- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Gastric Aspiration Models
Published: Vol 3, Iss 22, Nov 20, 2013 DOI: 10.21769/BioProtoc.968 Views: 20534
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
A Participant-Derived Xenograft Mouse Model to Decode Autologous Mechanisms of HIV Control and Evaluate Immunotherapies
Emma Falling Iversen [...] R. Brad Jones
Apr 5, 2025 2487 Views
Analysis of Vascular Permeability by a Modified Miles Assay
Hilda Vargas-Robles [...] Michael Schnoor
Apr 5, 2025 2499 Views
PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3923 Views
Abstract
The procedures described below are for producing gastric aspiration pneumonitis in mice with alterations for rats and rabbits described parenthetically. We use 4 different injury vehicles delivered intratracheally to investigate the inflammatory responses to gastric aspiration:
1) Normal saline (NS) as the injury vehicle control
2) NS + HCl, pH = 1.25 (acid)
3) NS + gastric particles, pH ≈ 5.3 (part.)
4) NS + gastric particles + HCl, pH = 1.25 (acid + part.)
The volume, pH, and gastric particle concentration all affect the resulting lung injury. In mice, we generally use an injury volume of 3.6 ml/kg (rat: 1.2 ml/kg, rabbit: 2.4 ml/kg), an injury pH (for the acid-containing vehicles) of 1.25, and a gastric particulate concentration (in the particulate-containing vehicles) of 10 mg/ml (rat: 40 mg/ml). In our hands this results in a maximal, non-lethal lung injury with ≤ 10% mortality for the most injurious vehicle (i.e., acid + part.) The maximum tolerable particulate concentration needs to be determined empirically for any new strains to be used, especially in genetically-altered mice, because an altered inflammatory response may have detrimental affects on mortality.
We have extensive experience utilizing these procedures in the outbred strain, CD-1, as well as many genetically-altered inbred stains on the C57BL/6 background. Choice of strain should be carefully considered, especially in terms of strain-specific immune bias, to assure proper data interpretation. The size of the mouse should be ≥ 20 g at the time of injury. Smaller mice can be attempted, if necessary, but the surgical manipulation becomes increasingly more difficult and the surgery survival rate decreases substantially. There are no size or strain constraints for rat and rabbit models, but we generally use Long-Evans rats at 250-300 g and New Zealand White rats at ≈ 2 kg at the time of initial injury.
Materials and Reagents
- Isoflurane
- Topical antiseptic microbicide prep solution (e.g. Medline Industries, catalog number: MDS093906 )
- 0.5% bupivicaine
- Hank’s Balanced Salt Solution (HBSS) with Ca2+, Mg2+ (e.g. Life Technologies, catalog number: 14025 )
- HBSS without Ca2+, Mg2+ (e.g. Life Technologies, catalog number: 14175 )
- Liquid nitrogen
- Bovine serum albumin (BSA)
- Cytospin filter cards (e.g. Thermo Fisher Scientific, catalog number: 5991022 )
- Diff Quik solutions kit (Fisher Scientific, catalog number: NC9943455 )
- Cytoseal 60 (VWR International, catalog number: 48212-154 )
- 100x protease inhibitor cocktail (e.g. Calbiochem®, catalog number: 80053-844 )
- Bupivacaine
- 100 mg/ml mouse (rat) gastric particles (see Recipes)
- Acid injury solution (acid) (see Recipes)
- Gastric particles injury solution (part.) (see Recipes)
- Acid + particles injury solution (acid + part.) (see Recipes)
- Phosphate buffered saline (PBS), pH 7.2 (see Recipes)
- Ammonium chloride lysis buffer (see Recipes)
- Lung homogenate buffer (see Recipes)
- 50 mM potassium phosphate buffer, pH = 6.0 (see Recipes)
- MPO homogenate buffer (see Recipes)
Note: All salts and other chemicals are from Sigma-Aldrich unless otherwise noted (however, any source for such chemicals is probably okay to use).
Equipment
- 1-0 braided silk material, bulk spool (e.g. Look catalog number: MBJF210)
- 12 mm x 75 mm x 1 mm microscope slides
- 2 x 2 gauze sponges, 8-ply (VWR International, catalog number: 82004-740 )
- Sterile 4 x 4 gauze sponges, 8-ply (VWR International, catalog number: 82004-742 )
- 6-0 monofilament polypropylene suture with P-13 cutting needle (e.g. Syneture, catalog number: SP-5695 )
- 60° incline dissection board (homemade out of plexiglass)
- Syringes (0.5, 1, 3, 5, 20 cc (cubic centimeter))
- Needles (14, 20, 22, 26, 29 gauge)
- Tracheal cannula (23 gauge x 1/2” stainless steel tubing adapter) (Becton, Dickinson and Company, catalog number: 408213 )
- 3” curved serrated forceps (2)
- 3” curved tissue (“toothed”) forceps
- 1.8 ml microfuge tubes
- 12 x 75 mm polystyrene tubes
- 22 x 22 mm #1.5 coverslips
- 4” curved micro dissecting scissors
- 37 °C water bath
- Disposable skin stapler (e.g. 3M, model: DS-25 )
- Hemocytometer or Coulter counter (e.g. Beckman Coulter, model: MultiSizer III )
- Cytocentrifuge with cytospin funnels (e.g. Shandon CytoSpin®)
- Tissue homogenizer (e.g. BrinkmannTMPolytronTM, model: PT2000 )
- Probe sonicator (e.g. Branson Sonifier®, model: 450 )
Procedure
- "Injury" procedure
- Fill a 0.5 cc syringe with 22 gauge needle with 0.2 ml air “chaser” then “injury” solution (rat: 1 cc syringe with 14 gauge needle with 0.5 ml air, rabbit: 5 cc syringe with 14 gauge needle with 2 ml air).
- Induce anesthesia in a chamber with 3-4% isoflurane in oxygen delivered at 1-2 L/min (rabbit: 30 mg/kg ketamine intramuscularly prior to 2% isoflurane) (Figures 1 and 2). Suspend mouse by its front teeth with a 1-0 suture strand on a 60° incline dissection board and maintain anesthesia with 2-2.5% isoflurane administration with nose cone.
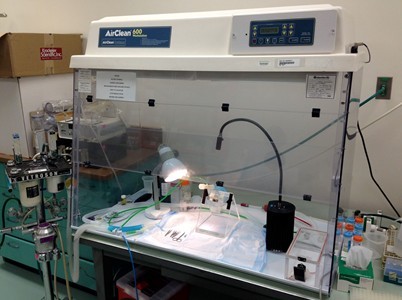
Figure 1. Lab bench set-up for surgical procedures. Procedures are performed in a fume hood with charcoal filtering for anesthetic gas scavenging that contains: small animal anesthetic gas exposure chamber (front right), lamps, and the plexiglass 60° incline dissection board (middle of hood). On the left of the picture, on a floor stand outside the hood, is the anesthetic vaporizer for delivering the isoflurane vapor.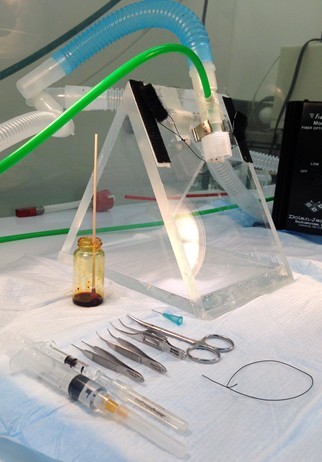
Figure 2. Instruments used for aspiration injury procedure. Plexiglass 60° incline dissection board is shown with nose cone setup to deliver isoflurane during the procedure. The green hose delivers the isoflurane in 100% oxygen to an inner nose cone, whereas the larger bore blue hose provides vacuum for scavenging the anesthetic through the outer nose cone. Also shown are (left to right): a 5 cc syringe with 26 gauge needle containing 1.5 ml normal saline for the subcutaneous fluid injection, a 0.5 cc syringe with 22 gauge needle containing the injury solution and 0.2 ml air “chaser”, 3 curved dissecting forceps (2 serrated and 1 “toothed”), curved dissecting scissors, a vial of antiseptic antimicrobial solution with cotton-tipped applicator, and a 6” strand of 1-0 braided silk suture material. Missing from picture: disposable skin stapler. - Shave ventral neck, paint with topical antiseptic prep solution and remove excess with gauze.
- Infiltrate future incision site with 100 μl 0.5% bupivacaine (provides local post-operative analgesia).
- Cut a 2 cm longitudinal incision in the skin with scissors (Figure 3).
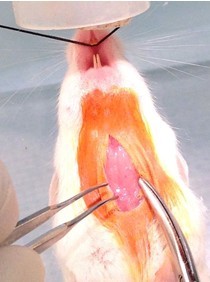
Figure 3. Neck incision. After suspending the mouse by its front incisors with a suture such that its nose is within the inner isoflurane nose cone (step A2), prepping the surgical site (steps A3 & A4), a 2 cm longitudinal incision is made with scissors and tissue forceps (step A5). - Expose trachea by blunt dissection with 2 curved toothless forceps (Figures 4, 5 and 6).
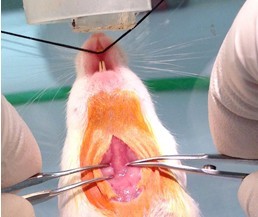
Figure 4. Tracheal exposure by blunt dissection. The fascia membrane is teased away with curved serrated forceps and the salivary glands are pulled to the side in order to expose the trachea (faint white vertical between the forceps) that is surrounded by paratracheal musculature (step A6).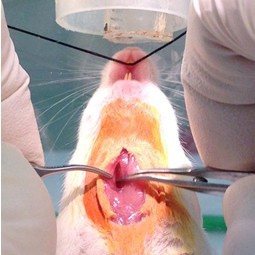
Figure 5. Tracheal exposure by blunt dissection, continued. Grasp one side of the paratracheal musculature and pull it to the side while rubbing along the muscle longitudinally. This will result in a separation of the muscle fiber bundles and allow the muscle to be reflected to the side (step A6). Notice the inner and outer nose cone configuration for delivering the anesthetic and scavenging. Mouse’s nose has been positioned lower than usual for clarity.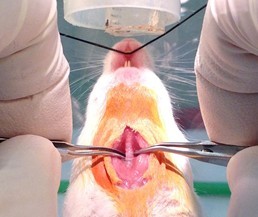
Figure 6. Tracheal exposure by blunt dissection, continued. Use the two curved serrated forceps to reflect the paratracheal musculature, fully exposing the trachea (step A6). - Work curved forceps under trachea (Figure 7). and use to pull a 6" strand of 1-0 silk suture material through (Figure 8).
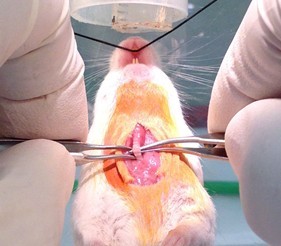
Figure 7. Work curved serrated forceps under trachea (step A7)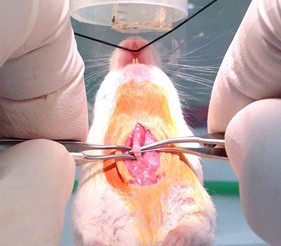
Figure 8. Use forceps to place suture under trachea (step A7) - Discontinue isoflurane administration by removing nose cone. Before instilling injury vehicle by the steps below, allow mouse’s plane of anesthesia to rise until it just starts reacting to a forceps toe pinch then instigate instillation steps quickly. Especially for the acid-containing injury vehicles, if the plane of anesthesia is too deep the animal will not begin to breathe spontaneously after the injury vehicle is instilled.
- Lift trachea up with suture to facilitate inserting injury syringe needle into the trachea (2-3 cartilage rings below the larynx) with bevel facing surgeon (advance until bevel of needle is just past the trachea insertion point) (Figure 9). It is important that the needle be as parallel with the trachea as possible, otherwise, the needle can easily pierce the other side of the trachea. This will result in the injury vehicle not being injected into the lungs and a high probability that the animal will not survive.
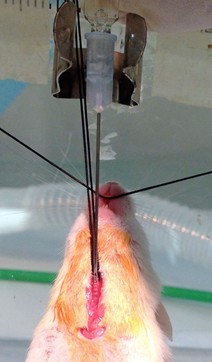
Figure 9. Use suture to facilitate injecting injury solution into trachea. Remove nose cone apparatus from incline board to provide unrestricted access to upper section of trachea. Lift trachea up with suture to facilitate inserting injury syringe needle into the trachea (2-3 cartilage rings below the larynx). Notice bevel of needle facing surgeon. Advance needle until bevel of needle is just past the trachea insertion point (step A9). It is important that the needle be as parallel with the trachea as possible, otherwise, the needle can easily pierce the other side of the trachea. This will result in the injury vehicle not being injected into the lungs and a high probability that the animal will not survive. While assistant squeezes rib cage (to expel most of vital capacity), quickly instill syringe contents. Release chest just before the injection begins. This maneuver, along with the air “chaser” assures deposition of the injury vehicle into the distal lung (step A10). - While assistant squeezes rib cage (to expel most of vital capacity), quickly instill syringe contents. Release chest just before the injection begins. This maneuver, along with the air “chaser” assures deposition of the injury vehicle into the distal lung.
- Leave animal on incline board until breathing commences and close incision with 2 staples (for rats and rabbits: trachea needle wound needs to be repaired with one 6-0 suture) (Figure 10).
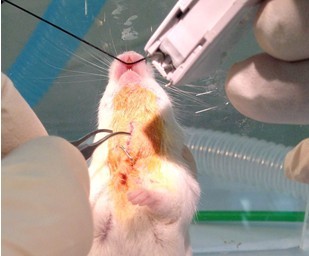
Figure 10. Close neck skin incision with surgical staples (step A11) - Inject 1 ml (rat: 10 ml, rabbit: 20 ml) sterile NS subcutaneously into the scruff of the neck for fluid resuscitation. There is virtually no fluid loss during the procedure, but the animal will not drink for a while after the procedure and can dehydrate (Figure 11).
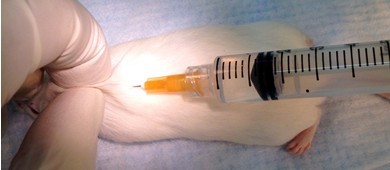
Figure 11. Fluid resuscitation. Inject 1 ml sterile NS subcutaneously into the scruff of the neck using a syringe with 26 gauge needle for fluid resuscitation (step A12). - Put into heated chamber (37 °C) perfused with 100% O2 until ambulatory (Figure 12).

Figure 12. Recovery chamber. Place mouse in recovery chamber that is continually perfused with 100% O2 (supplied by green hose) and maintained at 37 °C with heat lamp and temperature controller (notice thermistor probe in left chamber) until the mouse is ambulatory (step A13).
- Fill a 0.5 cc syringe with 22 gauge needle with 0.2 ml air “chaser” then “injury” solution (rat: 1 cc syringe with 14 gauge needle with 0.5 ml air, rabbit: 5 cc syringe with 14 gauge needle with 2 ml air).
- Harvest procedure (Figure 13)
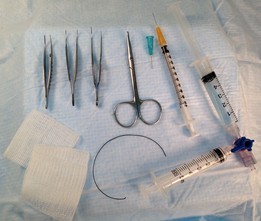
Figure 13. Instruments and materials used for harvest. From left to right: two serrated dissecting forceps, tissue (“toothed”) forceps, dissecting scissors, 23 gauge stainless steel blue-hubbed cannula, 1 cc blood collection syringe with 26 gauge needle, bronchoalveolar lavage apparatus (top syringe filled with 5 ml HBSS without Ca2+, Mg2+, left syringe is for fluid collection), two 2 x 2 gauze sponges (bottom left) for retracting abdominal organs, and 4” strand of 1-0 braided silk suture (bottom, middle). Missing from picture: 5 cc syringe with 26 gauge needle containing 5 ml HBSS with Ca2+, Mg2+ for flushing pulmonary vasculature.- Anesthetize mouse with isoflurane in 100% O2.
- When mouse is unresponsive, place on a dissecting board in supine position and continue isoflurane administration with a nose cone.
- Using forceps and scissors, make a longitudinal incision up the abdomen to the xyphoid.
- Make a latitudinal incision across the belly (Figure 14) and expose vena cava and abdominal aorta using gauze sponges to reflect abdominal organs (Figure 15).
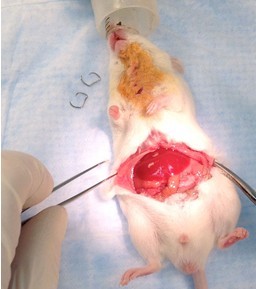
Figure 14. Abdominal incision for collecting blood. Using forceps and scissors, make a longitudinal incision up the abdomen to the xyphoid and a latitudinal incision across the belly (steps B3 & B4).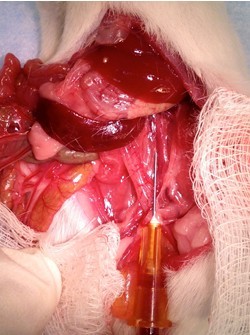
Figure 15. Collect blood. Expose vena cava and abdominal aorta using gauze sponges to reflect abdominal organs and collect blood using a 1 cc syringe with 26 gauge needle from either vessel (steps B4 & B5). - Harvest blood by (used to assess systemic inflammatory responses (e.g. serum or plasma cytokine levels)):
- Collect blood from abdominal aorta using 27 gauge needle on a 1 cc syringe (if plasma is to be isolated syringe must contain appropriate anticoagulant) (Figure 15).
- The vena cava can be used to collect blood, but generally, not as much blood can be collected as from the aorta (Figure 15).
- Dispense blood into 1.8 ml μ-fuge tube. Until blood can be processed, keep on ice if plasma will be prepared, or keep at room temperature if serum will be prepared. Process blood, as described below.
- Transect vena cava/abdominal aorta to euthanize by exsanquination.
- Collect blood from abdominal aorta using 27 gauge needle on a 1 cc syringe (if plasma is to be isolated syringe must contain appropriate anticoagulant) (Figure 15).
- Flush pulmonary vasculature by (performed to remove the blood from the pulmonary circulation so measurements of compounds in the processed lung tissue reflect only values from the pulmonary compartment that do not include a systemic contribution):
- Grasp xyphoid process with forceps, puncture diaphragm with tips of scissors to deflate lungs and carefully cut away ventral aspect of diaphragm (Figure 16).
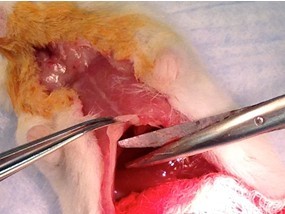
Figure 16. Cut-away diaphragm. Grasp xyphoid process with forceps, puncture diaphragm with tips of scissors to deflate lungs and carefully cut away ventral aspect of diaphragm (step B6a). - Continue the longitudinal incision up the sternum and through the neck.
- Cut away sternum to expose lungs (be careful not to puncture lungs) (Figure 17).
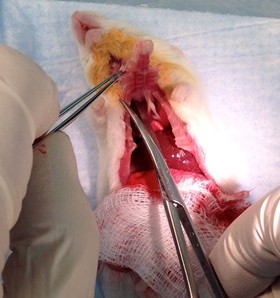
Figure 17. Perform sternotomy. Continue the longitudinal incision up the sternum and through the neck. Cut away sternum to expose lungs (be careful not to puncture lungs) (steps B6b-c). - Grasp apex of heart with toothless forceps and inject 5 ml (rat: 20 ml) 1x HBSS with Ca2+, Mg2+ (at 37 °C) into right ventricle (it will be on the left side of the heart since the animal is on its back) with 5 cc (rat: 20 cc) syringe + 26 gauge needle (Figure 18). The rate of injection should be fast enough to keep the heart “inflated”, but not so fast as to force the fluid out through the injection site. The calcium in the buffer, and its being at 37 °C, aids the heart to keep beating to facilitate flushing the pulmonary vasculature.
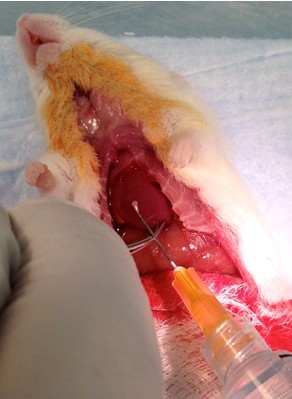
Figure 18. Flush pulmonary vasculature. Grasp apex of heart with serrated forceps (tissue forceps have a tendency to tear the heart tissue) and inject 5 ml 1x HBSS with Ca2+, Mg2+ (at 37 °C) into right ventricle (it is on the left side of the heart in this picture since the animal is on its back) with 5 cc syringe + 26 gauge needle. The rate of injection should be fast enough to keep the heart “inflated”, but not so fast as to force the fluid out through the injection site (step B6d).
- Grasp xyphoid process with forceps, puncture diaphragm with tips of scissors to deflate lungs and carefully cut away ventral aspect of diaphragm (Figure 16).
- Perform bronchoalveolar lavage (BAL) by (performed to collect cells and compounds secreted into the airspaces (i.e. alveoli and bronchial lumen) that can be assessed to determine the degree of pulmonary inflammation (i.e. neutrophil influx, various inflammatory cytokine levels (i.e. tumor necrosis factor-alpha (TNFα), interleukin-1beta (IL-1β), IL-6, macrophage inflammatory protein-2 (MIP-2), monocytic chemotactic protein-1 (MCP-1)), and lung injury (i.e. total protein or albumin concentration):
- Expose trachea and work tip of curved forceps under it to pull a 4" strand of 1-0 silk suture through.
- Insert a 23 gauge x 1/2” (rat: 14 gauge, rabbit: 3 mm ID, 4.5 mm OD) stainless steel cannula (rabbit: polyethylene catheter) into the trachea and secure with suture (Figures 19 & 20).
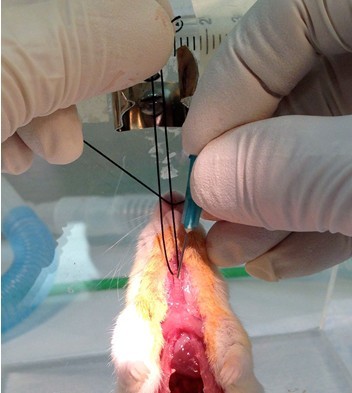
Figure 19. Insert tracheal cannula. Expose trachea and work tip of curved forceps under it to pull a 4" strand of 1-0 braided silk through, as described in the injury procedure (steps A6, A7 & A9). Use a 20 gauge needle to make a hole in the upper trachea and insert a 23 gauge 1/2” stainless steel cannula into the hole.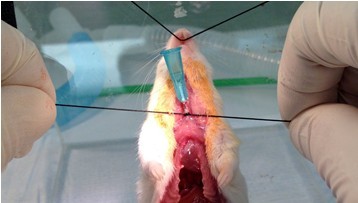
Figure 20. Secure tracheal cannula. Secure cannula in the trachea with the suture (steps B7a-b). Be sure the suture is tight and 2-3 mm below the insertion site so a good seal is made. Tip of cannula should be at least 3 mm before the carina (bifurcation of the trachea). If the 1/2” cannula is inserted just below the larynx, as depicted in the figure, the tip of the cannula will be in the proper position. - Connect lavage apparatus (2, 5 cc syringes connected to a 4-way stopcock) to catheter and slowly, lavage lungs with 5 x 1 ml (rat: 10 ml, rabbit: 50 ml) 1x HBSS without Ca2+, Mg2+ (at 37 °C) (Figures 21 & 22). The lack of calcium in the buffer facilitates harvesting of the airway cells by diminishing adherence.
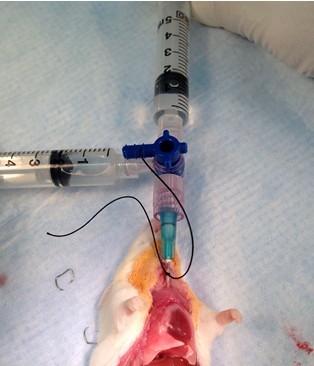
Figure 21. Perform bronchoalveolar lavage (BAL)-injection. Connect lavage apparatus to cannula and slowly inject 1 ml of 1x HBSS with Ca2+, Mg2+ to insulflate lungs (step B7c).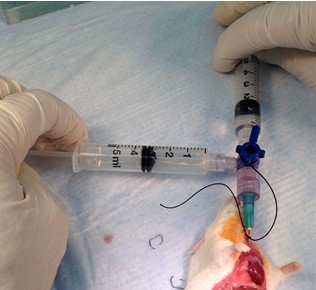
Figure 22. Perform BAL-collection. Switch stopcock valve and slowly collect the injected lavage fluid. Repeat the injection and collection process for a total of 5 lung washings (step B7c). - Dispense collected BAL fluid (BALF) into appropriately sized tube, record recovered volume (by weighing), and put on ice until BALF processing, described below.
- Expose trachea and work tip of curved forceps under it to pull a 4" strand of 1-0 silk suture through.
- Harvest lungs by (performed to assess the pulmonary inflammatory state distinct from the airspaces, i.e., parenchymal and interstitial tissue):
- Excise lungs, heart, and thymus, en bloc, by the trachea (Figure 23).
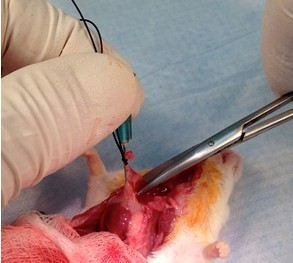
Figure 23. Harvest lungs, heart, and thymus, en bloc. Be sure clavicle has been cut away. Grasp cannula, trachea, and suture with fingers and cut trachea by larynx. While pulling up and out, work scissors dorsally under lungs and spread to disengage tissue connecting lungs and heart to thoracic cavity. Some cutting of connective tissue will be needed, as well as the esophagus and vessels to successfully remove the lungs, heart, and thymus, en bloc (step B8a). - Remove lung lobes by cutting lobe’s main stem bronchus at hilum (Figures 24 & 25), place in 2 ml cryovial, and flash freeze in liquid N2.
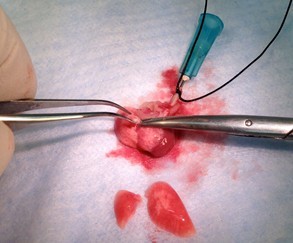
Figure 24. Harvest lung lobes. Collect individual lobes by using scissors and forceps to cut main stem bronchi at the lobe’s hilum (step B8b).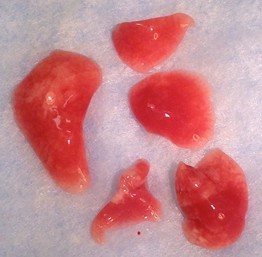
Figure 25. Mouse lung lobes. Clockwise from left: left lung lobe, right lung-cranial lobe, right lung-middle lobe, right lung caudal lobe, right lung-post-caval lobe. - Store at -80 °C until appropriate processing can be performed, described below.
- Excise lungs, heart, and thymus, en bloc, by the trachea (Figure 23).
- Anesthetize mouse with isoflurane in 100% O2.
- Blood processing
- Plasma
- Centrifuge at 2,000 x g for 4 min at 4 °C.
- Remove plasma with a pipet and dispense into 1.8 ml microfuge tube.
- Store at -80 °C.
- Centrifuge at 2,000 x g for 4 min at 4 °C.
- Serum
- Incubate at RT for 30 min then overnight at 4 °C to allow clot to contract.
- Centrifuge at 5,000 x g for 15 min at 4 °C.
- Remove serum with a pipet and dispense into 1.8 ml microfuge tube.
- Store at -80 °C.
- Incubate at RT for 30 min then overnight at 4 °C to allow clot to contract.
- Plasma
- BALF processing (keep samples on ice)
- Spin BALF at 1,500 x g for 5 min at 4 °C.
- Without disturbing pellet, collect supernatant with pipet (leave 50-100 μl residual volume of uncollected supernatant to prevent sucking-up cells) and dispense into equal volume aliquots in 1.8 ml microfuge tubes (the number and volume of the aliquots will depend on the assays that will be performed and should be designed to limit the amount of freeze/thaw cycles). Store at -80 °C.
- Resuspend pellet in the residual volume.
- Add 1 ml ammonium chloride lysis buffer (37 °C) and incubate for 2 min to lyse red blood cells.
- Layer entire volume on 4 ml ice cold 2% BSA in PBS in a 12 x 75 mm PS tube.
- Spin at 150 x g for 15 min at 4 °C.
- Aspirate supernatant (containing cell debris and lysed red blood cells), resuspend pellet in residual volume, and add 1 ml PBS. Removing the cell debris and lysed red blood cells by centrifuging the cells through 2% BSA makes counting the cells much easier and more accurate.
- Count cells on hemocytometer or Coulter counter.
- Prepare cytospin slide for microscopic viewing by:
- Add 5 x 104 white blood cells to 2% BSA in PBS in a cytospin funnel (300 μl total volume) attached to a microscope slide and filter card and spin at 28 x g (500 rpm) for 5 min.
- Remove slides and allow to air dry (IMPORTANT: stain immediately when dry).
- Stain with Diff Quik by:
- Dip slides 5 x 1 sec in fixative, Solution I, Solution II (Diff-Quik kit), rinse with H2OMQ & air dry.
- Mount slide with small drop of "Cytoseal 60" on cell spot and putting a 22 x 22 mm #1.5 coverslip on it (be sure no bubbles) and allow to air dry.
- Determine percentage of macrophages, neutrophils, lymphocytes, and eosinophils by light microscopy.
- Dip slides 5 x 1 sec in fixative, Solution I, Solution II (Diff-Quik kit), rinse with H2OMQ & air dry.
- Add 5 x 104 white blood cells to 2% BSA in PBS in a cytospin funnel (300 μl total volume) attached to a microscope slide and filter card and spin at 28 x g (500 rpm) for 5 min.
- Spin BALF at 1,500 x g for 5 min at 4 °C.
- Lung processing (Lungs can be processed in a number of different ways depending on what cellular compartment (e.g. whole lung homogenate, nuclear fraction, cytosolic fraction, etc.) and molecular species (e.g. protein, mRNA, etc.) are to be assayed and what assay techniques will be utilized (e.g. ELISA, Western blot, PCR, etc.). This procedure produces a lung homogenate supernatant that can be assayed for various cytokine protein levels by ELISA, as well as an extraction of myeloperoxidase from the lung homogenate pellet that can be assayed for enzymatic activity as a surrogate marker of neutrophil infiltration)
- Thaw frozen lungs and transfer to a round bottom centrifuge capable of withstanding 40,000 x g. Weigh tube before adding lungs then weigh the tube + lungs.
- Add enough cytokine homogenate buffer to bring the weight of the lungs + buffer to 3 g (rat: 10 g).
- Homogenize tissue with Polytron homogenizer with tube on ice to prevent heating of sample. Pick out any tissue caught in homogenizer blades with forceps. Rinse homogenizer with 70% EtOH and then sterile H2O between samples.
- Centrifuge homogenate at 40,000 x g for 10 min at 4 °C.
- Dispense supernatant into equal volume aliquots in 1.8 ml microfuge tubes and store at -80 °C.
- Add 2 ml MPO buffer to pellet and resuspend by vortexing.
- Store at -80 °C (in same tube) until MPO extraction can be performed.
- Thaw frozen lungs and transfer to a round bottom centrifuge capable of withstanding 40,000 x g. Weigh tube before adding lungs then weigh the tube + lungs.
- MPO extraction procedure
- Quick thaw sample in 37 °C water bath.
- Sonicate for 1 min, on ice to prevent sample heating, on maximum output at 50% duty cycle.
- Incubate at 55 °C for 2 h.
- Centrifuge at 40,000 x g for 15 min at 4 °C.
- Dispense into 0.5 ml aliquots in 1.8 ml microfuge tubes and store at -80 °C until MPO activity can be assessed
- Quick thaw sample in 37 °C water bath.
Recipes
- 100 mg/ml mouse (rat) gastric particles
- Harvest stomach from freshly necropsied mouse (rat) first thing in the morning and put in 50 ml tube on ice. Harvesting early in the morning assures a full stomach.
- In laminar flow hood
- Put stomach in Petri dish, cut longitudinally with scissors.
- Place stomach contents in a clean, 50 ml centrifuge tube.
- Add 10 ml sterile normal saline (NS) and vortex vigorously for 15 sec.
- Pour particle suspension through 8 layers of sterile gauze into a beaker.
- Use an additional 10 ml sterile NS to transfer residual particles in tube to gauze.
- Wring gauze to collect absorbed fluid.
- Transfer filtrate to a clean, 50 ml centrifuge tube.
- Put stomach in Petri dish, cut longitudinally with scissors.
- Spin filtrate at 5,000 x g for 5 min at 4 °C.
- Discard supernatant, and resuspend in 25 ml, sterile NS.
- Repeat steps 1c & 1d.
- Transfer particle suspension to an Erlenmeyer flask and autoclave 121 °C, 121 psi for 30 min (use an additional 25 ml, sterile NS to transfer residual particles).
- Transfer cooled, sterile particle suspension to a pre-weighed 50 ml centrifuge tube.
- Spin filtrate at 5,000 x g for 5 min at 4 °C.
- Decant supernatant and stand tube inverted on KIMWipe to remove all liquid.
- Re-weigh tube+particle pellet to determine particle wet weight.
- Add sterile NS to 100 mg/ml (particle wet weight/total volume).
- Store at 4 °C for up to 3 weeks until used for injury.
- Harvest stomach from freshly necropsied mouse (rat) first thing in the morning and put in 50 ml tube on ice. Harvesting early in the morning assures a full stomach.
- Acid injury solution (acid)
Adjust pH = 1.25 with sterile 1 N HClNormal saline (NS), sterile 9.44 ml 1 N HCl, sterile 562 μl
Make fresh - Gastric particles injury solution (part.)
Make fresh.Normal saline (NS), sterile 9 ml 100 mg/ml gastric particles in NS 1 ml (10 mg/ml) - Acid + particles injury solution (acid + part.)
Adjust pH = 1.25 with sterile 1 N HClNormal saline (NS), sterile 8.44 ml 1 N HCl, sterile 562 μl 100 mg/ml gastric particles in NS 1 ml (10 mg/ml)
Make fresh - Phosphate buffered saline (PBS), pH 7.2
H2OMQ to 1 LNaCl (58.44) 8 g (137 mM) Na2HPO4 (141.96) 1.15 g (8.1 mM) KCl (74.56) 200 mg (2.7 mM) KH2PO4 (136.09) 200 mg (1.5 mM)
Adjust pH = 7.2, filter sterilize
Stored at room temperature - Ammonium chloride lysis buffer
Mix powders together, thoroughlyNH4Cl (53.49) 4.13 g (154 mM) KHCO3 (100.1) 500 mg (10 mM) EDTA, tetrasodium salt (380.2) 18.5 mg (0.1 mM)
Dispense equal amounts into 10, 50 ml centrifuge tubes (0.46 g/tube)
On day of use, add 50 ml H2OMQ and mix until dissolved
Discard any unused solution - Lung homogenate buffer
H2OMQ to 1 LNaCl (58.44) 8.77 g (150 mM) Tris base (121.14) 1.82 g (15 mM) CaCl2.2H2O (147.02) 147 mg (1 mM) MgCl2.6H2O (203.3) 203 mg (1 mM)
Adjust pH = 7.4, autoclave at 121 °C, 15 psi, for 15 min
Stored at 4 °C
At time of use, add 1/100th volume of 100x protease inhibitor cocktail to volume of lung homogenate buffer needed for the day’s processing - 50 mM potassium phosphate buffer, pH = 6.0
H2OMQ to 500 mlKH2PO4 (136.01) 3.4 g (50 mM)
Adjust pH = 6.0 with 2 M NaOH
Stored at room temperature - MPO homogenate buffer
Stored at room temperature (Do not refrigerate)Hexadecyltrimethylammonium bromide (364.5) 2 g (0.5%) EDTA (372.24) 744 mg (5 mM) 50 mM potassium phosphate buffer, pH = 6.0 400 ml
Acknowledgments
The work presented here was supported by NIH grant HL048889, “Pathogenesis of Aspiration Pneumonitis” to Paul R Knight, M.D., Ph.D. and Bruce A. Davidson, Ph.D. To cite this protocol please also use the following reference: Davidson et al. (2013).
References
Mouse models
- Davidson, B. A., Vethanayagam, R. R., Grimm, M. J., Mullan, B. A., Raghavendran, K., Blackwell, T. S., Freeman, M. L., Ayyasamy, V., Singh, K. K., Sporn, M. B., Itagaki, K., Hauser, C. J., Knight, P. R. and Segal, B. H. (2013). NADPH oxidase and Nrf2 regulate gastric aspiration-induced inflammation and acute lung injury. J Immunol 190(4): 1714-1724.
- Guo, W. A., Davidson, B. A., Ottosen, J., Ohtake, P. J., Raghavendran, K., Mullan, B. A., Dayton, M. T. and Knight, P. R., 3rd (2012). Effect of high advanced glycation end-product diet on pulmonary inflammatory response and pulmonary function following gastric aspiration. Shock 38(6): 677-684.
- Hutson, A. D., Davidson, B. A., Raghavendran, K., Chess, P. R., Tait, A. R., Holm, B. A., Notter, R. H. and Knight, P. R. (2006). Statistical prediction of the type of gastric aspiration lung injury based on early cytokine/chemokine profiles. Anesthesiology 104(1): 73-79.
- Raghavendran, K., Davidson, B. A., Mullan, B. A., Hutson, A. D., Russo, T. A., Manderscheid, P. A., Woytash, J. A., Holm, B. A., Notter, R. H. and Knight, P. R. (2005). Acid and particulate-induced aspiration lung injury in mice: importance of MCP-1. Am J Physiol Lung Cell Mol Physiol 289(1): L134-143.
- Segal, B. H., Davidson, B. A., Hutson, A. D., Russo, T. A., Holm, B. A., Mullan, B., Habitzruther, M., Holland, S. M. and Knight, P. R., 3rd (2007). Acid aspiration-induced lung inflammation and injury are exacerbated in NADPH oxidase-deficient mice. Am J Physiol Lung Cell Mol Physiol 292(3): L760-768.
Rat models
- Davidson, B. A., Knight, P. R., Helinski, J. D., Nader, N. D., Shanley, T. P. and Johnson, K. J. (1999). The role of tumor necrosis factor-alpha in the pathogenesis of aspiration pneumonitis in rats. Anesthesiology 91(2): 486-499.
- Davidson, B. A., Knight, P. R., Wang, Z., Chess, P. R., Holm, B. A., Russo, T. A., Hutson, A. and Notter, R. H. (2005). Surfactant alterations in acute inflammatory lung injury from aspiration of acid and gastric particulates. Am J Physiol Lung Cell Mol Physiol 288(4): L699-708.
- Kennedy, T. P., Johnson, K. J., Kunkel, R. G., Ward, P. A., Knight, P. R. and Finch, J. S. (1989). Acute acid aspiration lung injury in the rat: biphasic pathogenesis. Anesth Analg 69(1): 87-92.
- Knight, P. R., Druskovich, G., Tait, A. R. and Johnson, K. J. (1992). The role of neutrophils, oxidants, and proteases in the pathogenesis of acid pulmonary injury. Anesthesiology 77(4): 772-778.
- Knight, P. R., Rutter, T., Tait, A. R., Coleman, E. and Johnson, K. (1993). Pathogenesis of gastric particulate lung injury: a comparison and interaction with acidic pneumonitis. Anesth Analg 77(4): 754-760.
- Knight, P. R., Davidson, B. A., Nader, N. D., Helinski, J. D., Marschke, C. J., Russo, T. A., Hutson, A. D., Notter, R. H. and Holm, B. A. (2004). Progressive, severe lung injury secondary to the interaction of insults in gastric aspiration. Exp Lung Res 30(7): 535-557.
- Nader-Djalal, N., Knight, P. R., Davidson, B. A. and Johnson, K. (1997). Hyperoxia exacerbates microvascular lung injury following acid aspiration. Chest 112(6): 1607-1614.
- Nader-Djalal, N., Knight, P. R., 3rd, Thusu, K., Davidson, B. A., Holm, B. A., Johnson, K. J. and Dandona, P. (1998). Reactive oxygen species contribute to oxygen-related lung injury after acid aspiration. Anesth Analg 87(1): 127-133.
- Nader, N. D., Knight, P. R., Bobela, I., Davidson, B. A., Johnson, K. J. and Morin, F. (1999). High-dose nitric oxide inhalation increases lung injury after gastric aspiration. Anesthesiology 91(3): 741-749.
- Nader, N. D., Knight, P. R., Davidson, B. A., Safaee, S. S. and Steinhorn, D. M. (2000). Systemic perfluorocarbons suppress the acute lung inflammation after gastric acid aspiration in rats. Anesth Analg 90(2): 356-361.
- Nader, N. D., Davidson, B. A., Tait, A. R., Holm, B. A. and Knight, P. R. (2005). Serine antiproteinase administration preserves innate superoxide dismutase levels after acid aspiration and hyperoxia but does not decrease lung injury. Anesth Analg 101(1): 213-219, table of contents.
- Rotta, A. T., Shiley, K. T., Davidson, B. A., Helinski, J. D., Russo, T. A. and Knight, P. R. (2004). Gastric acid and particulate aspiration injury inhibits pulmonary bacterial clearance. Crit Care Med 32(3): 747-754.
- Raghavendran, K., Davidson, B. A., Knight, P. R., Wang, Z., Helinski, J., Chess, P. R. and Notter, R. H. (2008). Surfactant dysfunction in lung contusion with and without superimposed gastric aspiration in a rat model. Shock 30(5): 508-517.
- Raghavendran, K., Davidson, B. A., Huebschmann, J. C., Helinski, J. D., Hutson, A. D., Dayton, M. T., Notter, R. H. and Knight, P. R. (2009). Superimposed gastric aspiration increases the severity of inflammation and permeability injury in a rat model of lung contusion. J Surg Res 155(2): 273-282.
- Raghavendran, K., Davidson, B. A., Hutson, A. D., Helinski, J. D., Nodzo, S. R., Notter, R. H. and Knight, P. R. (2009). Predictive modeling and inflammatory biomarkers in rats with lung contusion and gastric aspiration. J Trauma 67(6): 1182-1190.
- Shanley, T. P., Davidson, B. A., Nader, N. D., Bless, N., Vasi, N., Ward, P. A., Johnson, K. J. and Knight, P. R. (2000). Role of macrophage inflammatory protein-2 in aspiration-induced lung injury. Crit Care Med 28(7): 2437-2444.
Rabbit model
- Knight, P. R., Kurek, C., Davidson, B. A., Nader, N. D., Patel, A., Sokolowski, J., Notter, R. H. and Holm, B. A. (2000). Acid aspiration increases sensitivity to increased ambient oxygen concentrations. Am J Physiol Lung Cell Mol Physiol 278(6): L1240-1247.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Davidson, B. A. and Alluri, R. (2013). Gastric Aspiration Models. Bio-protocol 3(22): e968. DOI: 10.21769/BioProtoc.968.
Category
Immunology > Animal model > Mouse
Immunology > Animal model > Rabbit
Immunology > Animal model > Rat
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










