- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Qualitative Detection of Lipid Peroxidation in Mosquito Larvae Using Schiff’s Reaction: A Simple Histochemical Tool for In Situ Assessment of Oxidative Damage
Published: Vol 16, Iss 3, Feb 5, 2026 DOI: 10.21769/BioProtoc.5597 Views: 83
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Method to Injure, Dissect and Image Indirect Flight Muscle of Drosophila
Kunal Chakraborty [...] Rajesh Gunage
May 20, 2018 9981 Views

Protocol for the Implantation of Scaffolds in a Humanized Mouse Cutaneous Excisional Wound Healing Model
Dina Gadalla [...] David G. Lott
Sep 20, 2024 1457 Views

Dissection and Whole-Mount Immunofluorescent Staining of Mouse Hind Paw Muscles for Neuromuscular Junction Analysis
Rebecca L. Simkin [...] James N. Sleigh
May 20, 2025 3943 Views
Abstract
Lipid peroxidation (LPO) is a major indicator of oxidative stress and cellular damage, frequently associated with environmental and toxicological stressors and mechanistically linked to ferroptotic regulated cell death (RCD). This protocol describes a simple and reproducible method for the qualitative in situ visualization of LPO in mosquito larvae using Schiff’s reagent, which histochemically labels reactive aldehyde groups [such as malondialdehyde (MDA)] generated during lipid degradation. Although Schiff’s reagent detects aldehydes commonly associated with lipid peroxidation, these compounds are not exclusive to LPO and may also arise from other oxidative processes. The method preserves tissue integrity, enabling direct, spatially resolved observation of oxidative damage in whole larvae. Following staining, larvae are rinsed in a stabilizing sulfite solution to maintain the characteristic magenta coloration. Using this assay, Culex quinquefasciatus larvae exposed to ferroptotic cyanobacteria, such as Synechocystis sp., exhibit a marked accumulation of lipid-derived aldehydes consistent with lipid ROS–mediated damage. This oxidative response is specifically suppressed by pre-treatment with the canonical ferroptosis inhibitor Ferrostatin-1 (Fer-1), which inhibits lipid peroxidation and significantly reduces larval mortality. As a complementary approach to traditional spectrophotometric assays such as thiobarbituric acid reactive substances (TBARS), this qualitative method enables in situ visualization of lipid peroxidation without tissue homogenization, providing a rapid and biologically informative screening tool for assessing ferroptosis-associated oxidative damage in Cx. quinquefasciatus and other biological models exposed to multiple stressors.
Key features
• Direct qualitative assessment: Provides visual, histochemical evidence of lipid peroxidation in situ, enabling rapid evaluation of oxidative membrane damage at the tissue and whole-organism levels.
• Preservation of spatial context: Allows localization of oxidative damage without tissue homogenization, maintaining tissue architecture and spatial resolution.
• Environmental and toxicological screening: Optimized for rapid detection of oxidative stress in mosquito larvae exposed to larvicides, biocontrol agents, and other environmental stressors.
• Complementary to quantitative assays: Functions as a screening tool that complements spectrophotometric methods such as thiobarbituric acid reactive substances (TBARS).
Keywords: Oxidative stressGraphical overview
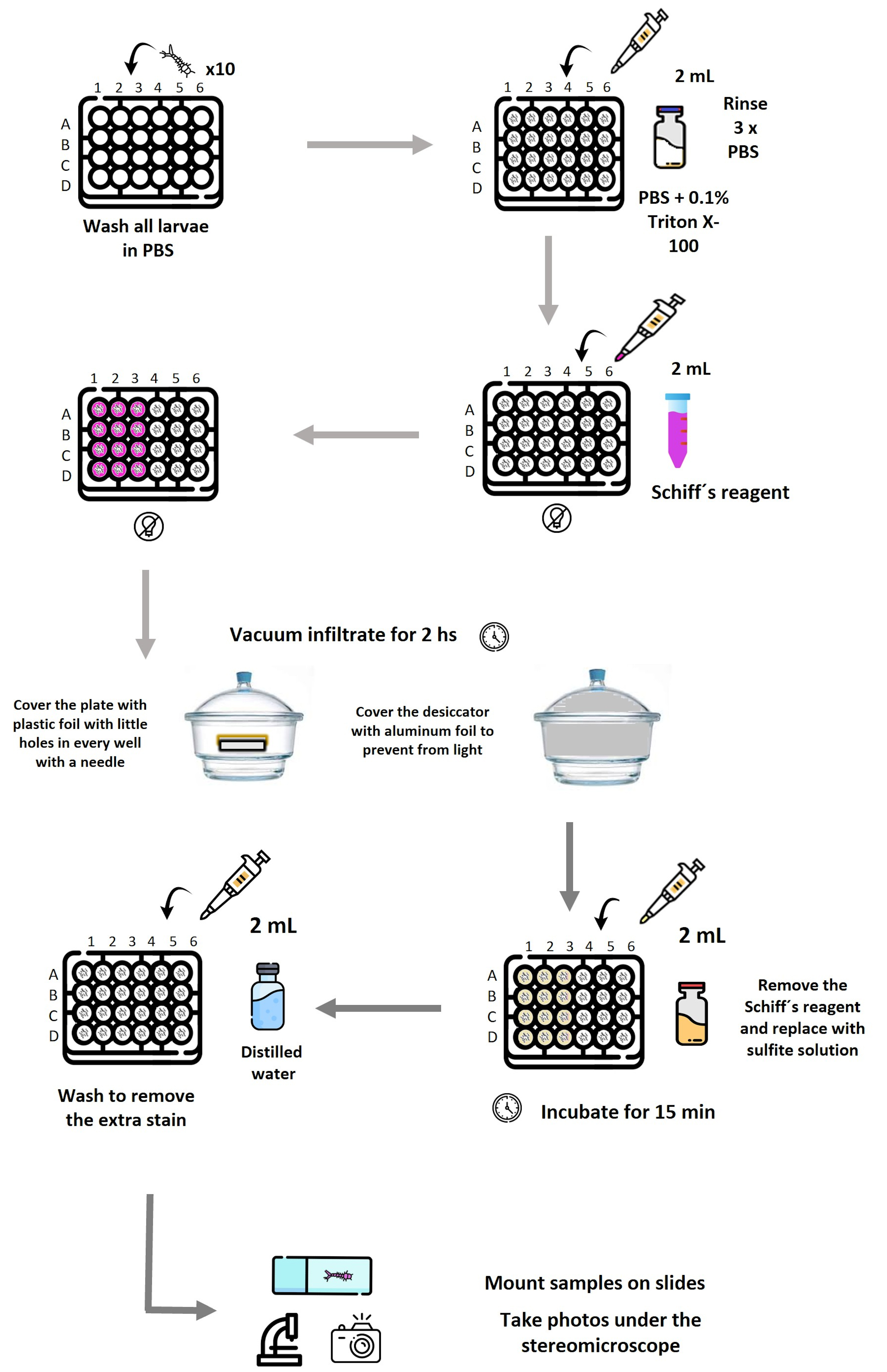
Schematic representation of the workflow used to detect lipid peroxidation–associated aldehydes in mosquito larvae by Schiff’s reaction. Larvae (10 per well) are distributed in a 24-well plate and washed in PBS, followed by permeabilization with PBS containing 0.1% Triton X-100 and subsequent PBS rinses. Schiff’s reagent is added where needed. The plate is covered with Parafilm, with holes in each well. Vacuum infiltration is applied in a desiccator covered with aluminum foil for 2 h to enhance reagent penetration. After staining, Schiff’s reagent is removed and replaced with sulfite solution to stabilize the coloration, followed by washing with distilled water to remove excess stain. Larvae are then mounted and photographed under a stereomicroscope. Reagent volumes (2 mL), incubation times, and light-protection steps are indicated at each stage. Plate layout: Row A, negative controls; A1–A3, stained with Schiff’s reagent; A4–A6, processed without Schiff’s reagent (unstained). Row B, positive controls exposed to 100 mM H2O2; B1–B3, stained; B4–B6, unstained. Row C, larvae exposed to live Synechocystis; C1–C3, stained; C4–C6, unstained. Row D, larvae exposed to ferroptotic Synechocystis; D1–D3, stained; D4–D6, unstained. Each experiment was performed in triplicate.
Background
The evaluation of oxidative stress and cellular damage is essential in fields such as toxicology, environmental biology, and insect physiology. Lipid peroxidation (LPO), the oxidative degradation of membrane lipids, represents a key biomarker of cellular damage induced by stressors, toxins, or pathogens [1].
In mosquitoes, LPO detection is particularly relevant for assessing oxidative stress induced by insecticide exposure and for understanding physiological mechanisms underlying tolerance or susceptibility. The thiobarbituric acid reactive substance (TBARS) assay remains a widely used quantitative approach to detect malondialdehyde (MDA) and requires tissue homogenization [2,3].
As a complementary approach that overcomes the limitations of TBARS-based analyses, this protocol employs Schiff’s reagent, a histochemical staining technique that permits the in situ localization and visualization of aldehyde functional groups, producing a visible magenta coloration upon reaction. It is crucial to note that while Schiff’s reagent detects reactive aldehydes generated by lipid peroxidation [such as malondialdehyde (MDA)], these aldehydes are not exclusive to LPO and may also arise from the oxidation of alcohols or the oxidative degradation of carbohydrates and amino acids under stress conditions [4]. Consequently, this staining provides a qualitative in situ assessment of oxidative damage and requires careful interpretation within the experimental context and the inclusion of appropriate controls [5]. The methodology, originally adapted from Awasthi et al. [6], has been specifically optimized for mosquito larvae, successfully preserving tissue architecture and enabling whole-organism visualization of oxidative damage patterns. The southern house mosquito (Culex quinquefasciatus) undergoes four distinct developmental stages—egg, larva, pupa, and adult—with the larval stage representing a fully aquatic, metabolically active phase particularly suitable for assessing oxidative stress responses. Under optimal conditions, development from egg to adult occurs within approximately 7–10 days, and the size of the larva increases as it molds to a new instar (Figure 1).
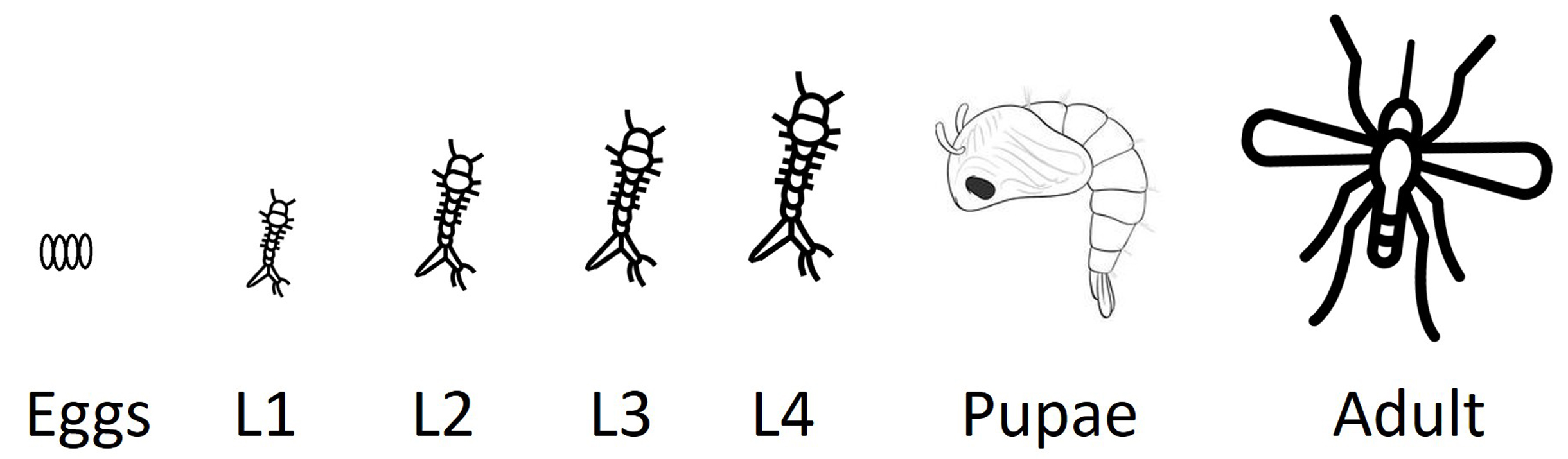
Figure 1. Life cycle of the southern house mosquito (Culex quinquefasciatus).Schematic representation of the mosquito life cycle, including egg, four larval instars (L1–L4), pupa, and adult stages. The protocol described here is optimized for the analysis of lipid peroxidation in larval stages, which represent a fully aquatic, metabolically active phase, particularly susceptible to oxidative stress induced by environmental and toxicological stressors.
Materials and reagents
Biological materials
1. Mosquito larvae at the stage of interest; in our study, second-stage larvae (L2) (Figure 1)
Reagents
1. Distilled water
2. Schiff’s reagent (Biopack, catalog number: 2000938600- 100 mL)
3. Hydrochloric acid (HCl) (Biopack, catalog number: 9632.08)
4. Hydrogen peroxide (H2O2) (Sigma-Aldrich, catalog number: H1009)
5. Sodium metabisulfite (Na2S2O5) (Sigma-Aldrich, catalog number: S9000)
6. Sodium hydroxide (NaOH) (Anedra, catalog number: 71690)
7. Potassium hydroxide (KOH) (Mallickrodt, catalog number: 6984)
8. Disodium phosphate (Na2HPO4) (Fluka, catalog number: 71645)
9. Monopotassium phosphate (KH2PO4) (Timper, catalog number: 6504)
Laboratory supplies
1. Standard 24-well flat-bottom microplate made of optically clear polystyrene
2. Microscope slides (Sail Brand, catalog number: 7102)
3. ParafilmTM M plastic wrap
4. Aluminum foil
Solutions
1. Phosphate-buffered saline (PBS) (see Recipes)
2. Permeabilization solution (see Recipes)
3. Schiff’s reagent staining solution [6] (see Recipes)
4. Sulfite solution 0.5% (w/v) (see Recipes)
5. BG11 medium (see Recipes)
Recipes
1. PBS
| Components | Quantity | Final concentration |
| NaOH (sodium hydroxide) | 0.4 g | 200 mM |
| KOH (potassium hydroxide) | 0.01 g | 3.57 mM |
| Na2HPO4 (disodium phosphate) | 0.072 g | 10.14 mM |
| KH2PO4 (monopotassium phosphate) | 0.001225 g | 0.18 mM |
| Sterile Mili-Q Water | To 50 mL |
2. Permeabilization solution
Add 0.1% Triton X-100 to PBS (Recipe 1).
This solution increases cell membrane permeability, allowing reagents to penetrate into cells or tissues.
3. Schiff’s reagent staining solution [6]
Prepare a 10% (v/v) Schiff’s reagent solution in distilled water and mix well. Prepare fresh.
4. Sulfite solution 0.5% (w/v)
Na2S2O5 in 0.05 M HCl.
5. BG11 medium
| Component | Quantity |
| NaNO3 | 1.5 g |
| K2HPO4·3H2O | 0.04 g |
| MgSO4·7H2O | 0.075 g |
| CaCl2·2H2O | 0.036 g |
| Citric acid | 0.006 g |
| Ferric ammonium citrate | 0.006 g |
| EDTA (disodium magnesium salt) | 0.001 g |
| Na2CO3 | 0.02 g |
| Trace metal mix A5 + Co* | 1 mL/L |
| Deionized water | 1,000 mL |
| pH (after autoclaving and cooling) | 7.4 |
* Trace metal mix A5 + Co contains (in g/L) H3BO3 2.86, MnCl2·4H2O 1.81, ZnSO4·7H2O 0.222, Na2MoO4·2H2O 0.390, CuSO4·5H2O 0.079, and Co(NO3)2·6H2O 0.0494
Equipment
1. Pipettes (Gilson PIPETMAN, FinnpipetteTM, 2-20, 20-200 and 100-1,000 μL)
2. pH meter (pH Tutor, Eutech Instruments)
3. Weighing balance (Sartorius, 0.1 mg to 220 g)
4. Vacuum pump and vacuum desiccator (SIGMA, model: Z119008)
5. Stereomicroscope (Nikon, model: SMZ800 stereoscope)
6. Digital camera (Olympus, model: DP72 with CellSens Entry imaging software)
7. Orbital shaker (Haimen Kylin-Bell Lab Instruments Co., Ltd., China, model: TS-8)
8. Brush (Artística Dibu, Marta 100 series, size 5/0, Argentina)
9. Insulin syringe needle (Micro-Fine, catalog number: 328418)
Procedure
A. Larval treatments
1. Select larvae of a known stage (see Figure 1) and pre-treat them according to experimental conditions (e.g., exposure to treatments, like ferroptotic Synechocystis) [5]. Include a positive control (larvae treated with 100 mM H2O2 for 20 min) and a negative control (larvae reared in BG11 medium) [7]. BG11 medium is used as a control because it is the Synechocystis growing medium (see Figure 2).

Figure 2. Histochemical detection of lipid peroxidation in Culex quinquefasciatus larvae using Schiff’s reagent under different experimental conditions. Representative images of larvae showing magenta staining indicative of lipid peroxidation. (A) Positive control: larvae treated with 100 mM H2O2 for 20 min. (B) Negative control: larvae reared in water. (C) Larvae reared on BG11 medium. (D) Larvae exposed to live Synechocystis. (E) Larvae exposed to ferroptotic Synechocystis (heat-treated at 50 °C). Larvae were fed with commercial fish food (Shulet Carassius). Each staining was performed in triplicate, and the entire procedure was independently repeated three times. Scale bars, 1 mm.
2. Prepare PBS (Recipe 1) and permeabilization solution (Recipe 2).
3. Transfer 10 larvae per well in a 24-well plate for each treatment and its corresponding controls.
Note: The experimental layout in the 24-well plate was organized as follows: Row A contained the negative control larvae; wells A1–A3 included larvae processed with Schiff’s reagent (stained negative controls), whereas wells A4–A6 contained larvae processed without Schiff’s reagent (unstained negative controls). Row B contained the positive control larvae exposed to 100 mM H2O2; wells B1–B3 were processed with Schiff’s reagent (stained positive controls), and wells B4–B6 were processed without Schiff’s reagent (unstained positive controls). Row C contained larvae exposed to live Synechocystis, and row D contained larvae exposed to ferroptotic Synechocystis; in both rows, wells 1–3 were processed with Schiff’s reagent (stained treatments), and wells 4–6 were processed without Schiff’s reagent (unstained treatments).
1. Larval preparation: Wash all larvae in 2 mL of PBS.
Caution: Larvae should be handled gently to prevent tissue damage. All liquid removal steps are performed using a pipette.
2. Permeabilization: Incubate larvae in 2 mL of permeabilization solution using a pipette for 15–30 min at room temperature with gentle agitation. Rinse three times in 2 mL of PBS for 5 min each to remove detergent.
Notes:
1. In this particular experiment, larvae are treated while alive and die later during the staining process. For larvae treated with ferroptotic Synechocystis and in the death process, staining is performed at 24 h post-treatment to assess lipid oxidation prior to death, which typically occurs between 48 and 72 h.
2. Gentle agitation is achieved with an orbital shaker. This should be performed occasionally, approximately every 2–3 min, throughout the 15–30 min incubation period.
3. Staining
a. Prepare fresh 10% (v/v) Schiff’s reagent solution. Protect from light.
Caution: Keep the reagent and the plate covered with aluminum foil during incubation in the dye to prevent light interference. It is important to emphasize that the primary components and reaction products of Schiff’s reagent are considered potentially toxic and carcinogenic. For this reason, Schiff’s reagent must be handled with appropriate personal protective equipment, including gloves, and in accordance with standard laboratory safety guidelines.
b. Add 2 mL of reagent, ensuring full immersion of larvae.
c. After immersion of larvae, cover the entire 24-well plate with Parafilm M, perforated with approximately 15 small needle holes per well to allow controlled air exchange while maintaining vacuum conditions. Place the covered plate inside a vacuum desiccator and apply vacuum infiltration for 2 h in darkness. To prevent light-induced degradation of the dye, cover the desiccator with aluminum foil for the entire duration of the vacuum incubation. The vacuum strength applied corresponds to standard laboratory desiccator conditions (approximately -70 to -80 kPa; 200–250 mmHg), which ensures effective reagent infiltration without damaging the larvae.
Caution: During vacuum infiltration, some larvae may adhere to the parafilm. If this occurs, gently detach them using a fine brush under PBS to avoid tissue damage.
4. Rinsing and mounting
a. Gently replace the reagent with 2 mL of sulfite solution (Recipe 4) using a pipette and incubate for 15 min. Wash the larvae by performing three consecutive rinses with distilled water. For each rinse, add ~2 mL of distilled water (or enough to fully immerse the larvae), gently agitate, and carefully aspirate the liquid with a pipette to avoid damaging the specimens.
b. Carefully mount the larvae on glass slides using a fine needle and a soft brush. Distilled water is used as the mounting medium. Coverslips are not required and are intentionally avoided to prevent compression of the larvae and to preserve their structural integrity. Acquire representative images under brightfield illumination using a light background (preferably white) to maximize contrast and allow reliable comparison across samples. Keep illumination conditions consistent among samples. Capture images using a stereomicroscope equipped with a 4× objective lens (see Figure 2).
Note: It is recommended to photograph the stained samples immediately after staining to ensure optimal image quality. If immediate imaging is not possible, samples may be stored at 4 °C and photographed within 24 h. Beyond this period, staining intensity may fade, and tissue integrity may deteriorate, which can compromise image quality.
Data analysis
For potential larvicidal evaluation for mosquito larval control, Schiff’s staining was applied to larvae exposed to the cyanobacterium Synechocystis sp. PCC 6803 subjected to ferroptosis (by thermal treatment). The staining revealed a widespread accumulation of oxidized lipids in larvae filtering ferroptotic Synechocystis, whereas those exposed to live cyanobacterial cells or BG11 medium exhibited coloration primarily restricted to the gastric caecum, with markedly lower intensity. These findings indicate that ferroptotic cyanobacteria induce elevated lipid peroxidation, correlating with increased larval mortality [5].
As this is a qualitative assay, results were documented by acquiring representative photographs under standardized imaging conditions, and interpretation was based on the presence, localization, and relative intensity of the staining signal, as described in Cuniolo et al. [5].
Validation of protocol
This protocol (or parts of it) has been used and validated in the following research article:
Cuniolo et al. [5]. Ferroptotic cyanobacteria as biocontrol agent of the southern house mosquito. Culex quinquefasciatus. J Invert Pathol.
General notes and troubleshooting
General notes
1. Light protection during incubation: Keep the plate covered with aluminum foil during incubation in the dye to prevent light interference.
2. Vacuum handling: When applying vacuum, wrap the uncovered plate with plastic wrap. Create small holes in the wrap to allow air entry. Larvae may adhere to the plastic wrap.
3. Light control: Cover the vacuum desiccator with aluminum foil to maintain darkness.
4. Photography tip: Use a light-colored background for clearer images. Ensure consistent lighting conditions during observation to achieve reliable results.
Acknowledgments
This study was partially supported by Grants of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), PUE 2017-0101, Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación (ANPCyT), PICT-2020-SERIE A-02641 and PICT-2021-GRF-TII-00106, and Universidad Nacional de Mar del Plata (800 202405 00109 MP).
Antonella Cuniolo: Writing—original draft, Writing—review & editing, Visualization, Validation, Methodology, Investigation, Formal analysis, Conceptualization.
Corina M. Berón: Writing—original draft, Writing—review & editing, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition.
María Victoria Martin: Writing—original draft, Writing—review & editing, Visualization, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization.
Competing interests
The authors declare no conflicts of interest.
Ethical considerations
The protocol for Cx. quinquefasciatus laboratory rearing was reviewed and approved by the CONICET Safe Procedures Policy (NPS: NBTC017-version 1.3). All experimental procedures should comply with institutional biosafety and environmental handling standards.
References
- Kagan, V. E., Mao, G., Qu, F., Angeli, J. P. F., Doll, S., Croix, C. S., Dar, H. H., Liu, B., Tyurin, V. A., Ritov, V. B., et al. (2016). Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 13(1): 81–90. https://doi.org/10.1038/nchembio.2238
- Ghani, M. A., Barril, C., Bedgood, D. R. and Prenzler, P. D. (2017). Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chem. 230: 195–207. https://doi.org/10.1016/j.foodchem.2017.02.127
- Malafaia, G., Da Luz, T. M., Guimarães, A. T. B. and Araújo, A. P. d. C. (2020). Polyethylene microplastics are ingested and induce biochemical changes in Culex quinquefasciatus (Diptera: Culicidae) freshwater insect larvae. Environ Contam. 15(1): 79–89. https://doi.org/10.5132/eec.2020.01.10
- Kazimírová, V. and Rebroš, M. (2021). Production of Aldehydes by Biocatalysis. Int J Mol Sci. 22(9): 4949. https://doi.org/10.3390/ijms22094949
- Cuniolo, A., Martin, M. V. and Berón, C. M. (2024). Ferroptotic cyanobacteria as biocontrol agent of the southern house mosquito Culex quinquefasciatus. J Invertebr Pathol. 207: 108225. https://doi.org/10.1016/j.jip.2024.108225
- Awasthi, J. P., Saha, B., Chowardhara, B., Devi, S. S., Borgohain, P. and Panda, S. K. (2018). Qualitative Analysis of Lipid Peroxidation in Plants under Multiple Stress Through Schiff’s Reagent: A Histochemical Approach. Bio Protoc. 8(8): e2807. https://doi.org/10.21769/bioprotoc.2807
- Rippka, R., Stanier, R. Y., Deruelles, J., Herdman, M. and Waterbury, J. B. (1979). Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria. Microbiology (N Y). 111(1): 1–61. https://doi.org/10.1099/00221287-111-1-1
Article Information
Publication history
Received: Oct 28, 2025
Accepted: Dec 30, 2025
Available online: Jan 15, 2026
Published: Feb 5, 2026
Copyright
© 2026 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Cuniolo, A., Berón, C. M. and Martin, M. V. (2026). Qualitative Detection of Lipid Peroxidation in Mosquito Larvae Using Schiff’s Reaction: A Simple Histochemical Tool for In Situ Assessment of Oxidative Damage. Bio-protocol 16(3): e5597. DOI: 10.21769/BioProtoc.5597.
Category
Environmental science > Environmental toxicology
Cell Biology > Tissue analysis > Tissue staining
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









