: 9/10 daphnids swimming
10%:10/10 daphnids swimming
5% 9/10 daphnids swimming
In this scenario, 10% of the EC50 is a concentration where over a 48-hour period there was no noticeable toxic effect of the drug. 10% of the EC50 may now be considered the EC048: the maximum concentration that is not toxic after 48 hours of exposure.
What if there is no dose comparable to the control? Are they all comparable to the control?
It is possible that the LC50 of the drug will be different at home due to differences in your husbandry conditions [8], or Daphnia line.
If none of the experimental conditions are comparable to the control then try again with greater dilutions. If they are all comparable to the control then try increasing the concentration.
6e. Prolonged toxicity test: Finding your EC07 Days
The length of duration is a critical component of a drug's function. This is very clear when the effective or lethal doses of drugs are compared between 24 and 48 hours [9]. The drug becomes more effective the longer the animal is exposed to it. For this reason, it is recommended that a longer trial be run to determine the effects of your EC048 hoursas dilutions of this dosage, with the expectation that its effects will be greater over longer exposure. At this stage, all of your conditions should be done in duplicate to increase your confidence in the results. Regular feeding and water/drug changes should take place. If none of your dosages have comparable survival to the control you will want to try again with lower dosages.
6f. Tracking Maturation
While finding the EC07 Days take the opportunity to track animal reproductive maturation i.e.: when do they start producing ephippia or neonates? Reproductive fitness is an indicator of overall fitness and if none of your dosages reach reproductive maturity within 4 days of the control restart this trial at lower doses. If you have dosages that have comparable survival to the control after 7 days but do not reach maturity, you will want to extend your trial to determine this.
Note the day that each group becomes reproductive. Any dose that has animals reaching sexual maturity within 4 days of the control is an appropriate dose for a longevity experiment. Continue to maintain experimental conditions for the lifespan of the animal recording whichever findings are relevant to your investigation (i.e. survival, fecundity, behavior). If no group reaches sexual maturity within a difference of 4 days then the experiment should be re-run with lower doses.
6g. Full scale longevity testing
Once these dosages have been confirmed and initial findings have been noted you are ready to run a full-scale longevity experiment. It is recommended that a second longevity experiment be run with these dosages which has a more robust number of replicates as described in section 4a (5, container dependent). This will make any findings significant enough to be reportable.
7. Future Work and Request for Specific Feedback
There are several directions we plan to develop in future versions of this protocol paper as we hear back from the citizen scientists and learn more about specific interest and experiences.
First, we plan to cover the experiments with magnifying glass and simply microscope such like the Fold-Scope, which would involve observing and smart phone video-recording of daphnids heartbeat, peristaltic movement and eye movements.
We did not discuss making up the media used in Daphnia labs for optimal conditions as it requires obtaining and properly mixing multiple salts and vitamins. These are known as ADAM and COMBO media and introduce needless complications for the beginners.
We have to develop open data web portal which allows data logging and user authentication as well as data agglomeration and analysis. The proper platform has not been identified.
We did not cover experimenting with drugs which are not water-soluble. Generally this involves first dissolving the drugs in a vehicle such as ethyl acetate or DMSO and then delivering the drugs to the animals. This can get quite involved and involves safety considerations.
8. Conclusions
One key advantage of Daphnia lifespan tests at home is that they are invertebrates. The laws and regulations of animal experimentation in most countries are limited to vertebrates. Another advantage is that Daphnia are simple to keep compared to other invertebrate models. For example, nematodes eat a medium that is required to culture bacteria which, at home, is extremely complex and is easily contaminated. Fruit flies are not ideal because they fly and can infest the home. However, some simple techniques were developed for schools, notably lifespan experiments [10].
Today, we know relatively little about the degree of relevance of Daphnia to human biology, only further experiments can help judge that. Though both nematodes (Caenorhabditis elegans) and fruit flies (Drosophila melanogaster) are heavily studied, the standard lab conditions were later found to have caveats when it comes to studying ageing. It was found that lab nematodes often die from infections by the bacteria that they are fed (leading to a swollen pharynx, [11]), which limits their relevance for the study of ageing. The causes of death of lab fruit flies is still unknown but similar patterns are found as in nematodes [12], suggesting similar issues. We hope that Daphnia will permit us to discover solutions to ageing rather than things that actually prevent circumstantial mortality but we do not know at this stage. It is therefore important to start with stable conditions that do not lead to short lived Daphnia: if some unfavorable condition were to exist, starting with longer lifespans suggests that the effect of the unfavorable conditions is minimized.
At home Daphnia studies would prove to be a good model for aging if mortality came primary from old age and not stressors and conditions. Even so, the degree to which Daphnia can serve as a model for humans will likely remain limited due to substantial differences between Daphnia and human physiology. Still, preliminary work can be completed in Daphnia and better inform studies on more comparable models.
Creating a community around Daphnia at home lifespan tests will help establish workflows and infrastructure to share experimental results and to limit frequent mistakes. The resulting large scale data on treatment results will be compared and contrasted with life extension results observed in rodents.
References
1. Cho, Y., Jonas-Closs, R. A., Yampolsky, L. Y., Kirschner, M. W., & Peshkin, L. (2022). Intelligent high-throughput intervention testing platform in Daphnia. Aging cell, 21(3), e13571. https://doi.org/10.1111/acel.13571
2. Sobral, O., Chastinet, C., Nogueira, A., Soares, A. M., Gonçalves, F., & Ribeir, R. (2001). In vitro development of parthenogenetic eggs: a fast ecotoxicity test with Daphnia magna?. Ecotoxicology and environmental safety, 50(3), 174–179. https://doi.org/10.1006/eesa.2001.2088
3. Ebert D. Ecology, Epidemiology, and Evolution of Parasitism in Daphnia [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2005. Available from: https://www.ncbi.nlm.nih.gov/books/NBK2036/
4. Betini, G. S., Wang, X., Avgar, T., Guzzo, M. M., & Fryxell, J. M. (2019). Food availability modulates temperature-dependent effects on growth, reproduction, and survival in Daphnia magna. Ecology and evolution, 10(2), 756–762. https://doi.org/10.1002/ece3.5925
5. Pirow, R., Wollinger, F., & Paul, R. J. (1999). The sites of respiratory gas exchange in the planktonic crustacean Daphnia magna: an in vivo study employing blood hemoglobin as an internal oxygen probe. The Journal of experimental biology, 202 Pt 22, 3089–3099. https://doi.org/10.1242/jeb.202.22.3089
6. Tkaczyk, A., Bownik, A., Dudka, J., Kowal, K., & Ślaska, B. (2021). Daphnia magna model in the toxicity assessment of pharmaceuticals: A review. The Science of the total environment, 763, 143038. https://doi.org/10.1016/j.scitotenv.2020.143038
7. OECD (2004), Test No. 202: Daphnia sp. Acute Immobilisation Test, OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris, https://doi.org/10.1787/9789264069947-en.
8. Kim, J., Park, J., Kim, P. G., Lee, C., Choi, K., & Choi, K. (2010). Implication of global environmental changes on chemical toxicity-effect of water temperature, pH, and ultraviolet B irradiation on acute toxicity of several pharmaceuticals in Daphnia magna.Ecotoxicology (London, England), 19(4), 662–669. https://doi.org/10.1007/s10646-009-0440-0
9. Harbottle, J., Strangward, P., Alnuamaani, C., Lawes, S., Patel, S., Prokop, A. (2016). Making research fly in schools: Drosophila as a powerful modern tool for teaching Biology. School Science Review 97, 19-23.
10. Stuhr, N. L., & Curran, S. P. (2020). Bacterial diets differentially alter lifespan and healthspan trajectories in C. elegans. Communications biology, 3(1), 653. https://doi.org/10.1038/s42003-020-01379-1
11. Zhao, Y., Gilliat, A. F., Ziehm, M., Turmaine, M., Wang, H., Ezcurra, M., Yang, C., Phillips, G., McBay, D., Zhang, W. B., Partridge, L., Pincus, Z., & Gems, D. (2017). Two forms of death in ageing Caenorhabditis elegans. Nature communications, 8, 15458. https://doi.org/10.1038/ncomms15458
12. Rera, M., Clark, R. I., & Walker, D. W. (2013). Why do old flies die? Aging, 5(8), 586–587. https://doi.org/10.18632/aging.100589
Supplementary Remarks
In France everything for a Daphnia lifespan study cab be bought for 16€ + S&H:
https://aquazolla.com/products/kit-elevage-daphnies
Related files
 DIY_02.pdf
DIY_02.pdf - Anderson, C, Jonas-Closs, R A, Matei-Dediu, B, Debonneuil, E and Peshkin, L(2023). DIY Intervention Testing in Daphnia. Bio-protocol Preprint. bio-protocol.org/prep2542.
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
