Advanced Search
Establishing a 3D printed spheroid model for anti-colon cancer drug screening
Last updated date: Aug 13, 2023 Views: 651 Forks: 0
Vaishnavi Gogineni 1, Chunhua Yang 1,2 *, and Didier Merlin 1,2
- Institute for Biomedical Sciences, Digestive Disease Research Group, Georgia State University, Atlanta, GA, 30303, USA; vgogineni@gsu.edu (VG.); cyang16@gsu.edu (CH.Y.); dmerlin@gsu.edu (DM.)
- Atlanta Veterans Affairs Medical Center, Decatur, GA, 30302, USA; Chunhua.Yang@va.gov (CH.Y.); Didi-er.Merlin@va.gov (DM.)
*Correspondence: cyang16@gsu.edu.
ORCID: 0000-0003-0805-1008
Keywords: Caco2-BBe cells, Colitis-associated cancer, Bioink, 3D cell culture
Abstract
Colitis-associated cancer (CAC) presents a significant clinical challenge for individuals with inflammatory bowel diseases (IBD). These patients face an elevated risk of developing CAC, which tends to emerge in younger individuals and is often diagnosed at more advanced stages. The search for effective therapeutic approaches to tackle CAC is complicated by the limitations of 2-dimensional (2D) colon tumor models. These models fail to accurately represent the true morphology of the tumor. In response to this challenge, researchers have turned to 3-dimensional (3D) bioprinting as a means to construct colon tumor models that closely mimic the physiological environment. This innovative approach offers a promising solution that bridges the gap between traditional 2D cell culture systems and in vivo tumor models. By more accurately replicating the in vivo tumor microenvironment, the bioprinted 3D cell model empowers scientists to delve into the underlying mechanisms of CAC and discover novel targeted drug therapies. The protocol outlined herein provides a comprehensive workflow for generating 3D bioprinted Caco-2/BBe spheroids. These spheroids were employed to evaluate the efficacy of candidate inflammatory drugs for treating CAC.
Background
The human epithelial cell line known as Caco-2/BBe holds significant relevance in cancer and toxicology research, serving as a valuable model for studying the intestinal epithelial barrier (Sakharov et al., 2019; van Breemen and Li, 2005; Wang et al., 2008). This cell line possesses the unique ability to undergo spontaneous differentiation, forming a polarized monolayer that closely resembles human colon cells in terms of morphology. Researchers frequently turn to 2-dimensional (2D) cultures of Caco-2/BBe cells to delve into aspects such as drug absorption, drug candidacy, transporter proteins, toxicology, and disease modeling (Sun et al., 2008). However, it's worth noting that these 2D cultures, while useful, consist of a single cell layer that's uniform and densely packed. This characteristic fails to accurately mirror the intricate three-dimensional (3D) structure and functionality seen in in vivo colon carcinoma (Jensen and Teng, 2020; Kapałczyńska et al., 2018).
In a concerted effort to enhance the utility of Caco-2/BBe cells for drug screening endeavors, our approach involved the utilization of 3D printed spheroids. The intention was to construct a colon tumor model that faithfully replicates the morphological intricacies within a microenvironment akin to in vivo conditions. This protocol outlines both the process of 3D bioprinting involving Caco-2/BBe cells and the subsequent post-printing characterization. The ultimate objective was to generate a more faithful representation of human epithelial cells, thereby bolstering the reliability of Caco-2/BBe cells in drug screening trials.
The creation of the 3D cultured spheroids began with initially cultivating the Caco-2/BBe cells in a 2D configuration, forming a densely packed single layer. Subsequently, these cells were detached and combined with an appropriate quantity of bioink, resulting in a bioink-cell mixture. The amalgam was then loaded into the printhead of the BioX 3D printer. Employing a bioprinting nozzle, bioink-cell droplets were meticulously deposited into a 96 well plate, with the addition of a crosslinking agent to each 3D cell droplet for the facilitation of stabilization and solidification. The final step encompassed the visual examination of the morphology of the 3D Caco-2/BBe cells via microscopy.
In a recent publication (Long et al., 2023), the application of 3D printed Caco-2/BBe cells took center stage in scrutinizing the effectiveness of potential inflammatory drug candidates for colorectal cancer (CAC) treatment. The integration of 3D bioprinting technology furnishes a more faithful platform for investigating CAC and appraising potential therapeutic interventions.
Materials and Reagents
- Caco-2/BBe cells (CRL-2102™, ATCC, Manassas, VA.)
- Dulbecco's Modified Eagle's Medium (DMEM) (10-103-CVR, Corning, Keene, NH.)
- Fetal Bovine Serum (S11150, Atlanta Biologicals [R&D Systems], Lawrenceville, GA.)
- Penicillin/Streptomycin solution (100X, Corning, Keene, NH.)
- Cell culture flask (T75 and T175, Fisher Scientific, Waltham, MA.)
- Phosphate Buffered Saline (PBS, 1X, 21-040-CVR, Corning, Keene, NH.)
- Trypsin EDTA solution (R001100, ThermoFisher Scientific, Waltham, MA.)
- Glutathione-6-shogaol (M13, BOC Sciences, Shirley, NY.)
- Ethanol (493511-1L, Sigma Aldrich, Burlington, MA.)
- Dimethyl Sulfoxide (BP231-100, Fisher Scientific, Waltham, MA.)
- Hydrogel Bioink (Ref #IK1020000303, Cellink, Gothenburg, Sweden.)
- Crosslinking Agent (Ref #CL1010006001, Cellink, Gothenburg, Sweden.)
- Cyto3DTM Live-Dead Assay Kit (Cat# BM01, TheWell Bioscience, North Brunswick, NJ.)
Equipment
- Laminar Flow Hood (LabGard ES NU-540 Class II Type A2 Biosafety Cabinet, Nuaire, Plymouth, MA)
- Forma Series II Water Jacket CO2 Incubator (Thermo Scientific, Waltham, MA)
- BioTek Synergy 2 Microplate Reader (BioTek Instruments, Inc., Winooski, VT)
- BioX 3D printer (Cellink, Sweden)
- Cartridge (Ref #CSC010300102, Cellink, Sweden)
- 22G printing nozzle (Ref #NZ5220505002, Cellink, Sweden)
- 96-well plate (Thermo Scientific, Waltham, MA)
- Keyence BZ-X700 series Fluorescent Microscope (Keyence Corporation, Osaka, Japan)
Software
- GraphPad Prism 8.0 (GraphPad, San Diego, CA)
- ImageJ software (NIH, Bethesda, MD)
Procedure
A. 2D Cell Culturing
- Prepare complete culture medium by adding 50 mL FBS and 5 mL Penicillin/Streptomycin solution (100x) to 500 mL DMEM.
- Place the prepared medium into 37 °C water bath for 30 mins.
- Take 1 vial of frozen Caco-2 cell from liquid N2 tank, immediately thaw the cells in the 37 °C water bath by shaking the vial inside the water bath for 1 min.
- Wipe the outside of the vial with 70% ethanol and transfer the vial into laminar flow hood.
- Using a sterile pipette, carefully transfer the contents of the vial into a sterile cultural flask (75 cm2).
- Add 15 mL of complete culture medium (warmed to 37°C) to the cultural flask.
- Place the flask into CO2 incubator.
- After overnight incubation, dispose of the current culture medium completely using an aspirating pipette (Figure 1).
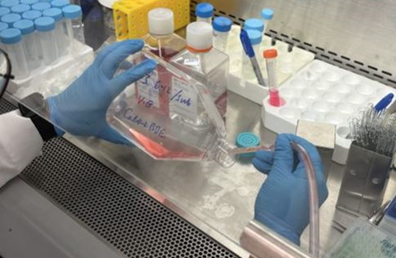
Figure 1. Dispose of culture medium from cultural flask using aspirating pipette.
9. Add fresh complete culture medium (warmed to 37°C) to the cultural flask.
10. Let the cells grow until reaching 80% confluence.
11. To subculture the cell, rinse the attached cells with 15 mL of PBS solution (Figure 2) and then dispose PBS solution using an aspirating pipette.
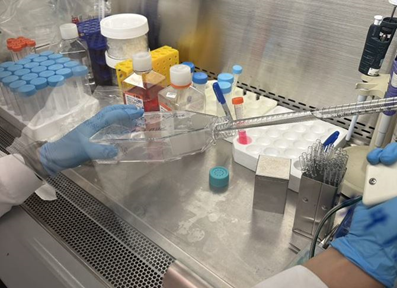
Figure 2. Rinse cells in cultural flask with PBS solution using a sterile pipette.
12. Prewarm frozen Trypsin-EDTA solution in a 37 °C water bath for 30 mins.
13. Using a micropipette, transfer 10 mL of Trypsin-EDTA solution to the cell vial (Figure 3), ensuring the solution completely covers the cells.
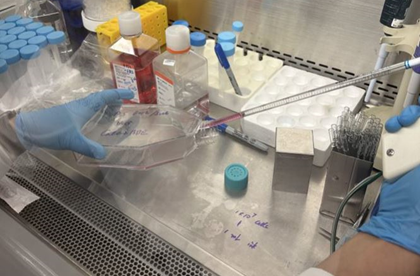
Figure 3. Transfer 10 mL Trypsin-EDTA solution to cultural flask using a sterile pipette.
14. Place the flask in a 5% CO2 incubator for 5 minutes, then add 10 mL complete culture medium to the flask.
15. Transfer cell suspension to two 15 mL tubes, and place both in centrifuge at 100 x g for 3 min.
16. Return to laminar flow hood and remove supernatant immediately using an aspirating pipette.
17. Add 2 mL of fresh complete culture medium to each tube and pipette gently to mix the cell suspension.
18. Transfer the contents of each tube to two large cell culture flasks and incubate in 5% CO2 incubator, replacing medium twice a week until reach 80% confluence.
B. 3D cell printing
- Warm the bioink in a cartridge to room temperature (25 °C).
- Transfer 33 million Caco-2/BBE cells (in 300 μL cell suspension) to the 1 mL cell syringe using a female/female Luer lock adaptor (Figure 4).
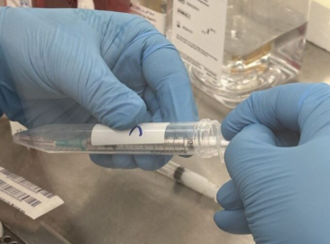
Figure 4. Transfer the cell suspension to a 1 mL sterile syringe.
3. Transfer 3 mL bioink to a 12 mL syringe using a female/female Luer lock adaptor (ten parts bioink works with one part cell suspension).
4. Connect the two syringes to the dispensing unit (Figure 5).
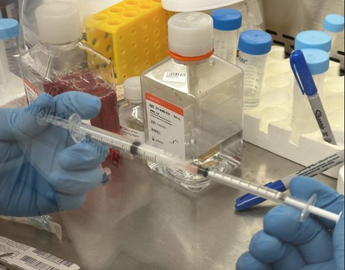
Figure 5. Connect two syringes and empty cartridge into a mixing unit.
5. Apply gentle pressure onto the Dispensing unit to mix the content, avoiding the introduction of air bubbles to the mixture (final cell concentration in the mixture is 10 million Caco-2/BBE cells per mL).
6. Remove the 1 mL syringe and connect an empty cartridge to the mixing unit. Push mixed content of syringes into the empty cartridge (Figure 6).
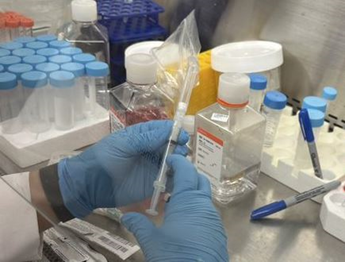
Figure 6. Load mixed content into the empty cartridge.
7. Cap the cartridge with a 22G (0.410 mm) bioprinting nozzle (Figure 7).
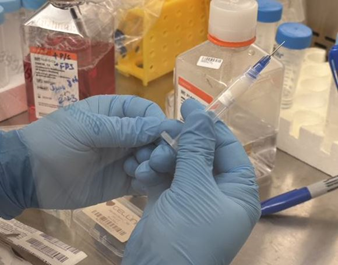
Figure 7. Cap the bioink.
8. Place the loaded cartridge in the printhead (Figure 8).
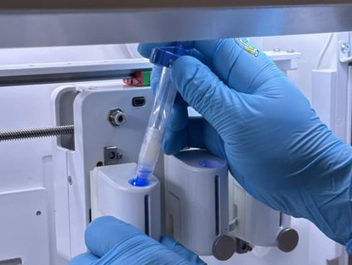
Figure 8. Place the cartridge.
9. Print 0.3 mm diameter spheroids into a 96-well plate (Figure 9), adjust the printer’s parameters to “Speed: 10 mm/s and Pressure: 30-50 kPa”.

Figure 9. Print 0.3 mm diameter spheroids on a 96 well plate.
10. Add 100 μL of crosslinking solution (CaCl2 solution) to the cell-laden constructs in each well (Figure 10) and remove the solution after 45 seconds.
Figure 10. Add 100 μL of crosslinking solution to each well.
11. Wash the plate with 100 μL of cell culture medium to remove the remaining crosslinking solution (Figure 11) and remove the washing medium.
Figure 11. Wash each well with 100 μL cell culture medium.
12. Add 200 μL fresh culture medium to the constructs and place the plate into an incubator.
13. Incubate the constructs in cell culture medium in standard culture conditions (37°C, 5% CO2 and 95% relative humidity).
14. Change the medium twice a week.
C. Visualization and characterization of 3D printed cells
- Bring the Cyto3D Live-Dead Assay Kit to room temperature for 15 mins.
- Add 2 μL of Cyto3D reagent to each well contains 100 μL of fresh DMEM.
- Incubate the spheroids at 37 °C for 10 minutes.
- Capture bright field images and fluorescent images with Keyence BZ-X700 series Fluorescent Microscope and combine image channels to create a new image (Figure 12).

Figure 12. Combined Image of a 3D cell spheroid.
5. Assess survival rate of 3D Caco-2/BBe cells by calculating mean fluorescence intensity of green fluorescence (live) and orange fluorescence (dead) in 3D spheroid (using ImageJ software) of each group with or with drug treatment.
Reference:
- Jensen, C. and Teng, Y. (2020). Is It Time to Start Transitioning From 2D to 3D Cell Culture? Frontiers in Molecular Biosciences 7. https://www.frontiersin.org/articles/10.3389/fmolb.2020.00033
- Kapałczyńska, M., Kolenda, T., Przybyła, W., Zajączkowska, M., Teresiak, A., Filas, V., Ibbs, M., Bliźniak, R., Łuczewski, Ł. and Lamperska, K. (2018). 2D and 3D cell cultures – a comparison of different types of cancer cell cultures. Archives of Medical Science 14(4): 910-919. https://doi.org/10.5114/aoms.2016.63743
- Long, D., Alghoul, Z., Sung, J., Yang, C. and Merlin, D. (2023). Oral administration of M13-loaded nanoliposomes is safe and effective to treat colitis-associated cancer in mice. Expert Opinion on Drug Delivery: 1-20. https://doi.org/10.1080/17425247.2023.2231345
- Sakharov, D., Maltseva, D., Knyazev, E., Nikulin, S., Poloznikov, A., Shilin, S., Baranova, A., Tsypina, I. and Tonevitsky, A. (2019). Towards embedding Caco-2 model of gut interface in a microfluidic device to enable multi-organ models for systems biology. BMC Systems Biology 13(1): 19. https://doi.org/10.1186/s12918-019-0686-y
- Sun, H., Chow, E. C., Liu, S., Du, Y. and Pang, K. S. (2008). The Caco-2 cell monolayer: usefulness and limitations. Expert Opin Drug Metab Toxicol 4(4): 395-411.
- van Breemen, R. B. and Li, Y. (2005). Caco-2 cell permeability assays to measure drug absorption. Expert Opin Drug Metab Toxicol 1(2): 175-185.
- Wang, L., Nagesha, D. K., Selvarasah, S., Dokmeci, M. R. and Carrier, R. L. (2008). Toxicity of CdSe Nanoparticles in Caco-2 Cell Cultures. Journal of Nanobiotechnology 6(1): 11. https://doi.org/10.1186/1477-3155-6-11
- Gogineni, V, Yang, C and Merlin, D(2023). Establishing a 3D printed spheroid model for anti-colon cancer drug screening. Bio-protocol Preprint. bio-protocol.org/prep2388.
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
