Advanced Search
Cell suspension preparation for flow cytometry and flow cytometry analysis
Last updated date: Jul 5, 2022 Views: 589 Forks: 0
Geissmann Lab, Immunology Program, Memorial Sloan Kettering Cancer Center Standard Operating Procedure | |||
| Protocol No.: 027 | Title: Isolation of adipose tissue immune populations and particularly macrophages for analysis by flow cytometry
|
Page 1 of 3 | |
| Effective Date: 02/02/2016 | |||
Revision Number: Version 1 Date & Initials: 02/03/2016 LC | |||
| Originator: Crozet Lucile | Approved by: (sign and date) | ||
Isolation of adipose tissue macrophges for analysis by flow cytometry
Materials
- Reagents
- PBS 1X Without Ca2+ Mg2+
- PBS 1X BSA 0.5%, filtered with 0.22μm stericup
- CaCl2 1mol/L
- Facs buffer: PBS 1X + 0.5% BSA + 2mmol/L EDTA, filtered with 0.22μm stericup
- Collagenase II (sigma, ref: C6885-1g), prepared at 4 mg/mL in PBS + 0.5% BSA + CaCl2 5mmol/L. (For 50mL: 0.2g of collagenase II + 250μL CaCl2 1mol/L + 49.75mL PBS 1X + 0.5% BSA)
- Materials
- 6-well plate (Falcon, ref: 353046)
- 96-well plate (Thermo scientific, ref: 163320)
- 50mL Falcon tubes
- 100 μm strainers (Falcon, ref 352360)
- 70 μm strainers (Falcon, ref 352350)
- 10mL pipettes
- 37°C bug incubator, or any other incubator with shaking option
- Antibodies – on page 2 -
Procedure
Once the mouse is dead, collect the desired fat pads (for our experiments: mWAT, eWAT, iWAT, iBAT) see reference: Orr et al below – page 3 -
1. Adipose tissues are collected in 2mL of cold PBS, in a 6-well plate on ice. NO more than 1.2g of tissue per well
2. Mince the fat tissues into small pieces and transfer into a clean 50mL falcon tube
3. Wash the wells of the 6-well plate with 1mL PBS and then add to the 2mL already in the 50mL falcon.
4. Add 3mL of Collagenase II mix per tube (final concentration for the collagenase 2 mg/mL)
Geissmann Lab Standard Operating Procedure | |||
| Protocol No.: 027 | Title: Isolation of adipose tissue immune cells and macrophages for flow cytometry - analysis
|
Page 2 of 3 | |
| Effective Date: 02/02/2016 | |||
Revision Number: 1 Date & Initials: 02/03/2016 LC | |||
| Originator: Crozet Lucile | Approved by: (sign and date) | ||
5. Incubate for 20min on shaker under agitation at 37°C
6. After incubation, add 10mL of ice cold PBS to the falcon tube and pipette up and down several times with a 10mL pipette
7. Filter the cell suspension through a 100μm strainer and then transfer the sample into a new 50 ml falcon tube
8. Spin the sample for 10min at 500g in a swinging-bucket centrifuge
9. Remove supernatant and resuspend the pellet in 50 μL of FACS buffer + FcBlock
10. Transfer the cell suspension into a 96-well plate and add 50μL of antibody mix, incubate for 30min on ice.
11. Following the incubation period spin down the samples for 7min at 320g in a swinging-bucket centrifuge
12. Wash the cell pellet with 200μL of FACS buffer
13. Repeat 3.9 and 3.10 steps
14. You are now done!! Remember to run the samples through a 70μm strainer prior to flow cytometry analysis
Antibody mix proposition
| Antigen | fluorochrome | Clone | Final dilution |
| CD45.2 | APC-Cy7 | 104 | 1/100 |
| CD3 | BV711 | 145-2C11 | 1/200 |
| CD19 | BV711 | 1D3 | 1/200 |
| NKp46 | BV711 | 29A1.4 | 1/200 |
| SiglecF | PE | E50-2440 | 1/200 |
| Ly6G | PE | 1A8 | 1/200 |
| F4/80 | BV605 | BM8 | 1/200 |
| CD11b | PE-Cy7 | M1/70 | 1/400 |
| Tim4 | APC | RMT4-54 | 1/200 |
| CD11c | BV421 | HL3 | 1/100 |
| MHCII | AF700 | M5/114.15.2 | 1/200 |
Geissmann Lab Standard Operating Procedure | |||
| Protocol No.: 027 | Title: Isolation of adipose tissue immune cells and macrophages for flow cytometry - analysis
|
Page 3 of 3 | |
| Effective Date: 02/02/2016 | |||
Revision Number: 1 Date & Initials: 02/03/2016 LC | |||
| Originator: Crozet Lucile | Approved by: (sign and date) | ||
References
- Protocol adapted from Orr, Jeb S., Arion J. Kennedy, et Alyssa H. Hasty. 2013. « Isolation of Adipose Tissue Immune Cells », JOVE no 75 (mai): e50707. doi:10.3791/50707.
- For our experiments: mWAT, eWAT, iWAT, iBAT
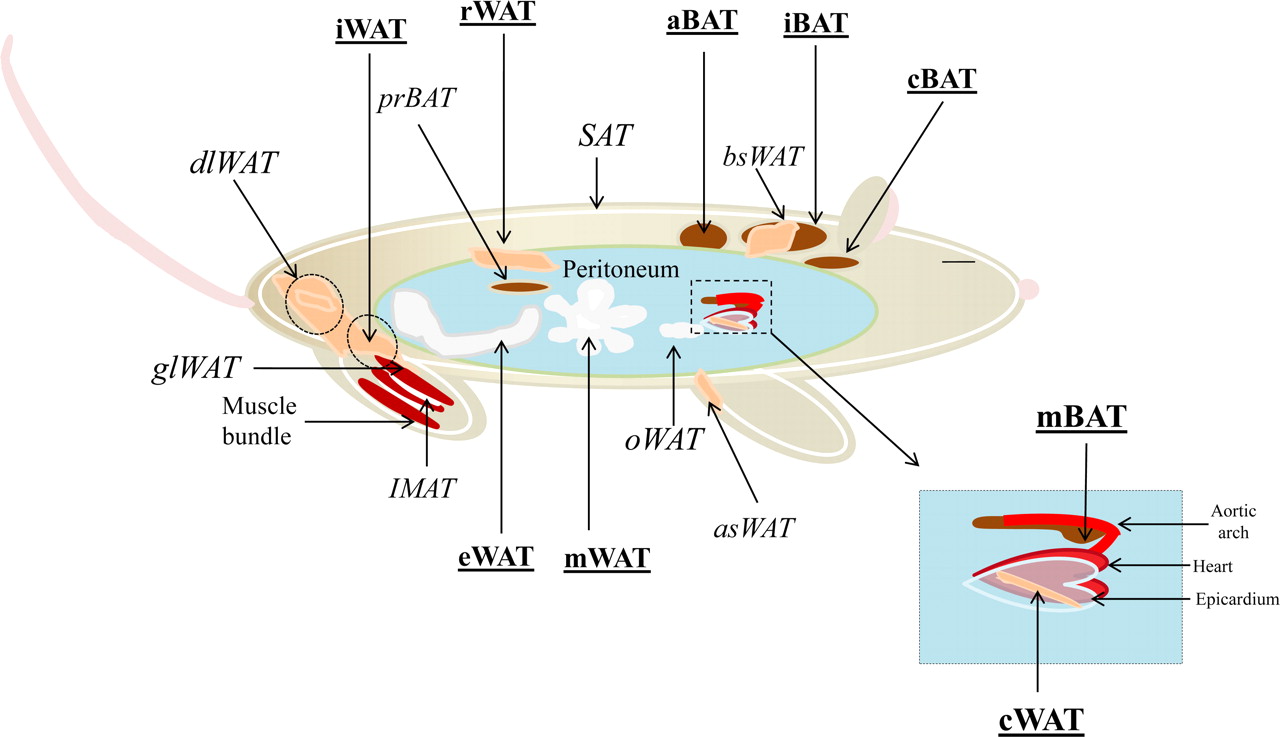
Schema from Waldén, Tomas B., Ida R. Hansen, James A. Timmons, Barbara Cannon, et Jan Nedergaard. 2012. « Recruited vs. Nonrecruited Molecular Signatures of Brown, “brite,” and White Adipose Tissues ». American Journal of Physiology - Endocrinology and Metabolism 302 (1): E19‑E31. doi:10.1152/ajpendo.00249.2011
- Cox, N and Geissmann, F(2022). Cell suspension preparation for flow cytometry and flow cytometry analysis. Bio-protocol Preprint. bio-protocol.org/prep1768.
- Cox, N., Crozet, L., Holtman, I. R., Loyher, P., Lazarov, T., White, J. B., Mass, E., Stanley, E. R., Elemento, O., Glass, C. K. and Geissmann, F.(2021). Diet-regulated production of PDGFcc by macrophages controls energy storage. Science 373(6550). DOI: 10.1126/science.abe9383
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
