Advanced Search
Animal experiments
Last updated date: May 3, 2021 Views: 918 Forks: 0
Preparation of single cells from tumors for immune phenotyping using flow cytometry
Hyungsoo Kim and Ze'ev A. Ronai
In our study, we assessed the abundance (number per gram of tumor) of immune cells in tumor infiltrating lymphocytes. Here, we describe the details of the experimental steps to prepare single-cell suspension from collected tumors for immune phenotyping using flow cytometry. Because of tumor tissue rigidity, two different dissociation methods were used for the B16F10 and YUMMER1.7 tumors.
Procedure
- Murine melanoma tumors (B16F10, YUMMER1.7) were collected on day12 or day17 after tumor cell engraftment.
- Tumor volume and weight were assessed.
- The collected tumors were dissected, as noted below, into single cells.
- B16F10 tumors; mechanical dissociation method
- Place the tumor onto cell strainer
- Chop tumors using scissors
- Mince chopped tumor tissue using a syringe plunger while dripping 10ml Ice-cold HBSS
- YUMMER1.7: enzymatic dissociation method
- Place the tumor on a 35mm sterile dish
- Chop tumors using scissors
- Transfer tumors to a 50ml tube with10ml of Collagenase D solution
- Incubate the tube with gentle mixing for 1hr at 30C
- Chop tumor tissue more by vigorous pipetting
- Mince tumor tissue using a syringe plunger and cell strainer
- B16F10 tumors; mechanical dissociation method
- Centrifuge collected cells (500g, 5min)
- Wash cells twice with ice-cold HBSS
- Resuspend cells with RBC lysing buffer and place on ice for 5 min
- Add HBSS and wash cells twice (500g, 5min)
- Resuspend cells with complete RPMI1640 media after the final wash
- Count cell number
- Cells (2 x 106) are transferred to a 96-well plate (round-bottomed) and centrifuged (500g, 5 min)
- Wash cells twice with FACS staining buffer (500g, 5min)
- Cells are stained with 50 ml of the indicated antibodies to surface markers in FACS staining buffer on ice for 20 min
- Wash cells twice with FACS staining buffer (500g, 5min)
- Cells are fixed with 1% formaldehyde in PBS for 15 min
- Wash cells twice with FACS staining buffer (500g, 5min) and
- Resuspend cells with FACS staining buffer and analyzed cells with flow cytometry
- To assess regulatory T cells, cells were stained with surface markers as described above (step 13), using Foxp3 antibody (Foxp3/transcription factor staining buffer set).
- To assess intracellular cytokine production in infiltrated CD4 and CD8 T cells, cells wer stimulated with the activation cocktail (PMA+Ionomycin+Bredfeldin A) for 16hr. Then, cells were stained with indicated antibody cocktails as described above (step 13). Intracellular cytokines were stained using cytofix/cytoperm solution kit.
- Expected cell number to get from a gram of tumor (Data from Fig. 3A and B in reference 2).
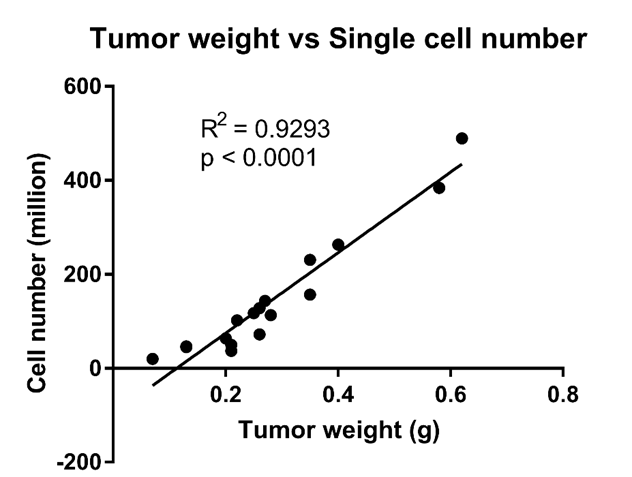
Equation: Y = 857.3*X - 96.67
Estimated number of single cells in a gram of tumor: ~700 million
CD45 positive cells are 5 ~ 30 % of single cell suspension
Reagents and materials
- Falcon Cell strainer, 70 mM Nylon (Falcon, REF352350)
- 35 mm dish (Falcon, 353003)
- Collagenase D solution: 0.1% (w/v) collagenase D (Roche, REF11088882001), 0.5% (w/v) BSA (Sigma, A9647), 100 mg/ml DNase I (Sigma, DN25) in phosphage buffered saline (PBS)
- Hank's balanced salt solution (HBSS)
- RPMI1640 complete medium: RPMI1640, 10% (v/v) fetal bovine serum, 1x penicillin/streptomycin
- Red Blood Cell Lysing Buffer Hybri-Max (Sigma, R7757)
- Foxp3/transcription factor staining buffer set (Invitrogen, 00-5523-00)
- Cytofix/Cytoperm (BD, 554714)
How to cite: Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
1. Kim, H., and Ronai, Z.A. (2020). Cell culture and treatment of melanoma cells to assess PRMT5 effect on DNA/RNA-induced STING activation and type I IFN (IFNI) response. Bio-protocol.
2. Kim, H., Kim, H., Feng, Y., Li, Y., Tamiya, H., Tocci, S., and Ronai, Z.A. (2020). PRMT5 control of cGAS/STING and NLRC5 pathways defines melanoma response to antitumor immunity. Sci Transl Med. DOI: 10.1126/scitranslmed.aaz5683
Copyright: Content may be subjected to copyright.
- Kim, H and Ronai, Z(2021). Animal experiments. Bio-protocol Preprint. bio-protocol.org/prep1052.
- Kim, H., Kim, H., Feng, Y., Li, Y., Tamiya, H., Tocci, S. and Ronai, Z. A.(2020). PRMT5 control of cGAS/STING and NLRC5 pathways defines melanoma response to antitumor immunity . Science Translational Medicine 12(551). DOI: 10.1126/scitranslmed.aaz5683
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
