Advanced Search
Cytotoxicity assay, cytokine production and continuos repeated stimulation
Last updated date: Jan 17, 2023 Views: 507 Forks: 0
Complete Cytotoxicity Assay
Materials and Reagents:
- TC plate 96 well, round base (Sarstedt. Ref 833925)
- RPMI culture medium supplemented with 3% human serum (Sigma-Aldrich), 1% penicillin/streptomycin
- White 96w Maxisorp Nunc plate (Thermo Scientific™)
- Bright-Glo™ Luciferase Assay Buffer
- Bright-Glo™ Luciferase Assay Substrate (lyophilized)
- Eppendorfs 1,5 ml
- Lysis Solution (from CytoTox 96® Non-Radioactive Cytotoxicity Assay)
- Multichannel pipette + tips
- Reagent reservoirs
- Vortex
Experimental procedure:
Day 0. Co-culture CAR T cells and tumoral cells.
- Resuspend and count tumoral, CAR T and UTD cells. Take the appropriate cell number.
Example Fig. 1: In the below example we use 5000 tumoral cells/well with initial an E:T ratio of 5:1 and 1:3 dilutions of CAR T cells. All by triplicate. For that we will need:
√ 528000 tumoral cells
√ 125000 UTD/ CART cells - Centrifuge 5 min, 1500 rpm. Discard supernatant and add required medium. In the example:
√ 5'28 ml to tumoral cells
√ 248 µl to UTD/ CART cells - Make dilutions: Prepare eppendorfs with 165 µl complete medium RPMI (5 eppendorfs for each CART/UTD group). Add 83 µl of cells to the next tube. Vortex and repeat.
- In a p96w with round base, add 50 µl/well of tumoral cells to all wells. Add 50 µl of medium, CART or UTD cells to the correspondent wells.
- Incubate at 37°C, for 24 h.
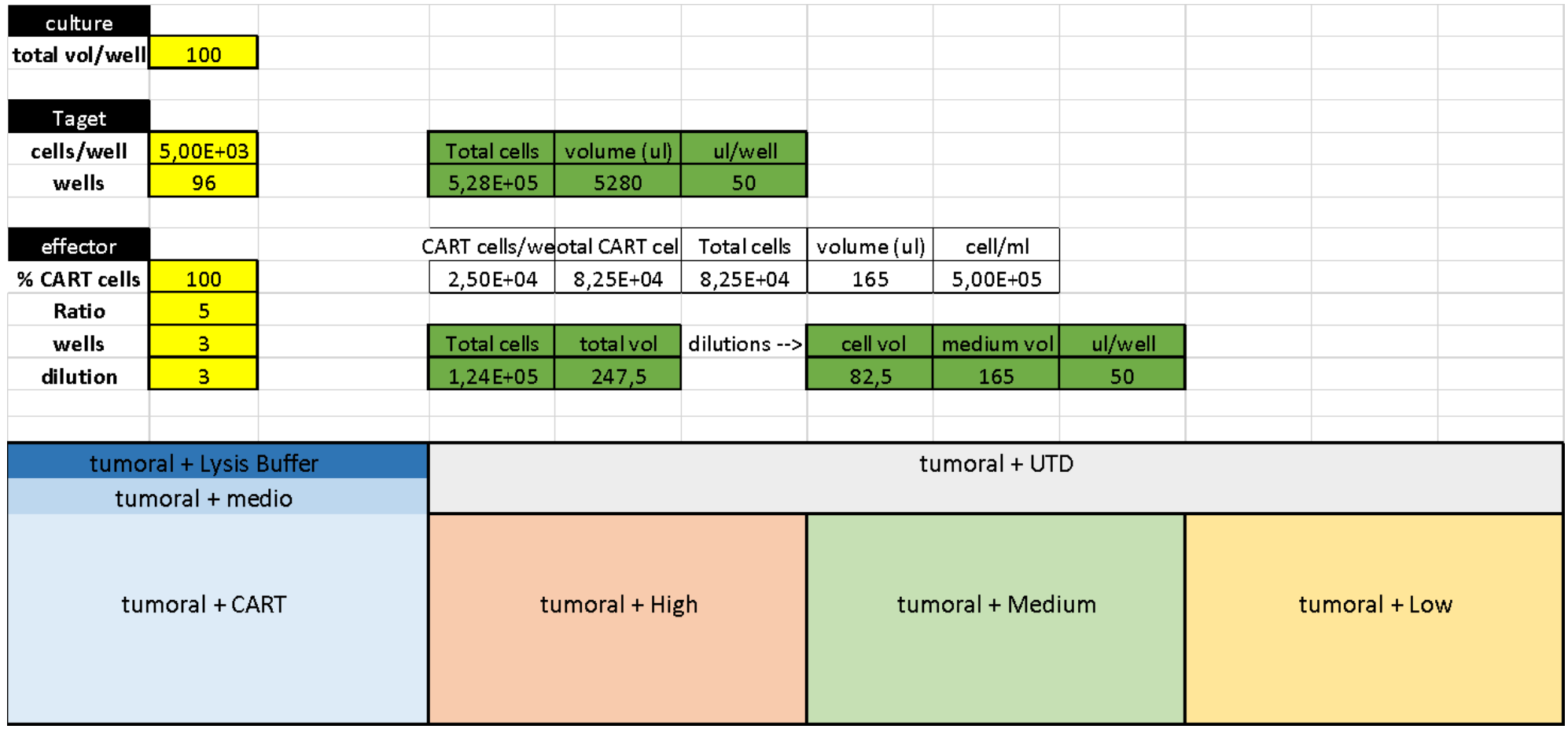
Fig. 1. Cytotoxicity example
Day 1. Bright-Glo Luciferase Assay (Cytotoxicity assay)
- Add 10 µl of Lysis Buffer to the maximum lysis wells (tumoral + lysis buffer). Incubate at 37°C, for 45 min.
- Equilibrate the medium RPMI 10% FBS to room temperature.
- Equilibrate the reagent to room temperature, which is near the temperature optimum of luciferase. Transfer the contents of one bottle of Bright-Glo™ Buffer (RT in the lab) to one bottle of Bright-Glo™ Substrate* (-20℃ in the lab). Mix by inversion until the substrate is thoroughly dissolved.
· Equilibration of the reagent prior to use is unnecessary when the buffer is stored at room temperature.
· If the reagent is stored frozen after reconstitution, the most convenient and effective method to thaw is to place it in a water bath at room temperature. Mix well after thawing. - Centrifuge multiwell plates for 5 min, 800 ×g. Put the supernatants in another p96w plate and store at -20°C (aprox 100 µl/well) for cytokine production measurement.
- Resuspend the cells with 100 µl of PBS (Wash step).
- Centrifuge 5 min, 800 ×g. Throw supernatant by plate inversion.
- Add 50 µl of medium RPMI 10% to each well, resuspend the cells and put them in a white 96w NUNC plate.
- Add 50 µl of reagent and mix. Wait 5 minutes to allow complete cell lysis, and measure in the luminometer.
- Store the remaining Bright-Glo™ Reagent at -80℃.
- To obtain the % of lysis, first, the average absorbance for the maximum lysis wells and for the spontaneous lysis wells (tumoral + media) is obtained from the 3 replicates. Then, the % of lysis of the experimental data is calculated for each well with the following formula:
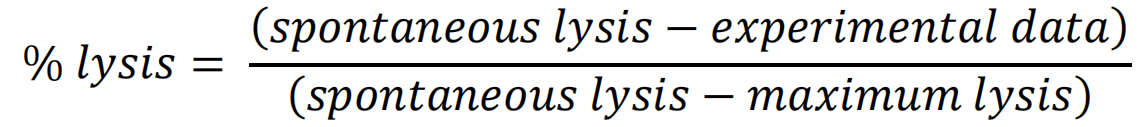
*Approximate stability of Bright-Glo™ Reagent after reconstitution: 10% loss of luminescence per 5 hours at room temperature, 10% loss per 24 hours at 4°C, and <5% loss after one month at -70°C. The reagent may be subjected to up to seven freeze-thaw cycles with no effect on potency. Check the manufacture instructions for more details.
Cytokine production measurement (ELISA)
Materials and Reagents:
- F96 Maxisorp Nunc-Immuno plate (Thermo Scientific™). Ref. 442404
- Plate sealers (R&D systems). Catalog nº DY992
- BD OptEIA™ Reagent Set B (Cat. No. 550534)
- OptEIA™ Sets for human interleukin-2 (IL-2), tumor necrosis factor (TNF-α), interferon- gamma (IFN-γ), etc.
- Eppendorfs 1,5 ml
- Multichannel pipette + tips
- Reagent reservoirs
- Vortex
Experimental procedure:
Day 0. Coating
Coat microwells with 100 µl per well of Capture Antibody diluted in Coating Buffer. For recommended antibody coating dilution, see lot-specific Instruction/Analysis Certificate. Seal plate and incubate overnight at 4℃.
Day 1.
- Aspirate wells and wash 3 times with ≥ 300 µl /well Wash Buffer. After last wash, invert plate and blot on absorbent paper to remove any residual buffer.
- Block plates with ≥ 200 µl /well Assay Diluent. Incubate at RT for 1 hour.
- Prepare standard and sample dilutions in Assay Diluent. See “Standards Preparation and Handling” from technical data sheet.
- Aspirate/wash as in step 2.
- Pipette 100 µl of each standard, sample, and control into appropriate wells. Seal plate and incubate for 2 hours at RT.
- Aspirate/ wash as in step 2, but with 5 total washes.
- Add 100 µl of Working Detector (Detection Antibody + Streptavidin-HRP reagent) to each well. Seal plate and incubate for 1 hour at RT.
- Aspirate/ wash as in step 2, but with 7 total washes. NOTE: In this final wash step, soak wells in wash buffer for 30 seconds to 1 minute for each wash.
- Add 100 µl of Substrate Solution to each well. Incubate plate (without plate sealer) for 30 minutes at room temperature in the dark.
- Add 50 µl of Stop Solution to each well.
- Read absorbance at 450 nm within 30 minutes of stopping reaction. If wavelength correction is available, subtract absorbance at 570 nm from absorbance 450 nm.
Continuous repeated stimulation
CARHigh T and CARLow T cells were co-culture with irradiated tumor cells for 21 days.
Materials and Reagents:
- 12 well cell culture plates
- RPMI culture medium supplemented with 3% human serum (Sigma-Aldrich), 1% penicillin/streptomycin
Experimental procedure:
- Count sorted CARHigh T and CARLow T cells. Take 1.5×106 cells of each population separately and centrifuge 5 min at 500 ×g. Discard supernatant and resuspend in 750 µl of complete RPMI culture medium.
- Take 3×106 cells ARP1-LucGFP and irradiate at 54 cGy.
- Centrifuge ARP1-LucGFP cells 5 min at 500 ×g. Discard supernatant and resuspend in 1.5 ml of complete RPMI culture medium.
- Co-culture 1.5×106 CARHigh T or CARLow T cells, at a 1:1 ratio, with 1.5×106 cells in RPMI culture medium in a total volume of 1.5 ml in a 12 well plate.
- Incubate at 37°C, for 72 h.
- Count CAR T cells and add ARP1-LucGFP tumoral cells irradiated at 54 cGy at a 1:1 effector: target ratio.
- Repeat steps 5 and 6 every three days, until day 21 is reached.
- Hernaez, M, Rodriguez-Madoz, J and Prosper, F(2023). Cytotoxicity assay, cytokine production and continuos repeated stimulation. Bio-protocol Preprint. bio-protocol.org/prep2122.
- Rodriguez-Marquez, P., Calleja-Cervantes, M. E., Serrano, G., Oliver-Caldes, A., Palacios-Berraquero, M. L., Martin-Mallo, A., Calviño, C., Español-Rego, M., Ceballos, C., Lozano, T., San Martin-Uriz, P., Vilas-Zornoza, A., Rodriguez-Diaz, S., Martinez-Turrillas, R., Jauregui, P., Alignani, D., Viguria, M. C., Redondo, M., Pascal, M., Martin-Antonio, B., Juan, M., Urbano-Ispizua, A., Rodriguez-Otero, P., Alfonso-Pierola, A., Paiva, B., Lasarte, J. J., Inoges, S., Lopez-Diaz de Cerio, A., San-Miguel, J., Fernandez de Larrea, C., Hernaez, M., Rodriguez-Madoz, J. R. and Prosper, F.(2022). CAR density influences antitumoral efficacy of BCMA CAR T cells and correlates with clinical outcome. Science Advances 8(39). DOI: 10.1126/sciadv.abo0514
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
