Advanced Search
Protocol for Construction of Transwell-based blood-brain barrier model
Last updated date: Jun 18, 2023 Views: 1397 Forks: 0
Protocol for Construction of Transwell-based blood-brain barrier model
Margarita Shuvalova1,2,3
1Center for Precision Genome Editing and Genetic Technologies for Biomedicine,
Pirogov Russian National Research Medical University, Moscow, 117997, Russia
2Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences,
Moscow, 117997, Russia
3 ‘Federal Center of Brain Research and Neurotechnologies’ of the Federal Medical and Biological Agency,
Moscow, 117997, Russia
Correspondence: margarita22zelenskaya@gmail.com
Summary
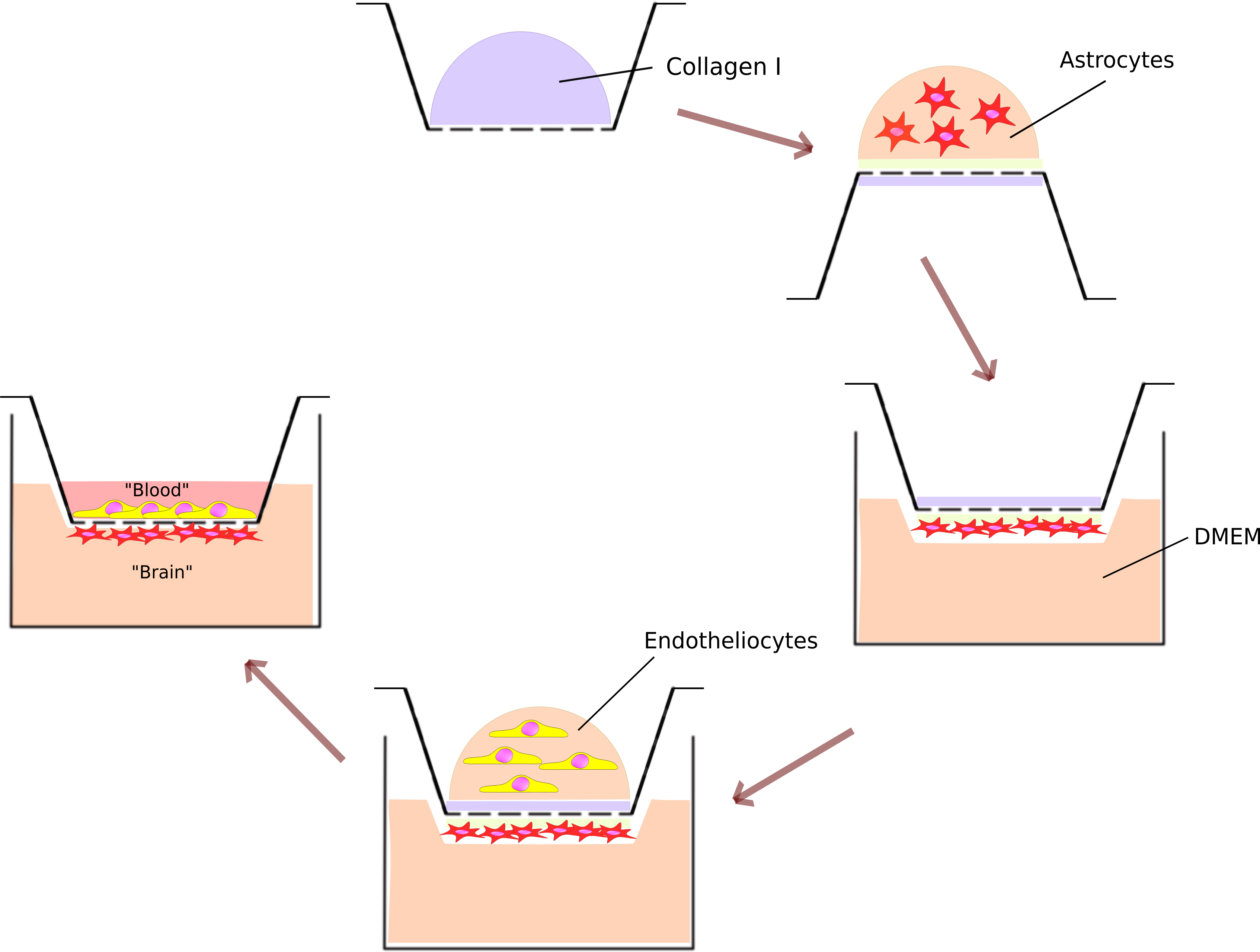
Here, we present a protocol for the construction of a blood-brain barrier (BBB) model using a Transwell culture insert, primary murine astrocytes, and bEnd.3 cells. In brief, the luminal (apical) side of the porous membrane of the insert was coated with collagen Ι. A 100 μl suspension containing 10^5 primary murine astrocytes was placed on the abluminal side of the membrane of the inverted insert and left for three hours to attach. Then, the insert was flipped to the normal position and placed in a 24-well culture plate with full DMEM medium. Next, 10^4 bEnd.3 cells were seeded on the luminal side of the porous membrane. The BBB model was allowed to mature for 5-7 days with medium changes twice a week. To assess the barrier properties of the model, the permeability coefficient for the fluorescent dye Lucifer yellow was calculated.
Graphical abstract
Materials and Equipment
Before starting this protocol prepare all media, solutions, and necessary materials as instructed in this section.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals | ||
| Dulbecco`s Modified Eagle Medium (DMEM) | GibcoTM | Cat#31966-021 |
| Fetal bovine serum (FBS) | GibcoTM | Cat#16000044
|
| Penicillin/streptomycin | PanEco | Cat#А065п |
| GlutaMAX™ | Thermo Fisher Scientific | Cat#35050061
|
| PBS 1x | Gibco | Cat#15374875 |
| Trypsin-EDTA (0.25%) | PanEco | Cat#1П034 |
| Versene solution | PanEco | Cat#Р080п |
| DNase I | Merck | Cat#10104159001 |
| Trypan blue dye (0.4%) | Thermo Fisher Scientific | Cat#T10282 |
| Hoechst 33342 | Thermo Fisher Scientific | Cat#H3570 |
| Lucifer Yellow | Thermo Fisher Scientific | Cat#L453 |
| NaCl | ЛенРеактив | Cat#533000 |
| KCl | ЛенРеактив | Cat#100432 |
| MgCl2 * 6Н2О | ЛенРеактив | Cat#120070 |
| CaCl2 | ЛенРеактив | Cat#101564 |
| HEPES | Диаэм | Cat#3350.1000 |
| D-Glucose | neoFroxx | Cat#LC-5938.1 |
| Collagen I | N/A | N/A |
| Other | ||
| Corning® Transwell® Inserts for a 24-well plate with polycarbonate membrane with a pore size of 3 µm | Corning Incorporated | Cat#3415 |
| 24-well cultural plate | Corning Incorporated | Cat#3524 |
| Fluorescent inverted microscope Evos M7000 | Thermo Fisher Scientific | AMF7000 |
| Varioskan LUX multimode microplate reader | Thermo Fisher Scientific | Cat#VL0000D0 |
| Black 96-well plate | Sigma-Aldrich | P8741 |
| Centrifuge | Eppendorf | Centrifuge 5430 R |
| Countess Automated Cell Counter | Thermo Fisher Scientific | AMQAF1000 |
| Countess™ Cell Counting Chamber Slides | Thermo Fisher Scientific | Cat#C10283 |
| 37°C, 5–10% CO2 incubator | N/A | N/A |
| Test tube, 15 mL | Nest | Cat#N-601002 |
| T-25 culture flask, treated | Thermo Fisher Scientific | Cat# 156499 |
Preparation of full DMEM medium: mix reagents as described in the table below. Prepare in a sterile environment, store at 4° C, do not use longer than 3 months.
Full DMEM medium
| Reagent | Final concentration | Amount |
|---|---|---|
| DMEM | 87.5% (v/v) | 438 mL |
| Fetal bovine serum | 10% (v/v) | 50 mL |
| GlutaMAX™ | 2 mM | 5 mL |
| Penicillin/Streptomycin | 100 IU/mL | 5 mL |
| Total | 500 mL |
Preparation of Krebs-Ringer modified buffer: mix reagents as described in the table below. Prepare in a sterile environment, store at 4° C.
Krebs-Ringer modified buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| NaCl | 140 mM | 8.182 g |
| KCl | 2.4 mM | 0.179 g |
| MgCl2 * 6Н2О | 1.4 mM | 0.282 g |
| CaCl2 | 2.5 mM | 0.277 g |
| Glucose | 10 mM | 1.8 g |
| HEPES | 10 mM | 2.383 g |
| ddH2O | N/A | 1000 mL |
| Total | 1000 mL |
- Lucifer Yellow 100 mM solution: dissolve 45,7 µg of Lucifer Yellow in 10 mL of Krebs-Ringer modified buffer. Store at 4° C in dark place.
- Lucifer Yellow 1 mM solution: add 100 µl of 100 mM Lucifer Yellow solution in 10 mL of Krebs-Ringer modified buffer. Store at 4° C in dark place.
Maintenance of primary astrocytes and bEnd.3 cells
We use healthy, exponentially growing bEnd.3 cells as endothelial cells and primary murine astrocytes (passage 1-5) for our BBB model. Cells can be stored for years in a liquid nitrogen storage tank. Cells grow in T-25 culture flasks in full DMEM. When confluence reaches 90-100%, cells are passaged using a standard trypsinization protocol.
Note: We have observed that bEnd.3 cells grow slowly initially after being frozen. It may take up to one week to obtain a 100% confluent culture in a T25 flask from 0.5 million frozen cells.
CRITICAL: The time of trypsinization of bEnd.3 cells may be increased due to the presence of tight junctions, which slowly succumb to digestion. Check the cells using an inverted microscope every 5 minutes and only proceed to the next step when the cells are detaching from the plate and become round.
Construction of the model
Note: For BBB modeling, we used Corning® Transwell® Inserts with a polycarbonate membrane and a pore size of 3 µm for a 24-well plate. Inserts with other size and pore diameter can also be used.
1. Coating with collagen I
Time: 1.5 h
a. Place the Transwell inserts in a 24-well cultural plate.
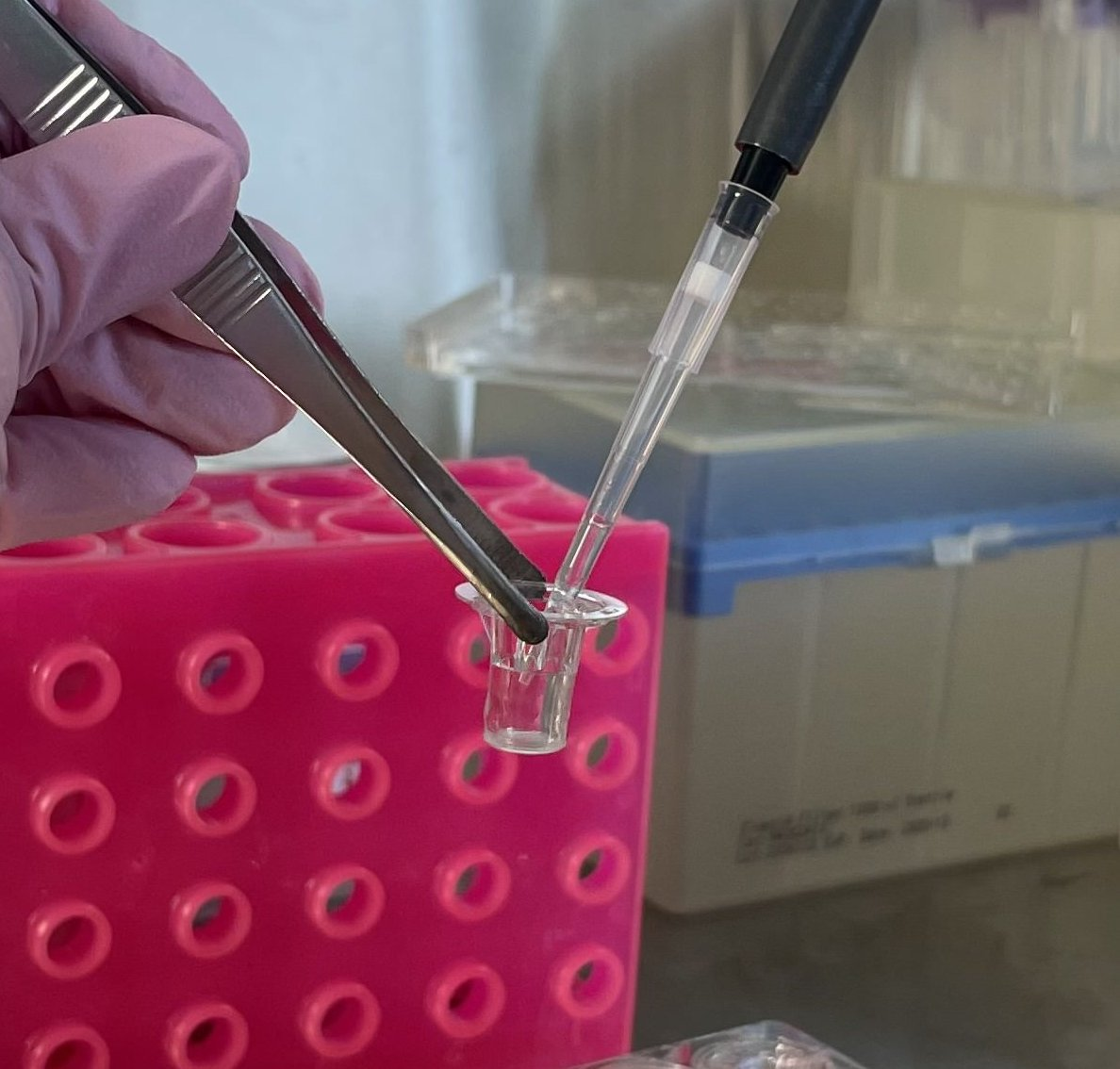
CRITICAL: when transferring inserts, use sterile tweezers with blunt ends. Hold the insert as shown in the figure 1. Do not touch the membrane to avoid damaging it.
b. Add 100 µL of 5 mg/mL collagen I to each Transwell insert.
CRITICAL: ensure that the drop of collagen is placed on the membrane and not on the walls of the insert.
CRITICAL: use sterile tweezers with blunt ends when transferring inserts and hold the insert as shown in the figure. Do not touch the membrane to avoid damaging it (fig.1).
c. Incubate at room temperature for at least 30 minutes.
d. Add 500 µL of PBS to the lower compartment and incubate for at least 30 minutes.
e. Add 200 µL of PBS in the apical compartment.
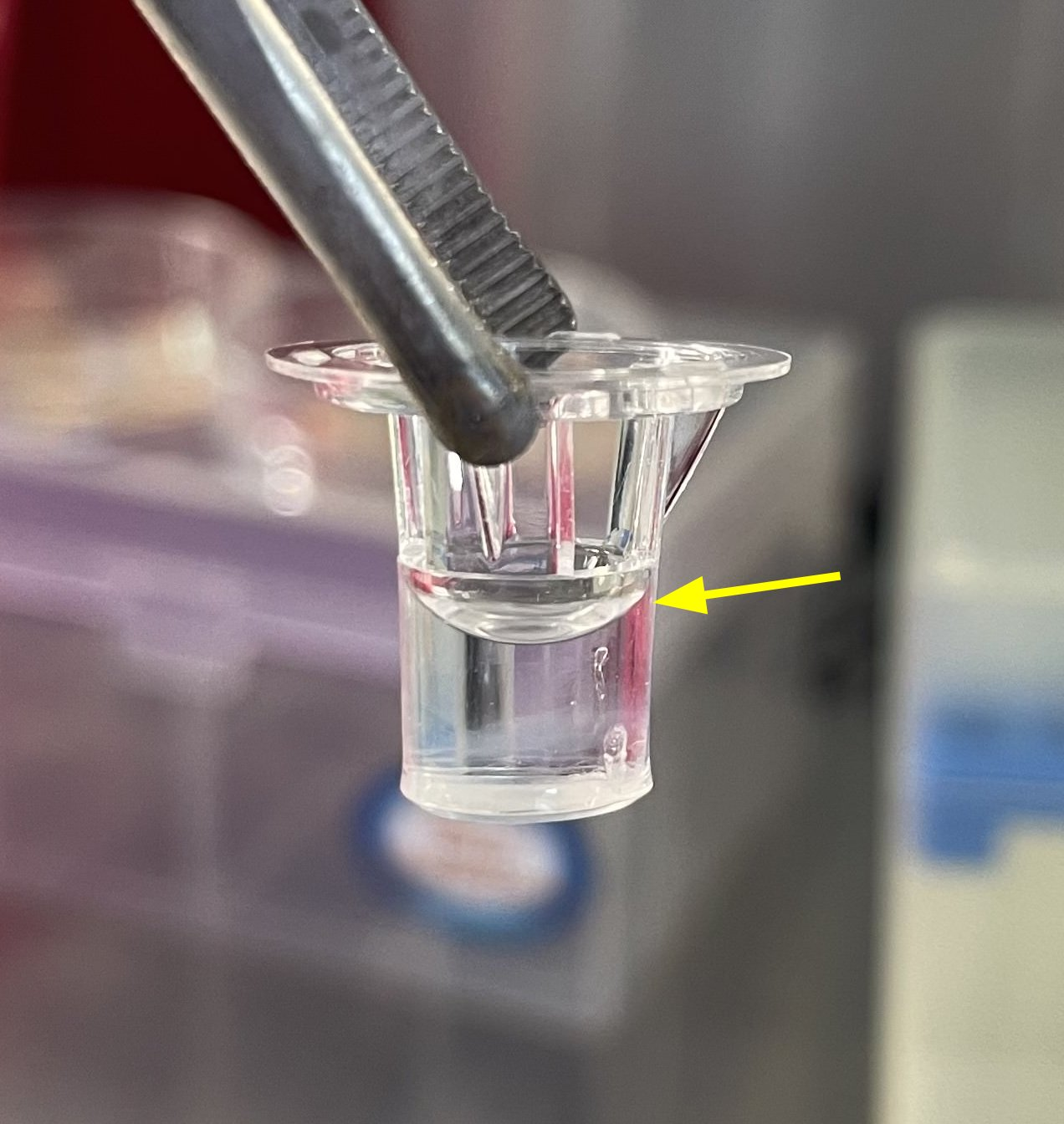
Note: if collagen polymerization is successful, the liquid column will hold for some time (fig.2).
f. Aspirate PBS from the lower compartment using a pipette or aspirator.
g. Aspirate PBS from the apical compartment carefully by tilting the Transwell with tweezers and avoiding touching the bottom of the insert to prevent destruction of the polymerized collagen layer (fig.3).
CRITICAL: do not use an aspirator when aspirating any substances from the insert as it can destroy the membrane, collagen, and cell layers.

Optional: Transwells coated with collagen can be stored in PBS in sterile conditions at +4C for 3 days.
2. Seeding of the astrocytes.
Time: 4 h
a. Detach astrocytes from the culture flask using the standard trypsinization protocol.
b. Stop digestion by adding full DMEM.
c. Centrifuge at 300 x g for 5 minutes. Aspirate the supernatant and resuspend the cells in a minimal volume of full DMEM.
CRITICAL: Be careful not to aspirate the cell pellet during the removal of the supernatant.
d. Count the live cells using Trypan blue dye and an automated cell counter.
e. Adjust the concentration of astrocytes to 10^6 cells/mL. For one Transwell, 100 µL of suspension (i.e., 10^5 astrocytes) will be required.
f. Place Transwells in a 6-well cultural plate in an inverted position (fig.4).

g. Place 100 µL of astrocyte suspension on the membranes, making sure that the drop stays on the membrane.
CRITICAL: Act quickly to prevent the drops from draining.
h. Once astrocytes have been added to all Transwells, carefully close the lid. The drop should be between the lid and the membrane (fig.5). The surface tension will prevent it from draining.
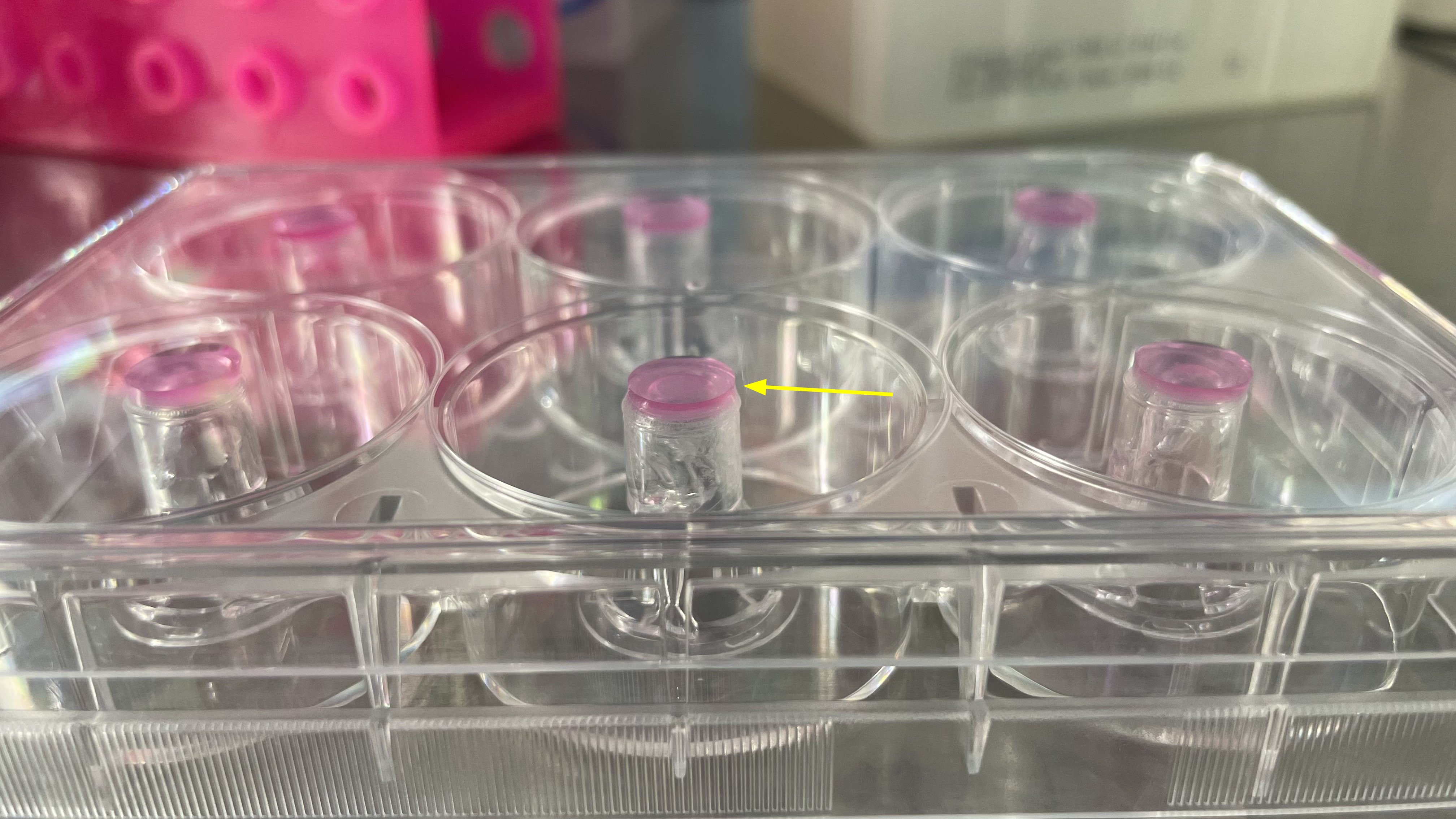
i. Incubate in a CO2 incubator for 3-4 hours for astrocyte attachment to the membrane.
h. After incubation, transfer Transwells to a 24-well plate with 1 mL of full DMEM per well.
Optional: At this point, the protocol can be paused until the next day.
Optional: To evaluate the effectiveness of astrocyte attachment, apply 50 µl of Hoechst 33342 to the abluminal side of the membrane of one of the Transwell. Then insert the Transwell into a 24-well plate with 1 ml of PBS and analyze it under a fluorescent microscope. The cell nuclei on the membrane will be visible (fig.6).
3. Seeding of bEnd.3 cells.
Time: 30 min
a. Detach bEnd.3 cells from the culture flask using the standard trypsinization protocol.
b. Stop digestion by adding full DMEM.
c. Centrifuge at 300g for 5 minutes, aspirate the supernatant, and resuspend cells in a minimal volume of full DMEM.
d. Count live cells using Trypan blue dye and an automated cell counter.
e. Adjust the concentration of bEnd.3 cells to 10^5 cells/mL. 100 µL of suspension (i.e., 10^4 bEnd.3 cells) will be required for one Transwell.
f. Add 100 µL of suspension to each Transwell.
g. Cultivate BBB models in a CO2 incubator and replace the medium twice a week.
Evaluation of barrier integrity
Time: 1.5 h
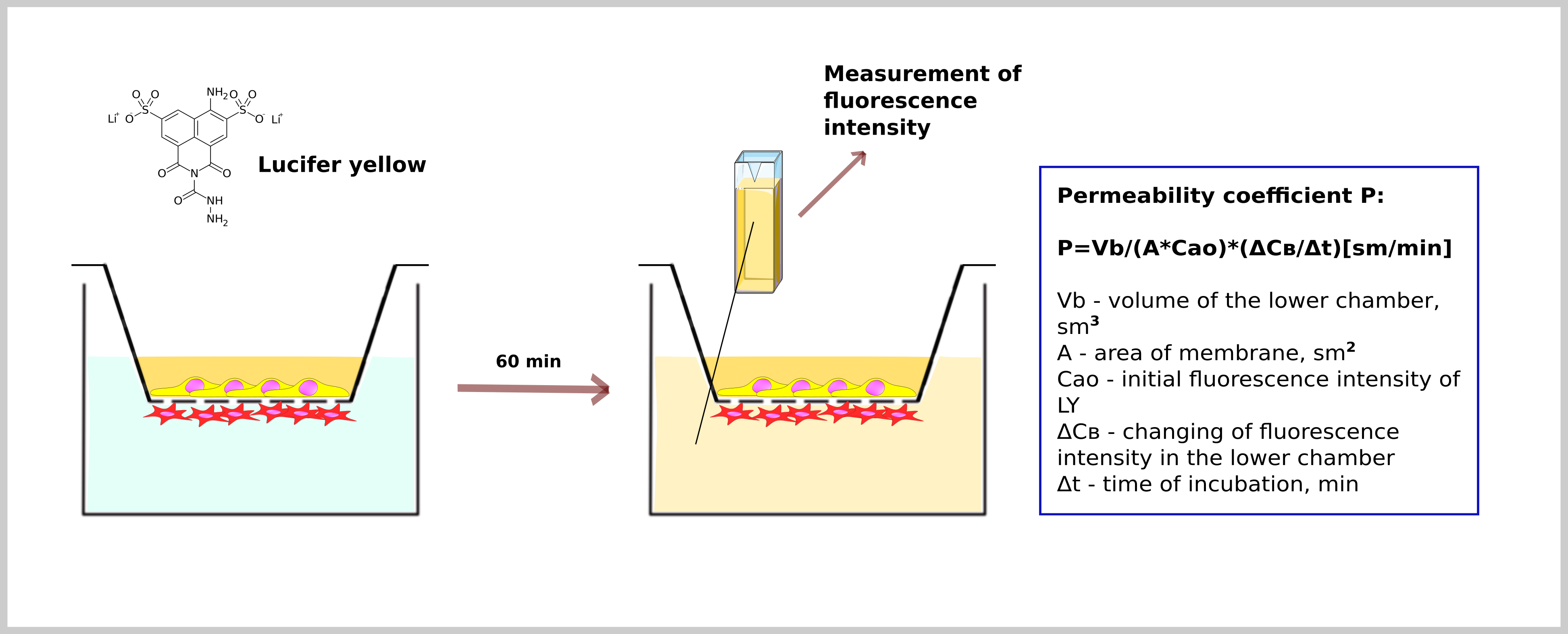
For evaluation of barrier integrity, we measure the coefficient of permeability (P) using the fluorescent dye Lucifer Yellow (fig.7).
a. Take a new 24-well plate and add 1 mL of pre-warmed (37°C) Krebs-Ringer buffer to each well.
b. Aspirate all medium from the upper and lower chambers of the BBB models.
CRITICAL: Do not use an aspirator when aspirating any substances from the insert. It can destroy the membrane, collagen, and cell layers.
CRITICAL: Aspirate from the upper compartment as shown in the figure.
Optional: Use a Transwell without any cells as a control.
c. Wash the BBB models with cultural medium. Add 1 mL of pre-warmed (37°C) Krebs- Ringer buffer to the lower chamber and 200 µL to the upper chamber.
d. Aspirate all buffer from the upper and lower chambers.
CRITICAL: achieve almost complete removal of the liquid from the upper chamber to avoid dilution of the dye solution and errors in the analysis.
e. Using tweezers, take the first Transwell and add 200 µL of LY solution to it.
f. Place the Transwell in the 24-well plate from step a.
g. Repeat steps e-f with all tested Transwells.
CRITICAL: Apply the dye very carefully and do not allow drops of dye to get on the outside of the insert.
CRITICAL: Act quickly to prevent a big difference in the incubation time of the first and last Transwell.
h. Incubate in a CO2 incubator for 1 hour.
CRITICAL: The incubation time must be timed accurately. Use a timer.
i. When incubation stops, replace all Transwells in a new 24-well plate.
CRITICAL: Fix the position of each Transwell to later find out the permeability coefficient for each and, if necessary, exclude some models from the analysis.
j. Aspirate the dye from the upper chamber.
k. Add 1 mL of full DMEM to the lower chamber and 200 µL to the upper chamber.
l. Place the BBB models in a CO2 incubator.
m. There will be a solution left in the wells of the well plate in which the incubation was taking place. This solution will be further analyzed.
n. Add 100 µL of solution from step m to a 96-well black plate.
o. Add 100 µL of the initial solution of LY to one well.
p. Add 100 µL of Krebs-Ringer buffer to another well for subtraction of background.
q. Measure the fluorescent signal using a plate reader (excitation 428 nm, emission 543 nm).
r. Use the following equation to calculate the permeability coefficient (P):
P = Vb/(А*Сао)*(ΔСв/Δt) [sm/min]
Vb - a volume of lower compartment, sm3 (1 sm3 in our model)
А - an area of the porous membrane, sm2 (0,33 sm2 in our model)
Сао - the initial intensity of fluorescence of added LY solution
ΔCв - change of fluorescence in the lower compartment
Δt - time of incubation, min (60 min in our model).
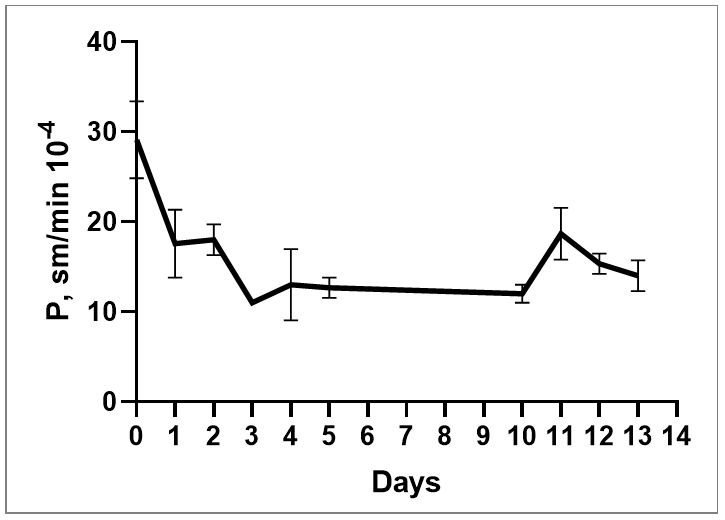
s. To evaluate barrier integrity, monitor the permeability coefficient during model maturation and when exposed to test substances. Typical values of the permeability coefficient are shown in the figure 8.
Declaration of interests
The author declares no competing interests.
Related files
 Protocol for Construction of Transwell-based blood-brain barrier model.docx
Protocol for Construction of Transwell-based blood-brain barrier model.docx - Shuvalova, M L(2023). Protocol for Construction of Transwell-based blood-brain barrier model. Bio-protocol Preprint. bio-protocol.org/prep2340.
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link

