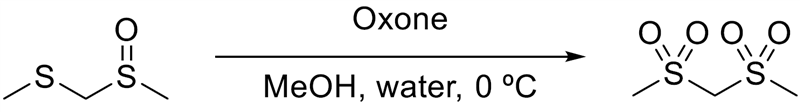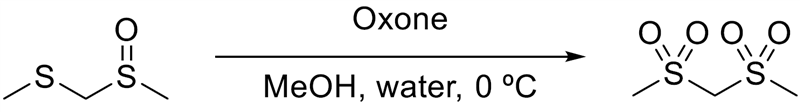
Bis(methylsulfonyl)methane
A solution of methyl (methylsulfinyl)methyl sulfide (2.07 g, 16.7 mmol) in methanol (65 mL) at 0 ºC was treated with a slurry of Oxone, monopersulfate compound (44.6 g, 145 mmol) in water (97 mL) in four portions. The suspension was stirred at 0 ºC for 7 hours. The suspension was treated with brine (50 mL) and extracted with EtOAc (2 x 50 mL, 2 x 100 mL). The combined organic phase was washed with brine. An aliquot of the organic phase mixed with water gave a strong response on starch KI paper. The organic phase was treated with 10% aqueous sodium sulfite solution (50 mL) and further solid sodium sulfite (to saturate the solution) and shaken vigorously. The yellow solution turned colourless and was allowed to stand overnight. An aliquot of the EtOAc layer mixed with water did not stain KI starch paper. The phases were separated and the organic phase was washed with brine (50 mL), dried (Na2SO4) and concentrated to give the title compound as a white solid, which was used without purification (1.88 g, 10.3 mmol), 62% yield.
1H NMR (400 MHz, DMS0-d6) δ 5.44 (2 H, s), 3.21 (6 H, s). MS (ES-) [M-H]- 171.
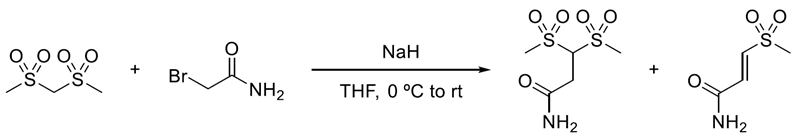
3,3-Bis(methylsulfonyl)propanamide
A solution of bis(methylsulfonyl)methane (200 mg, 1.16 mmol) in THF (10 mL) at 0 ºC was treated with sodium hydride (60% in mineral oil) (55 mg, 1.4 mmol). The mixture was stirred at 0 ºC for one hour, then treated dropwise with a solution of 2-bromoacetamide (192 mg, 1.4 mmol) in THF (1.5 mL). The reaction mixture was allowed to warm slowly to room temperature and stirred overnight, then quenched with saturated aqueous ammonium chloride (6 mL) and extracted with EtOAc (3 x 50 mL). The combined organic phase was dried (Na2SO4), concentrated onto silica and purified by column chromatography on a Combiflash Rf machine (0-10% MeOH-dichloromethane) to give 3,3-bis(methylsulfonyl)propanamide (11.4 mg, 0.047mmol, 4% yield) as a white solid. (E)-3-methylsulfonylprop-2-enamide (16.3 mg, 0.10 mmol, 9% yield) was also obtained.
3,3-Bis(methylsulfonyl)propanamide
1H NMR (500 MHz, DMS0-d6) δ 7.64 (1 H, s), 7.20 (1 H, s), 5.48 (1 H, t, J = 5.9 Hz), 3.28 (6 H, s), 2.97 (2 H, d, J = 5.9 Hz). MS (ES-) [M-H]- 228.
(E)-3-methylsulfonylprop-2-enamide
1H NMR (500 MHz, DMS0-d6) δ 8.04 (1 H, s), 7.61 (1 H, s), 7.45 (1 H, d, J = 15.1 Hz), 6.91 (1 H, d, J = 15.1 Hz), 3.14 (3 H, s). MS (ES-) [M-H]- 148.