Advanced Search
Serum antibodies pre-absorbed with Escherichia coli powder extract represents a reliable method that decreases non-specific responses in immunological tests
Last updated date: Oct 11, 2022 Views: 421 Forks: 0
Abstract
In order to evaluate the immunity of infectious diseases, techniques based on detection of antigen or antibodies are applied. A very important technique used to analyzed immunogenic biological fractions is the western blot, which combines the selective of polyacrylamide electrophoresis, with the sensibility of immune-enzymatic, showing the antibody-antigen interactions.
Mammals usually have been exposed to bacteria, so they have a widely diverse repertoire of antibodies, that in some cases recognize E. coli proteins, and this bacteria is usually used as expression system for recombinant proteins. This could be a problem when part of E. coli proteins co-elute in the purification of the expressed protein, giving a positive signal in WESTERN BLOTS or even ELISA tests, and produce false positives or overrated results respectively.
This work demonstrates how pre-absorb serum antibodies using a powder extract from Escherichia coli, eliminate either unspecific bands in western blot and overestimated signals in ELISA keeping only the antibodies that recognize our recombinant protein.
Keywords: Recombinant Protein, antibody, Inmune absortion, Escherichia coli powder extract, Westernblot, Elisa
[Background]
Western blotting is a technique that comes from the separation of proteins (based on size) through polyacrylamide gel electrophoresis under denaturing conditions (SDS-PAGE), and them being transfer and immobilized in membrane support, containing its replica of denatured proteins. Their selective detection consists of the use of an antibody-mediated system (Mahmood and Yang, 2012; Biji et al. 2011). Western blotting or so-called immunoblotting was introduced by Towbin et al. in 1979 (Towbin, Staehelin and Gordon, 1979).
This technique could be useful in the identification of a specific protein, maybe in order to verify the expressions patterns to have a better understanding of molecular and cellular events or just to verify an expression system (Taylor and Posch, 2014). Would be also useful in a selection of a specific antibody for immunodetection protocols from complex biological samples (Moore, 2009; Roitt, 1997).
The discovery, that certain enzyme-substrate combinations produced quantifiable colorimetric changes, led to a shift in immunodetection. This combination of enzyme-substrate that is linked to antibodies which in turn could detect, by epitope-para epitope interactions, a specific analyte was developed (Avrameas, 1969). In 1971, two independent research groups in Europe published papers that described the step-by-step process of performing an enzyme-linked immunosorbent assay (ELISA) (Engvall and Perlmann, 1971; Van Weemen and Schuurs, 1971).
The ELISA is now routinely used to detect and quantify specific molecules, by antibody-antigen interactions. The antigen is directly immobilized in a microplate well, or by specific antibody known as capture antibody. A “primary detection antibody” is added, forming an antigen-antibody complex. The primary detection antibody is either directly labeled with an enzyme direct ELISA, or the enzyme is attached to a secondary antibody known as secondary detection antibody, indirect ELISA. The measurement of the optical density is proportional to the quantity of antigen in the sample (Shah and Maghsoudlou, 2016).
The antibodies used in both techniques (Western Blotting or ELISA), comes from mammals that usually have been exposed to bacteria and pathogens, activating in some cases the adaptive immune system, so they have a widely diverse repertoire of antibodies. Some of these antibodies recognize E. coli proteins; which is the bacteria usually used as expression system, and when this proteins co-elute in the purification of the expressed protein, a cross-reaction could take place, giving a false positive signal in WESTERN BLOTS or even overestimation of ELISA results, producing false positives or overrated results respectively.
In order to eliminate these nonspecific signals, a pre-absorption of serum´s antibodies using the powder extract from Escherichia coli is needed, keeping only the antibodies that recognize our recombinant protein. This method will be explained in this work and demonstrated performing an immunological evaluation of the antibody against the Brucella melitensis´s protein (Omp31).
Materials and Reagents
- LB medium (Fisher Scientific, Catalog number: BP1425-500
- NaCl (Promega, catalog number: H5271)
- Acetone (Fisher Scientific, catalog number: 02-002-157)
- PBS 10X (Thermo Fisher Scientific, Catalog number: AM9624)
- Horseradish peroxidase anti-bovine immunoglobulin conjugate (Sigma-Aldrich, Catalog number: A8917-2ML)
- Eppendorf microcentrifuge tubes (Sigma-Aldrich, Catalog number: Z666505-100EA)
- Cellulose blotting membranes (Fisher Scientific, catalog number: 45-002-817)
- Whatman Filter Paper (Fisher Scientific, catalog number: 09-927-155)
Equipment
- Eppendorf Centrifuge 5417R rotor: FA-45-30-11 Marshall Scientific, catalog number: 22 62 180-7)
- Protein mini gel cassettes (Bio-Rad Laboratories, catalog number: 1658000FC).
- Pippettes
Procedure to produce the Escherichia coli powder
- Grow Escherichia coli cells in 100 ml of LB medium cultures until reach 0.8 OD (0.8-1.0 OD at 600nm would be OK).
- After reaching 0.8 OD, place the cell into microcentrifuge tubes and spin at 12,000 rpm for 10 min at 4 ºC.
- Discard supernatant, recollect the pellet and weight the pellet (net weight of the pellet should be around 1.0 g).
- Resuspend in 1.8 ml of 0.9% NaCl and transfer to 50 mL centrifuge tube.
- Used 1 ml 0.9% NaCl to rinse the microtube and transfer to 50 mL centrifuge tube in order not to lose cells.
- Complete the volume to 4ml using 0.9% NaCl.
- Add 16ml of cold acetone to the 4ml resuspension (16 ml cold acetone for each 4ml resuspension).
- Incubate on ice for 30 min shaking around each 5 min.
- Dispense in microcentrrifuge tubes and spin at 12,000 rpm for 10 min at 4 ºC.
- Discard supernatant, recollect the pellet in 50 mL centrifuge tube and resuspend pellet with 20 ml cold acetone.
- Leave it on ice for 10 min.
- Dispense in microcentrrifuge tubes and spin at 10,000 g for 10 min at 4 ºC.
- Discard supernatant.
- Remove the pellet from the tubes onto a piece of filter paper or weighting paper.
- Crush until pulverize the pellet and no more chunks are seem
- Dry at room temperature until the paste transform into a powder and transfer it to a 1.5ml microcentrifuge tube.
- Store it at –20 ºC.
Procedure to pre-absorb the Ab:
- To pre-absorb, dilute antibody depending of the concentration you want to gets, for example, if you need a high concentration Ab dilute 10 fold, 10uL Ab + 90uL with PBS 1X (1:10); or low concentration Ab dilute 50 fold 10uL Ab+490 uL PBS 1X, (1:500).
- Add acetone powder to 1% (W/V) (example 100uL Ab-solution + 1 mg acetone powder).
- Incubate at room temperature for 30 min.
- Spin at max speed for 5 min.
- Use the supernatant for your assay.
- If necessary, transfer the supernatant to a new tube, and repeat step 2-4.
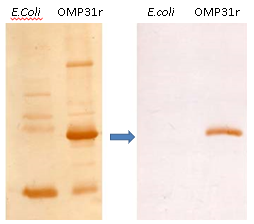
Fig.1 Wertern blot with the recombinan protein of Brucella melitensis (OMP31r) before annd after of the pre-absorption of serum´s antibodies using the powder extract from E. coli.
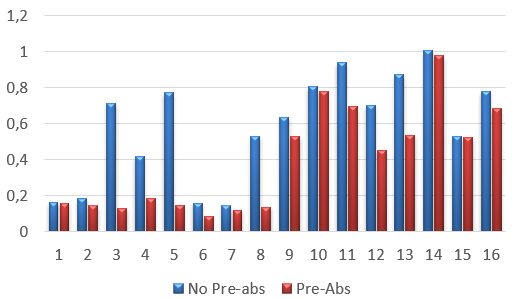
Fig. 2. Elisa analisys. Blue bars represents the bovine´s serum without pre-absortion and red ones represents bovine´s serum after the method of pre-absortion using the powder extract of E. coli. Samples 1-8 corresponds to bovine´s serum negative for brucella sp. and samples 9-16 are bovine´s serum positive for brucella sp.
Discussion:
We used a recombinan protein of Brucella melitensis (OMP31r), cloned in pET28a and expressed in E. coli, but had plenty of problems when the bovine´s seruns regcognize not only the OMP31r but also the proteins from the exprssiion system. The pre-absortion of the non-specific OMP31r antibodies in the bovine´s serums with the method of the powder extract from Escherichia coli was very useful, obtaining good results performing an immunological evaluation of this protein.
In Fig. 1, we see a westernblotting results, where in the left blotting it can observe the recognition of co-eluted E. coli proteins, and in the right one blotting after the pre-absortion method, there is a clean result and OMP31r specific recognition. In Fig. 2, an ELISA was performed, and we demonstrate a cut-off behind 0,2 OD, in bovine´s serum 3, 4, 5 and 8 negative for brucellosis but with immune recognition of E. coli proteins.
This method was also used in the article already published “The Omp31r Recombinant Protein of Brucella melitensis and Immunological Evaluation for its Possible Use for the Diagnosis in Bovine Brucellosis” (Jose-David Rosales et al, 2021). The expression of the Omp31r protein was performed in a prokariota expression system using the pET28a vector.
Acknowledgments
This work was possible thanks to the IDEA foundation, and the Molecular Biology laboratory of the Center of Agriculture and Alimentary Security.
Competing interests: The author declares no conflict of interest.
REFERENCES
- Avrameas S (1969) Coupling of enzymes to proteins with glutaraldehyde. Use of the conjugates for the detection of antigens and antibodies. Immunochemistry 6: 43–52
- Biji T. Kurien, Yaser Dorri, Skyler Dillon, Anil Dsouza, and R. Hal Scofield. (2011). Signal Transduction Immunohistochemistry: Methods and Protocols, Methods in Molecular Biology, vol. 717.
- Engvall E, & Perlmann P. (1971) Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry 8: 871–4
- Mahmood T, Yang PC (2012) Western blot: technique, theory, and trouble shooting. N Am J Med Sci 4:429–434
- Moore, C. (2009) Introduction to westernblotting. AbD serotec. www.abdserotec.com/uploads/WesternBlottingBrochure.pdf.
- Roitt A; Brostoff J. & Male, D. (1997). Inmunología. 4ta. ed. Edit. Harcourt-Brace. Madrid-España
- Shah, K., & Maghsoudlou, P. (2016). Enzyme-linked immunosorbent assay (ELISA): the basics. British Journalof Hospital Medicine, 77(7), C98–C101. doi:10.12968/hmed.2016.77.7.c98
- Thomas S. Hnasko and Robert M. Hnasko. (2012). Robert Hnasko (ed.), ELISA: The Western Blot, Methods and Protocols, Methods in Molecular Biology, vol. 1318,DOI 10.1007/978-1-4939-2742-5_9, © Springer Science+Business Media New York 2015.
- Towbin, H., Staehelin, T. and Gordon, J. (1979) “Electrophoretic transfer of proteins frompolyacrylamide gels to nitrocellulose sheets: procedure and some applications,” Proceedings of the National Academy of Sciences of the United States of America, vol. 76, no.9, pp. 4350–4354.
- Van Weemen BK, & Schuurs AH. (1971) Immunoassay using antigen-enzyme conjugates. FEBS Lett 15: 232–6
- Jose-David Rosales., et al. “The Omp31r Recombinant Protein of Brucella melitensis and Immunological Evaluation for its Possible Use for the Diagnosis in Bovine Brucellosis”. EC Veterinary Science 6.5 (2021): 06-16.
- Taylor, SC. & Posch, A. (2014). The Design of a Quantitative Western Blot Experiment. BioMed Research International. Volume 2014, Article ID 361590.
- Ochoa, A C, Fernández-Gomez, R, Freites, J C, Serrano, A T and Rosales, J(2022). Serum antibodies pre-absorbed with Escherichia coli powder extract represents a reliable method that decreases non-specific responses in immunological tests. Bio-protocol Preprint. bio-protocol.org/prep1987.
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
