Advanced Search
Pseudovirus (PSV) Assay
Last updated date: Nov 6, 2023 Views: 2451 Forks: 0
Pseudotyped lentivirus protocol for coronaviruses
1) Generation of host cells
- For human coronavirus infection, 3 stable expression cell lines were used: HeLa-ACE2 (for SARS-CoV-1, SARS-CoV-2, NL63), HeLa-DPP4 (for MERS) and HeLa-ANPEP (for 229E). The cell lines were generated from WT HeLa cells (CCL-2, ATCC) by VSV-G lentivirus infection (see part 2 for viral packaging details), and FACS sorted with the corresponding spike probe conjugated with FITC for high expression (top 1%) populations of the respective viral receptors. The cell lines were cultured in DMEM complete medium (10% FBS, 1%Pen/Strep and 1% Glutamax).
2) SARS-CoV-1, SARS-CoV-2, MERS, NL63, 229E pseudotyped viruses (PSVs) production
- Seed HEK293T cells to 6-well plate (1 million/well) or 10cm dish (5 million cells/dish) one day before transfection.
- Check for cell viability and confluency before transfection. The cells should be 70-80% confluent when you start to do viral packaging.
- Gently aspirate media, add 2 ml (6-well plate) or 10 mL (10cm dish) fresh DMEM (Gibco, 10569010) complete medium (with 10% Heat Inactivated FBS and 1% P/S) to 6-well plate or 10cm dish before transfection.
- Tube A: Prepare a mixture of the 3 transfection plasmids in 200uL (or 1mL, if using 10cm dish) Opti-MEM medium:
(The viral packaging plasmids should be prepped by transfection grade kit to remove any possible contamination of endotoxin.)
a) Envelope:
SARS2-D18 (Addgene # 170442): 0.5ug for 6-well plate; 2.5ug for 10cm dish
SARS1-D28 (Addgene # 170447): 1ug for 6-well plate; 5ug for 10cm dish
VSV-G (Addgene # 8454): 0.5ug for 6-well plate; 2.5ug for 10cm dish
MERS-D12 (Addgene # 170448): 1ug for 6-well plate; 5ug for 10cm dish
NL63-D14 (Addgene # 172666): 1ug for 6-well plate; 5ug for 10cm dish
229E-D15 (addgene # 188908): 1ug for 6-well plate; 5ug for 10cm dish
b) Gag/Pol:
pCMV-delta-R8.2 (Addgene # 12263)
2.5ug for 6-well plate, 12.5ug for 10cm dish.
c) Reporter:
pBOBI-FLuc (Addgene # 170674) for lentivirus
2ug for 6-well plate; 10ug for 10cm dish
- Use Lipofectamine2000 from Invitrogen to do the transfection. Add 2.5uL Lipo2000/ 1ug plasmid into a new tube (tube B) of Opti-MEM (same volume as tube A). Incubate at room temperature for 3-5 min, and mix tube B with tube A. Incubate at RT for 15-20min, then add the mixture gently into cell culture. Be careful not to disperse the cells.
(Other transfection reagents may be used according to manufacturer’s instructions. However, as we have tested, Lipo2000 / Lipo3000 work better than PEI)
- 12-16 hours post transfection, gently aspirate the medium and add 5mL (or 25mL) fresh medium to each well of 6-well plate or 10cm dish respectively.
- 48 hours post transfection, collect the supernatant and spin down at 1500g for 10min to get rid of any cell pellets (Alternative: pass through 0.45um filter). Collect the supernatant and aliquot and freeze at -80C for long-term storage. The pseudotyped virus produced can be stored at -80C for up to 6 months.
Optional: You can add another 5 / 25mL fresh medium, incubate for another 24 hours and collect PSV again (72 hours post transfection).
3) PSV infection assay
Layout template. 
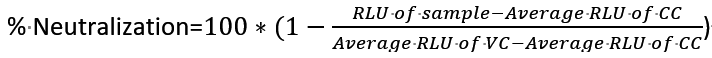
Note: If you still have questions, please refer to the Protocol for Neutralizing Antibody Assay for HIV-1 in TZM-bl Cells. You can find the equation to calculate % neutralization of serum or antibody. |
Figures
 |  |
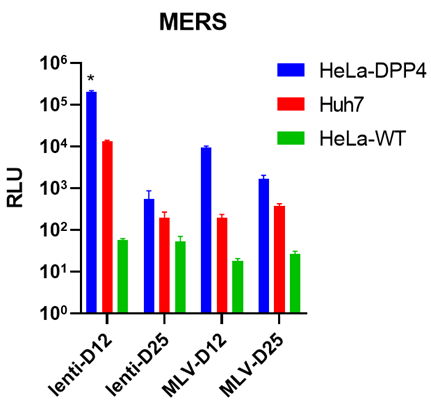 | |
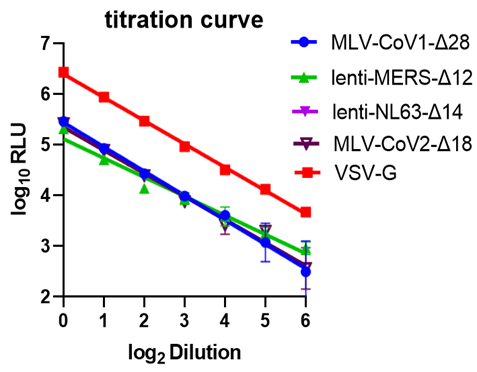
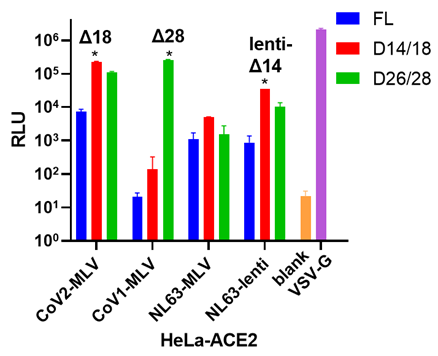
Viral titer measure: (a) Serial dilution (x2 fold) for different PSVs involved. RLU: relative luminescence unit. (b) Different c-terminal deletions affect viral titer. FL: full length. Δn: n amino acid deletion from the c-terminal. Each version with the highest viral titer is marked with an asterisk. All the PSVs were tested on HeLa-ACE2 cells. (c) MERS viral titer in different cell lines. | |
Protocol credit to Deli Huang, Linghang Peng and David Nemazee
Contact nemazee@scripps.edu for more information
Related files
 CoV PSV assay_DH.docx
CoV PSV assay_DH.docx - Rogers, T, Sok, D, Jardine, J and Burton, D(2023). Pseudovirus (PSV) Assay. Bio-protocol Preprint. bio-protocol.org/prep2494.
- Rogers, T. F., Zhao, F., Huang, D., Beutler, N., Burns, A., He, W., Limbo, O., Smith, C., Song, G., Woehl, J., Yang, L., Abbott, R. K., Callaghan, S., Garcia, E., Hurtado, J., Parren, M., Peng, L., Ramirez, S., Ricketts, J., Ricciardi, M. J., Rawlings, S. A., Wu, N. C., Yuan, M., Smith, D. M., Nemazee, D., Teijaro, J. R., Voss, J. E., Wilson, I. A., Andrabi, R., Briney, B., Lais, E., Sok, D., Jardine, J. G. and Burton, D. R.(2020). Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science 369(6506). DOI: 10.1126/science.abc7520
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
