Advanced Search
Protocol for isolation of primary murine astrocytes
Last updated date: Jun 4, 2023 Views: 994 Forks: 0
Margarita Shuvalova1,2,3
1Center for Precision Genome Editing and Genetic Technologies for Biomedicine, Pirogov Russian National Research Medical University, Moscow, 117997, Russia
2Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, Moscow, 117997, Russia
3 'Federal Center of Brain Research and Neurotechnologies' of the Federal Medical and Biological Agency, Moscow, 117997, Russia
Correspondence: margarita22zelenskaya@gmail.com
Summary
Here we present a simple and affordable protocol for obtaining of primary murine astrocytes.
Institutional permissions
All animal experiments should be reviewed and approved by the Laboratory Animal Management and Ethics Committee of your institute. All manipulations with animals were conducted according to the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (1986, ETS 123) and approved by the Institutional Animal Care and Use Committee of Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry (approval no. 331).
Materials and Equipment
Before starting this protocol prepare all media, solutions, and necessary materials as instructed in this section.
Key resources table
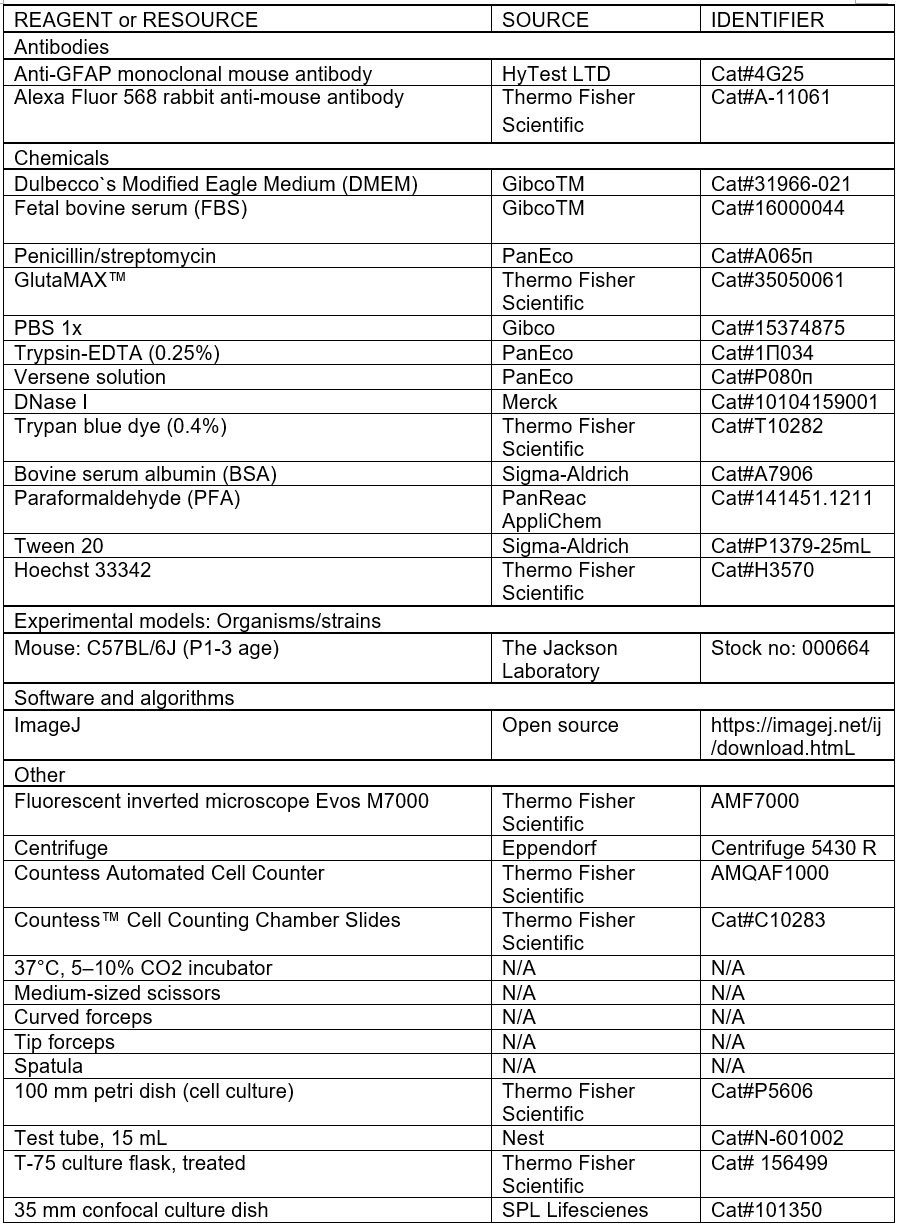
Preparation of full DMEM medium: mix reagents as described in the table below. Prepare in a sterile environment, store at 4° C, do not use longer than 3 months.
Preparation of fixation solution: dissolve 2 g of PFA in 50 mL of 1x PBS. Store at 4° C in dark place.
Preparation of blocking solution: dissolve 5 g BSA in 100 mL PBS, add 50 µl Tween 20. Store at 4°C.
Obtaining of primary murine astrocytes
Timing: 2-5 weeks
1. Isolation of primary astrocytes (passage 0)
CRITICAL: Brain isolation can take time due to inexperience. Perform only one decapitation at a time and complete step ‘h’ before next decapitation to ensure efficiency and a healthy brain culture.
- Before starting the dissection, cool the PBS on ice.
- Gently hold the mouse pup and spray the neck with 70% ethanol to prevent contamination.
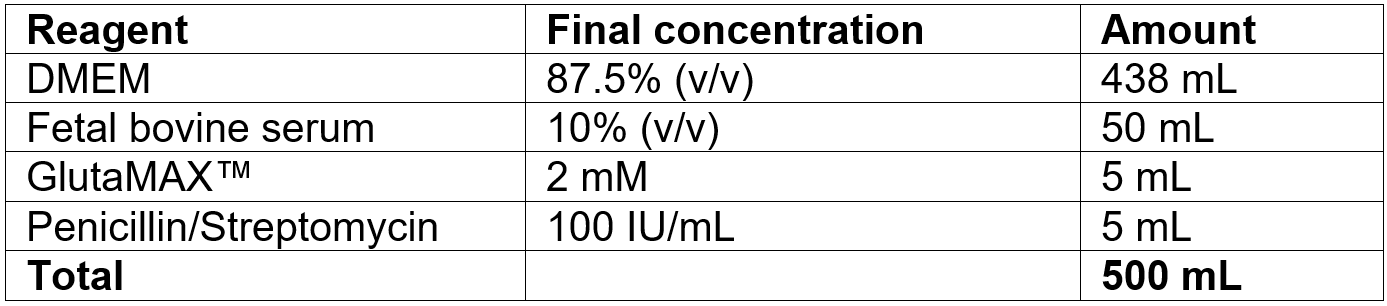
- Decapitate the mouse using scissors in one cut (fig.1a).
- Fix the head using anchoring forceps at the orbital cavities (fig.1b).
- Using sterile spatula, remove the skull and cranium from the top of the head (fig.1c, d).
- Using sterile forceps, carefully remove the brain from the skull (fig.1e).
- Once the brain is isolated, perform all next protocol steps in sterile conditions.
- Place the brain in a 10 cm Petri dish containing ice-cold PBS. The brain should be completely submerged in the liquid (fig.1f).
- Using scissors and forceps, remove the meninges and cerebellum (fig.1g).
- Cut the cortex into pieces about 2-3 mm in size (fig.1h).
- Place the cortex pieces into a 15 mL tube using a pipette and add 1 mL of Trypsin-EDTA and 1 µl of DNase I (fig.1i).
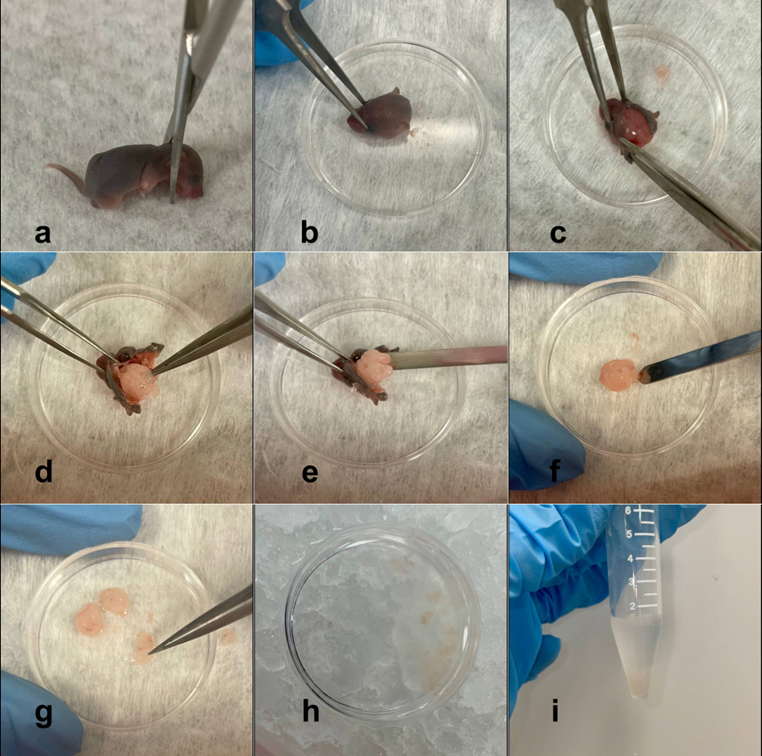
Figure 1. Obtaining of suspension of murine brain tissue.
CRITICAL: The volume of Trypsin-EDTA and DNase I depends on the number of brains in one tube. The volume of Trypsin-EDTA and DNase I should be 1 mL and 1 µl, respectively, for 1-3 brains, 2 mL and 2 µl for 4-6 brains, and 4 mL and 4 µl for 7-10 brains.
l. Incubate the tube at 37°C for 30-45 minutes with gentle shaking every 10 minutes.
m. Add 4 mL of full DMEM to stop the digestion.
n. Triturate the tissue with a P1000 pipette tip to obtain a single-cell suspension.
o. Filter the cell suspension through a 70 μm cell strainer into a new 50 mL conical tube.
p. Centrifuge at 300g for 5 minutes and aspirate the supernatant.
q. Suspend the pellet in full DMEM.
r. Plate the cell suspension in a T-75 cell culture flask and place the flask in a CO2 incubator.
CRITICAL: Plate the cell suspension at a rate of 3 brain per one T-75 flask.
s. The next day, remove all medium.
t. Wash cells with PBS to remove debris.
u. Add 5 mL of full DMEM and place the flask with cells in a CO2 incubator.
2. Passaging of Astrocytes
- Monitor the formation of monolayer of astrocytes under a microscope (figure 2).
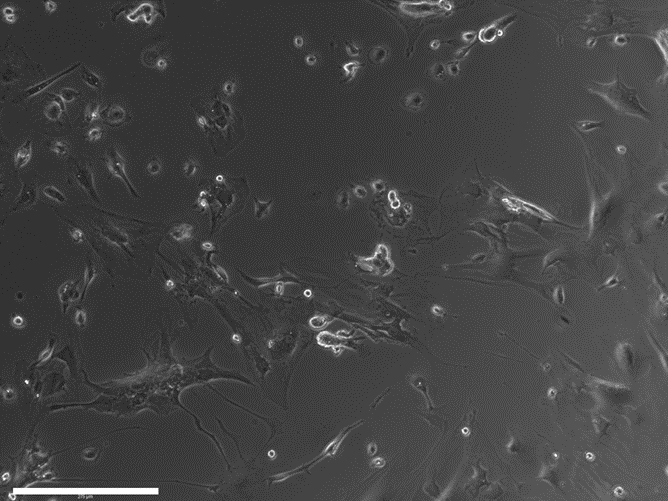
Figure 2. Cells the day after isolation. Scale bar – 125 μm.
Note: It typically takes 5-7 days for astrocytes to reach 70-80% confluence.
b. When confluence reaches 70-80%, remove all medium.
c. Wash cells with 2 mL of Versene solution.
d. Add 2 mL of Trypsin-EDTA.
e. Incubate at 37°C in CO2-incubator for 5-15 minutes.
CRITICAL: Check the cells using an inverted microscope every 5 minutes and only proceed to the next step when the cells are detaching from the plate and become round.
f. Stop trypsinization by adding 2 mL of full DMEM.
g. Resuspend detached cells and place the suspension into a 15 mL tube.
h. Centrifuge at 300g for 5 minutes and aspirate the supernatant.
i. Resuspend cells in 1 mL of full DMEM.
j. Take 10 μL of cell suspension and mix with 10μL of trypan blue.
k. Load the mix in a cell counting chamber slide and count cells in the Countess automated cell counter.
l. Take the volume of suspension containing 10^5 cells.
m. Place this volume in a new T-25 flask, add 5 mL of full DMEM, and place the flask in a CO2 incubator.
n. Change the medium 2 time a week.
Verification of purity of culture
To confirm the purity of the culture of astrocytes, use immunofluorescent staining for the astrocyte marker GFAP.
- For 3 days prior to immunostaining, seed astrocytes onto confocal culture dishes.
- At the 3rd day, remove all medium and wash the cells with PBS.
- Add 500 μL of fixation solution, incubate for 20 minutes at room temperature.
- Remove fixation solution, wash with PBS twice.
- Add 1 μL of blocking solution. Incubate for 1 hour at room temperature.
- Remove blocking solution. Add primary antibodies solution against GFAP (we used 1:500). Incubate for 1 hour at room temperature.
- Remove primary antibodies solution.
- Wash with PBS three times.
- Add secondary antibodies solution (we used 1:500). Incubate for 1 hour at room temperature.
- Remove secondary antibodies solution.
- Wash with PBS three times.
- Optional: add Hoechst 33342 for visualization of nuclei.
- Examine stained cells under fluorescent microscope (fig3).
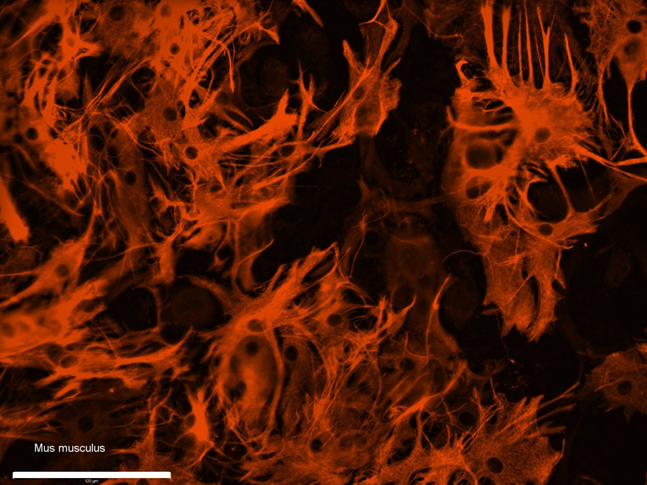
Figure 3. Immunostaining of obtained culture of astrocytes (p2) against GFAP. Scale bar – 125 μm.
Declaration of interests
The author declares no competing interests.
- Shuvalova, M L(2023). Protocol for isolation of primary murine astrocytes. Bio-protocol Preprint. bio-protocol.org/prep2327.
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link

