- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Low-Stress, Long-Duration Stable Tail Vein Catheterization and Precise Drug Delivery Protocol for Awake, Freely Moving Mice
(*contributed equally to this work) Published: Vol 16, Iss 3, Feb 5, 2026 DOI: 10.21769/BioProtoc.5585 Views: 105
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Construction of Activity-based Anorexia Mouse Models
Maria Consolata Miletta and Tamas L. Horvath
Aug 5, 2023 1772 Views

Binging from Food to Alcohol: A Sequential Interaction Between Binging Behaviors in Male Wistar Rats
Sergio Cuesta-Martínez [...] Cruz Miguel Cendán
Aug 5, 2023 1345 Views

The Mouse Social Frailty Index (mSFI): A Standardized Protocol
Charles W. Collinge [...] Alessandro Bartolomucci
Apr 20, 2025 1820 Views
Abstract
Tail vein catheterization in mice is a standard technique for precise drug delivery in pharmacological research, offering high accuracy and reproducibility. However, existing techniques face significant limitations in maintaining long-term stable catheter patency in awake, freely moving mice, and there is currently no standardized, detailed protocol for tail vein catheterization. Current methods suffer from high rates of catheter dislodgement, increased animal stress from repeated injections, and movement restrictions, all of which introduce confounding variables in behavioral and pharmacological studies. We have developed a simple and efficient fixation method that maintains stable tail vein catheter patency for more than 60 min while allowing complete freedom of movement. This protocol employs a strain relief loop design and multi-point fixation strategy, effectively preventing catheter dislodgement during extended periods while minimizing animal stress. This protocol has been successfully applied across multiple research areas, including metabolic studies, behavioral assessments, and neuropharmacological research in awake mice, achieving >95% catheter retention with normal animal behavior, providing a reliable technical platform for long-term awake-state research applications.
Key features
• Maintains catheter stability for over 60 min in freely moving awake mice without physical restraint.
• Catheter placement accommodates natural mouse behaviors (grooming, walking, standing).
• Compatible with swivel systems for continuous drug infusion during behavioral testing.
• Applicable to diverse research applications (metabolic studies, behavioral assessments, neuropharmacological research).
Keywords: Mouse tail vein catheterizationGraphical overview
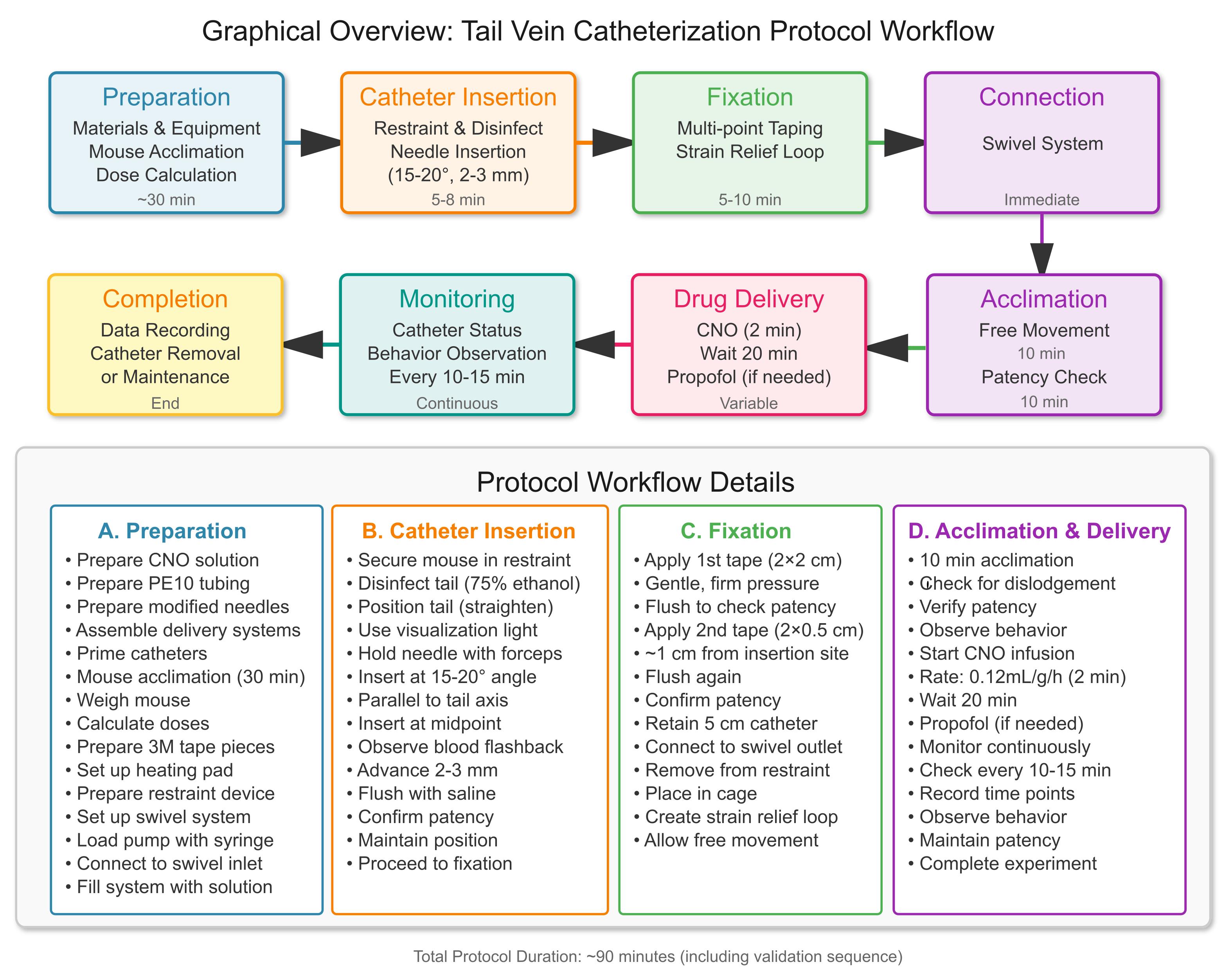
Tail vein catheterization protocol workflow
Background
Tail vein catheterization in mice is a fundamental technique in pharmacological research, providing precise and reproducible drug delivery for studying drug effects, pharmacokinetics, and pharmacodynamics [1,2]. This method is particularly valuable in neuropharmacological studies, where maintaining stable plasma drug concentrations is crucial for understanding drug mechanisms and therapeutic effects [3,4]. However, traditional tail vein injection methods face significant limitations when applied to long-term studies requiring continuous drug administration, particularly in awake, freely moving animals [5,6], due to technical challenges in catheterization, inadequate fixation methods, and long-duration limitations. Current tail vein catheterization methods primarily rely on single injections [6] or short-term catheter placement under anesthesia, which are insufficient for studies requiring extended drug exposure in conscious animals [7]. Due to technical challenges associated with tail vein catheterization, many researchers have adopted alternative approaches, such as intraperitoneal injection instead of intravenous delivery [8] or single bolus injection instead of continuous stable drug administration [7]. While these alternatives may be technically easier to implement, they introduce significant limitations: intraperitoneal injection exhibits slower and unpredictable drug absorption compared to intravenous delivery [8,9], and a single injection cannot maintain the stable plasma drug concentrations required for pharmacokinetic studies and chronic treatment protocols [8,9]. Repeated injections, while providing some temporal control, introduce significant animal stress that may confound behavioral and physiological measurements [10,11]. Short-term catheterization techniques typically maintain patency for less than 10 min in awake animals, which is insufficient for most experimental protocols requiring extended observation periods [12]. Collectively, these commonly used approaches are suboptimal for experiments requiring sustained, precisely controlled intravenous exposure in awake animals, because they either fail to maintain stable plasma drug concentrations over extended periods (bolus or non-IV routes) or introduce restraint- and handling-related stress (restraint-based infusion or repeated injections). Therefore, there remains a need for a low-stress method that supports continuous tail vein infusion for ≥60 min in awake, freely moving mice.
The technical challenges of long-term tail vein catheterization in awake mice stem from several factors. First, the small size and delicate nature of mouse tail veins make them prone to catheter dislodgement during normal animal movements such as grooming, walking, and standing [12]. Second, traditional fixation methods typically require pharmacological restraint, which introduces stress variables that affect experimental outcomes and animal welfare [13,14]. Third, there is currently no standardized protocol that effectively addresses both the issue of catheter stability during extended periods and animal comfort [11,15].
Developing a reliable long-term tail vein catheterization protocol for awake mice would significantly advance research capabilities across multiple fields [16]. Such a protocol would enable continuous drug delivery during behavioral testing, allow maintenance of stable plasma drug concentrations, and minimize animal stress by maintaining natural movement patterns. This is particularly important for diverse research applications, including neuropharmacological research, metabolic studies, behavioral assessments, and neuroimaging studies, where the effects of drugs on behavior, cognition, and neural function need to be evaluated in awake animals without the confounding effects of anesthesia or restraint stress.
Materials and reagents
Biological materials
1. Mice, 6–18 weeks of age, body weight 20–35 g (Beijing Vital River Laboratory Animal Technology Co., Ltd.)
Reagents
1. Sterile normal saline (0.9% NaCl) (Beyond, catalog number: ST341-500ml or equivalent)
2. Experimental drugs (as needed, such as CNO, propofol, etc.)
3. Clozapine N-oxide (CNO) [Sigma-Aldrich, catalog number: C0832; ≥98% (HPLC), or equivalent]
4. Propofol injection (10 mg/mL) (Fresenius Kabi Deutschi and GmbH Hafnerstraβe 36, A-8055 Graz, Austria, or equivalent)
5. Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D8418) for preparing drug solutions
6. 75% ethanol (Sigma-Aldrich, catalog number E7023 or equivalent) for disinfection
Solutions
1. CNO working solution (see Recipes)
Recipes
1. CNO working solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| CNO | 0.075 mg/mL | 0.3 mg |
| DMSO | 0.25% | 10 μL |
| Normal saline | 0.9% | 3,990 μL |
| Total | - | 4,000 μL |
Note: CNO working solution is used for i.v. infusion. Prepare a CNO stock solution by dissolving 0.3 mg of CNO in 10 μL of DMSO and store protected from light at -20 °C. Prepare the working solution by diluting the stock solution with 3,990 μL of sterile normal saline to a final volume of 4,000 μL. The final CNO concentration is 0.075 mg/mL, and the final DMSO concentration is 0.25% (v/v) (10 μL of DMSO in 4,000 μL of total volume). CNO is light-sensitive; therefore, prepare and handle solutions with minimal light exposure. The working solution is best prepared fresh; if not used immediately, store at 4 °C protected from light and use within 48 h. Freezing the working solution and repeated freeze–thaw cycles are not recommended due to potential loss of stability.
Laboratory supplies
1. Mouse restraint device (for tail vein procedures); dimensions: approximately 120 × 120 × 60 mm, inner diameter ~35 mm, with an adjustable tail access port (JiShuo, model: ZK-XGD-2, or equivalent)
2. PE10 polyethylene tubing, 0.28 mm inner diameter, 0.61 mm outer diameter (Scientific Commodities, Inc., catalog number: BB31695-PE/8)
3. 3M paper tape (for catheter fixation)
4. 30 G BD PrecisionGlide needles (Becton Dickinson, catalog number: 305106, 30G × 1/2” or equivalent)
5. Fine forceps (ophthalmic forceps) (RWD, catalog number: F12006-10) for holding needles during tail vein puncture
6. 1 mL syringes (Beyotime, catalog number: FS801-30pcs or equivalent)
7. Digital balance (for weighing mice)
8. Heating pad (for maintaining mouse body temperature)
Equipment
1. Microinjection pump (any precision infusion pump)
2. 30GA single-channel plastic swivel with welded 30 G needle (for continuous infusion during free movement)
Procedure
A. Pre-procedure preparation (timing: 30 min)
1. Material preparation
a. Prepare CNO working solution according to Recipe 1 (final concentration 0.075 mg/mL).
b. Prepare different lengths of PE10 polyethylene tubing segments (5 cm, ~20 cm, ~40 cm).
c. Prepare 30 G BD PrecisionGlide needles with three different modifications (see Figure 1A):
i. Type (a): Remove the tail only (retain the complete needle tip and remove plastic tail interface only, for mouse catheterization).
ii. Type (b): Remove both the needle tip and the tail (use hemostatic forceps to break off the tip by 2–3 mm, then grip the needle base to break the entire needle and remove the plastic tail; retain the middle segment ~5–10 mm; for catheter-to-catheter connection).
iii. Type (c): Remove the needle tip only (use hemostatic forceps to break off the needle tip by ~2–3 mm, for connecting syringes to catheter systems).
d. Assemble drug delivery system: connect in sequence drug-filled syringe → Type (c) needle → PE10 tubing (~40 cm) → swivel → PE10 tubing (~40 cm) → Type (b) needle (see Figure 1B, left).
e. Fill catheter components with drug solution and verify integrity and airtightness.
f. Connect the 20-cm PE10 tubing to the swivel outlet.
g. Start the injection pump to fill the catheter system with drug solution.
h. Load the syringe onto the microinjection pump and connect the tubing to the swivel inlet.
i. Assemble the saline delivery system: connect in sequence saline-filled syringe → Type (c) needle → PE10 tubing (5 cm) → Type (b) needle→ PE10 tubing (~15 cm) → Type (a) needle (see Figure 1B, right).
j. Fill the saline catheter components and verify integrity and airtightness.
k. Prepare the digital balance and heating pad.
l. Cut 3M surgical tape into small pieces (2 cm × 2 cm and 2 cm × 0.5 cm) and attach one corner to a convenient position for easy access.

Figure 1. Preparation of modified needles and assembly of PE10 catheter systems. (A) Components for the catheter system. Insets show three modified 30 G needles: Type (a), hub removed with intact tip; Type (b), tip and plastic hub removed (mid shaft retained); Type (c), tip removed. Also shown are PE10 tubing segments used for mouse tail indwelling and line connections. (B, C) Assembled delivery lines. Example of a pump line with an inline connector and strain relief loop (B): drug delivery line, syringe → Type (c) needle → PE10 tubing → swivel → PE10 tubing → Type (b) needle. (C) saline line for catheterization, syringe → Type (c) needle → PE10 tubing → Type (b) needle → PE10 tubing → Type (a) needle.
2. Mouse preparation
a. Transfer mice to the experimental room 30 min before the experiment for acclimation.
b. Observe mouse status to ensure health and normal behavior.
c. Weigh each mouse and record the weight for dose calculation.
d. Calculate the required CNO solution volume and propofol infusion rate based on weight.
Note: Ensure all materials are at room temperature before use to prevent animal heat shock.
B. Tail vein catheterization (timing: 1–3 min) (see Figures 2 and 3)
Animal welfare and analgesia considerations: This procedure uses a 30 G needle for tail vein puncture, which is minimally invasive with minimal tissue trauma, similar to routine tail vein blood sampling or injection. According to IACUC guidelines for minor procedures, routine systemic analgesia is generally not required. Local anesthetic is not recommended due to the small wound size and potential impact on research outcomes. Animals are closely monitored according to institutional IACUC/veterinary guidelines, with rescue analgesia provided when needed. Postoperative monitoring is performed at least twice daily, including assessment of behavior, body weight, food intake, and catheter/skin condition. If signs of pain or stress appear (e.g., abnormal posture/crouching, vocalization, persistent struggling, reduced grooming/feeding, self-injury), preset interventions should be implemented per the approved protocol (analgesia and withdrawal from experiment). All procedures have received IACUC ethical approval. The progressive catheter insertion and fixation process is illustrated in Figure 2.
1. Preparation
a. Restrain the mouse using a restraint device.
b. Disinfect the mouse tail with 75% ethanol cotton balls.
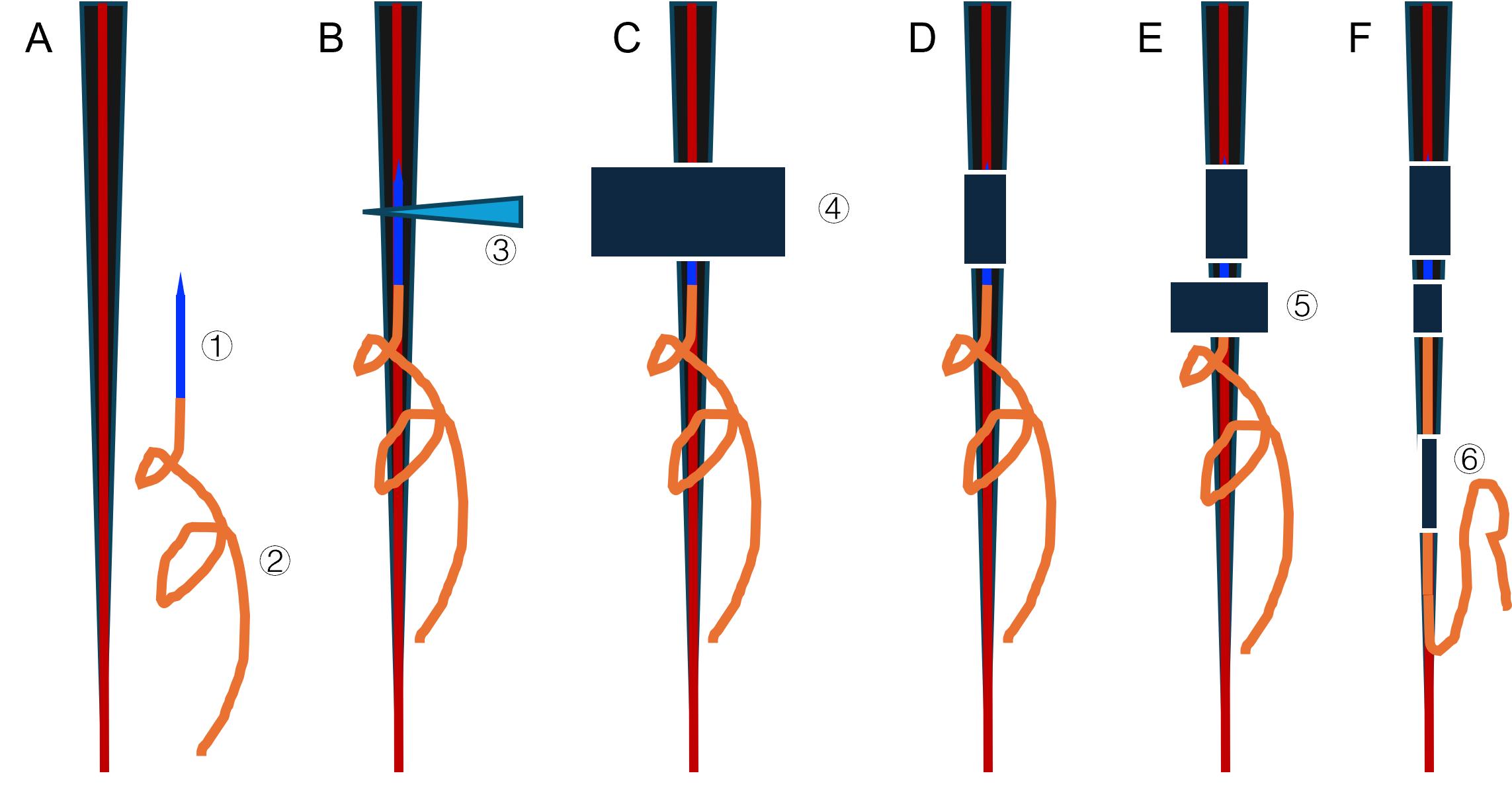
Figure 2. Step-by-step illustration of catheter insertion and multi-point fixation strategy. Progressive catheter insertion and fixation process. Panels A through F show the tail cross-section with the venous structure (red line) and 30 G needle (blue). The orange spiral represents the PE10 catheter with its strain relief design. (A) Schematic before insertion. (B) Schematic after insertion. (C–D) Schematics showing positions before and after the first tape fixation. (E–F) Schematics showing the second and third tape fixations. 1: 30G needle. 2: PE10 catheter. 3: Modified needle hub. 4: First fixation tape. 5: Second fixation tape. 6: Second fixation tape. This stepwise schematic highlights the critical progression from single- to multi-point fixation and illustrates how the fixation strategy helps prevent catheter dislodgement during free movement.

Figure 3. Photographic workflow of mouse tail vein catheterization and multi-point fixation. (A) Steps 1–3: Insert the needle to target the lateral tail vein until blood flashback is observed. (B) Steps 4–9: Apply the first fixation over the puncture site and verify patency after each step. (C) Steps 10–12: Add 1–2 additional fixations proximal and distal to the puncture site. (D) Steps 13–15: Recheck patency and connect to the system line to complete the setup.
2. Catheter insertion [see Figure 2A–B and Figure 3 (2–4)]
a. Use the assembled saline delivery system.
b. Position mouse tail: grip the tail base with the middle and index fingers and hold the distal end with your thumb and ring/pinky fingers.
c. Straighten the tail so that the lateral vein faces upward and is parallel to the work surface.
d. Hold a 30 G needle with ophthalmic forceps, keeping it parallel to the mouse tail.
e. Insert the needle at a shallow angle of 15–20° relative to the tail surface, keeping the needle body parallel to the longitudinal axis of the tail.
f. Insert the needle into the vein at the midpoint between the tail base and the tip.
g. Advance 2–3 mm after observing blood flashback.
h. Flush with saline to confirm catheter patency.
i. Maintain current position and immediately proceed to catheter fixation.
Note: Maintain aseptic technique throughout the procedure to prevent infection. Operation time depends on operator proficiency. A tail vein visualization light source placed below the tail can notably assist observation, especially for less experienced operators.
Critical: Maintain the relative position of the needle and tail unchanged until catheter fixation is complete to prevent the needle tip from dislodging from the vein.
C. Catheter fixation (timing: 2–5 min)
1. Insertion site fixation [see Figure 2C–E and Figure 3 (4–13)]
a. Maintain a straight tail position.
b. Apply one piece of 2 cm × 2 cm 3M paper tape to the mouse tail from above the needle insertion site.
Note: Apply the tape snugly enough to immobilize the catheter, but not tight enough to impair tail circulation (e.g., no swelling, discoloration, or coolness). Common pitfalls include tape that is too tight (impaired circulation), too loose (insufficient stabilization), taping before confirming venous entry, insufficient tape–skin contact/poor adhesion, or taping at an angle that introduces traction on the catheter/PE10 tubing.
c. After tape application, flush again with saline to ensure patency.
d. Apply an additional 2 cm × 1 cm 3M surgical tape approximately 1 cm from the insertion site for extra fixation.
e. After tape application, flush again with saline to ensure patency.
Note: Proceed to the next step only after confirming patency.
2. Mouse–catheter–swivel–pump connection [see Figure 2F and Figure 3 (13–15)]
a. Retain only a 5-cm tubing segment connected to the indwelling needle; remove all other tubing and syringes.
b. Connect the remaining 5-cm tubing on the mouse tail to the swivel outlet tubing using a 30 G needle (tip and tail removed). Remove the mouse from the restraint device and place it in the cage for free movement (see Figure 4).
Note: Allow mouse adaptation time (see Figure 5). If the mouse attempts to bite the catheter, provide appropriate intervention. Mice typically adapt to the swivel system within 5–10 min after connection, but for first-time experiments or more sensitive individuals, a brief 10–15 min acclimation period is recommended.

Figure 4. Complete setup for continuous drug infusion in freely moving mice. Diagram of the complete apparatus chain: infusion pump → delivery line → swivel (mounted on an adjustable stand) → in-cage PE10 tubing → indwelling tail catheter in the mouse. The swivel is positioned above the cage and can rotate freely to prevent line entanglement; in the cage, leave sufficient PE10 tubing for the mouse to move freely according to the cage size.

Figure 5. Normal behaviors in mice after tail vein catheterization. The catheterization protocol maintains low stress and allows unrestricted natural behaviors in mice, which is critical for behavioral and pharmacological studies. (1) Mouse in feeding position. (2) Mouse in rearing posture, stretching upward to explore. (3–9) Mouse in resting/low position while feeding. Additional assessment methods: While this protocol primarily evaluates stress by monitoring normal behaviors and the absence of abnormal behaviors, readers may consider supplementary quantitative assessments depending on experimental requirements. The Mouse Grimace Scale (MGS) provides a standardized approach to assess pain- or distress-related facial expressions. In addition, automated behavioral tracking systems can quantify locomotor activity and other behavioral parameters before and after catheterization, offering objective metrics to support low-stress evaluation. These complementary approaches may be particularly useful for studies requiring detailed behavioral phenotyping or for comparisons across different experimental conditions. This figure validates the protocol’s low-stress design, demonstrates successful catheter retention during natural movements, and supports compatibility with a range of behaviors (e.g., grooming, walking, standing, feeding), which is essential for long-term awake-state research without confounding restraint stress.
3. Stability test
a. Allow the mouse to move freely for 10 min to recover from catheterization stress.
Note: The 10-min acclimation period allows mice to recover from brief handling/catheterization and re-establish baseline behavior, and provides time to detect early issues (e.g., leakage, occlusion, or loosening) before drug infusion.
b. Observe for catheter dislodgement.
c. Check catheter patency.
Critical: Multi-point fixation is crucial for preventing dislodgement during free movement.
D. Continuous drug delivery setup (timing: 3–5 min)
1. Continuous drug delivery parameter settings
a. Set infusion rate and duration according to experimental requirements.
b. Use a microinjection pump to control the drug delivery rate.
c. Ensure that the drug solution fills the entire catheter system.
2. Drug delivery monitoring
a. Check catheter integrity every 10–15 min (if bitten, liquid beads will seep out).
b. Observe mouse behavior changes to confirm no abnormal reactions.
c. Record key operation time points (catheterization time, drug delivery time, etc.).
Note: For situations with limited equipment, mice can be temporarily disconnected from the main equipment. Seal the catheter using an occluded 30 G needle (tip and tail removed) to prevent bleeding. Reconnect before the next drug delivery, but first flush manually with saline to ensure catheter patency.
Pause point: The protocol can be paused here for an extended period. Ensure appropriate catheter maintenance during the pause.
Validation note: This catheter system has been successfully applied to various studies, including chemogenetic studies using CNO and anesthesia studies using propofol, demonstrating its reliability in maintaining stable drug infusion (see Validation of protocol section for details).
Data analysis
The protocol’s performance was evaluated using three primary metrics. The catheter insertion success rate was >90%, indicating reliable technical performance. The catheter retention rate was >90% during the 90-min validation workflow, supporting the protocol’s suitability for long-duration infusion experiments. In addition, >90% of mice displayed normal behaviors after catheterization, consistent with minimal stress and discomfort under the conditions tested. These values are based on our validation experiments and prior application experience, and may vary with operator proficiency, mouse strain, and experimental conditions.
Note: The above data are based on specialized validation experiments from our team and application experience in published research. Specific success and retention times may vary based on operator proficiency, mouse strain, experimental conditions, and other factors. Operators are advised to practice sufficiently before starting formal experiments to master the key technical points.
Validation of protocol
We performed systematic validation in transgenic mice expressing light-sensitive inhibitory constructs (n = 19), simulating the full experimental workflow. After tail vein catheterization, we first flushed with saline and allowed 10 min of free movement for acclimation. We then performed a 2-min infusion at 0.12 μL/g/h of either saline (control) or CNO (0.3 mg/kg) for chemogenetic activation, followed by 20 min of free movement to allow sufficient time for CNO-mediated effects to develop, consistent with commonly used regimens in prior studies [17]. Next, propofol was administered at 10 mg/kg/min for 5.5 min to induce anesthesia, during which the time to Loss of Righting Reflex (LORR) was recorded. Anesthesia was then maintained at 3 mg/kg/min for 10 min, after which the time to Recovery of Righting Reflex (RORR) was recorded. Mice were then allowed to move freely for 20 min to confirm full recovery. Finally, saline was infused through the catheter to verify patency before removing the catheter and achieving hemostasis. The entire validation sequence lasted approximately 90 min. This duration was chosen to simulate a representative experimental workflow that exceeds the 60-min infusion target and to challenge catheter stability across awake–anesthetized–awake transitions. Only one catheter dislodged near the end during free movement; overall catheter retention exceeded 90%, and animals displayed normal behavior without stress indicators throughout. These results confirm protocol stability across awake–anesthetized–awake state transitions and reliability during prolonged infusion. See Figure 6 for the pharmacological validation plot (LORR/RORR under saline vs. CNO pre-treatment).
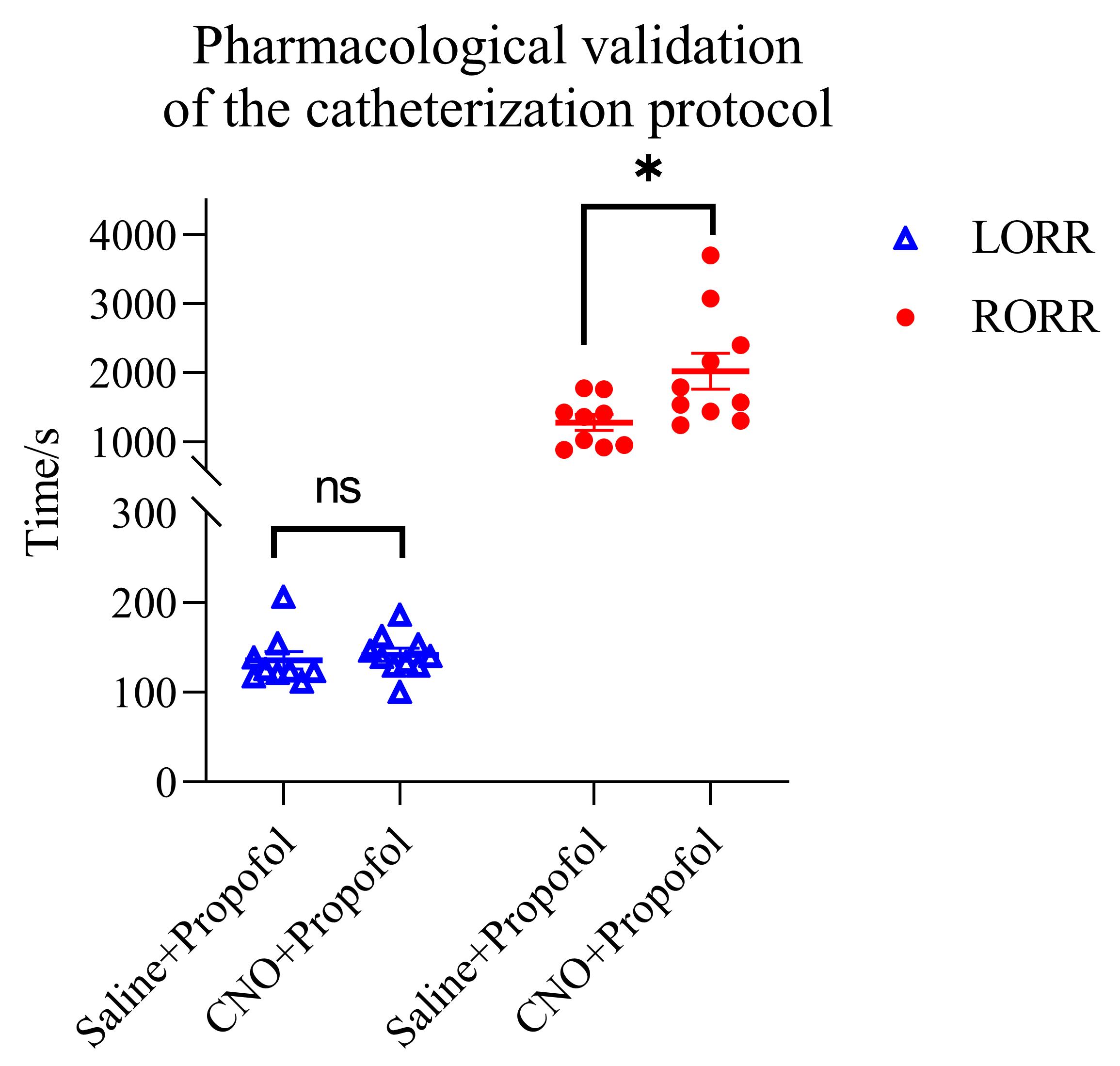
Figure 6. Effects of clozapine N-oxide (CNO) after viral delivery of a pan-inhibitory chemogenetic construct in a specific brain region on propofol-induced anesthesia. Scatter plots (mean ± SEM) of time to Loss of Righting Reflex (LORR) (blue triangles) and Recovery of Righting Reflex (RORR) (red circles) under two conditions: saline + propofol and CNO + propofol. LORR: saline 135.98 s vs. CNO 142.03 s (unpaired t-test, p = 0.601). RORR: saline 1280.89 s vs. CNO 2022.70 s (unpaired t-test, p = 0.0167). CNO group, N = 10; saline group, N = 9. Note: ns, no statistical significance p > 0.05; *p < 0.05 (unpaired t-test).
This tail vein catheterization method has been successfully applied and validated in the following research articles (as technical support):
• Wang et al. [18]. Oxidation of ethanol in the rat brain and effects associated with chronic ethanol exposure. Proc Natl Acad Sci USA. 110(35): 14444–14449. https://doi.org/10.1073/pnas.1306011110
• Yang et al. [19]. Exploring cerebral structural and functional abnormalities in a mouse model of post-traumatic headache induced by mild traumatic brain injury. Zool Res. 45(3): 648–662. https://doi.org/10.24272/j.issn.2095-8137.2023.323
• Chen et al. [20]. Circadian Regulation of the Lactate Metabolic Kinetics in Mice Using the [1H-13C]-NMR Technique. Mol Neurobiol. 61(8): 5802–5813. https://doi.org/10.1007/s12035-024-03927-w
• Liu et al. [21]. Regional Metabolic Patterns of Abnormal Postoperative Behavioral Performance in Aged Mice Assessed by 1H-NMR Dynamic Mapping Method. Neurosci Bull. 36(1): 25–38. https://doi.org/10.1007/s12264-019-00414-4
• Guo et al. [22]. Investigation of metabolic kinetics in different brain regions of awake rats using the [1H-13C]-NMR technique. J Pharm Biomed Anal. 204: 114240. https://doi.org/10.1016/j.jpba.2021.114240
Note: The above references demonstrate the successful application of this tail vein catheterization technique across multiple research scenarios, validating the method's stability and reliability. These validation studies demonstrate that this technique can support the experimental needs of long-term awake mouse studies.
General notes and troubleshooting
General notes
1. Catheter length considerations: PE10 tubing length can be adjusted as needed.
2. Fixation method: Two or more fixation points must be used after tail catheter placement to prevent catheter dislodgement.
3. Strain relief loop: Not essential construction; operators can manually create alternatives.
4. Operation time: Minimize operation time to reduce animal stress.
5. Environmental temperature: Maintain appropriate temperature to prevent animal hypothermia.
6. Applicability: This protocol is suitable for mice, rats, etc. Adjust injection needles and catheter tubing accordingly.
7. Safety precautions: Take necessary protective measures throughout to avoid bites when handling mice.
8. Compatibility: 30 G needles, PE10 tubing, and 30GA swivel with welded 30 G needle are relatively matched. If one size needs to be changed, all must be replaced; otherwise, sealing will be affected, leading to inaccurate pump doses.
9. Reporting and practical considerations: During protocol development and optimization, we did not prospectively record the aggregate number of catheterization attempts across all operators, and we did not perform a formal comparison of success rates between novice and experienced operators. In our experience, once key technical steps are reliably achieved (successful venous entry, correct multi-point fixation, and appropriate PE10 slack/strain relief), outcomes become more consistent, and operator experience primarily affects procedural speed and ease. Leakage most commonly occurs following partial catheter displacement or loosening and is therefore closely related to fixation quality. Catheter occlusion due to clotting may occur when the line remains idle; in our practice, if no infusion or flushing is performed for approximately 20 min after placement, occlusion may occur. To mitigate this risk, consider periodic small-volume saline flushing (e.g., every ~5 min) or maintaining minimal continuous flow when appropriate, and consider anticoagulation strategies when longer-duration catheterization is required, in accordance with institutional/IACUC and veterinary guidance. Although dislodgement was uncommon within our typical experimental window (≤90 min), the risk may increase with longer durations due to gradual loss of tape adhesion and tail morphology; therefore, for longer sessions, minimize prolonged no-infusion intervals and increase monitoring and fixation checks.
Troubleshooting
Problem 1: After indwelling needle placement, mice repeatedly and vigorously bite the indwelling site.
Possible cause: Needle tip has dislodged.
Solution: Re-catheterize.
Problem 2: After indwelling needle placement, mice show a tendency to sniff or mildly bite the indwelling site.
Possible cause: Normal reaction.
Solution: Use a glass rod or similar to prevent biting behavior.
Problem 3: The catheter is patent before drug delivery, but cannot be pumped during pump infusion after the mouse's free movement.
Possible cause: Clot formation.
Solution: For very long-term (>30 min) drug delivery intervals, using an anticoagulant is recommended.
Problem 4: A large amount of leakage from the needle puncture site on the mouse’s tail during pump infusion.
Possible cause: The indwelling needle has dislodged.
Solution: End experiment, re-fixate, and re-catheterize.
Problem 5: No blood flashback observed during tail vein puncture.
Possible causes:
1) Needle has not entered the vein.
2) Venous pressure is too low.
3) Bubbles present in the catheter system.
Solutions:
1) Re-position vein location and ensure the needle fully enters the vein.
2) A tail vein visualization light can be used to assist positioning.
3) Ensure the catheter system is filled with saline and free of bubbles before puncture.
4) If the vein entry is confirmed but there is no blood flashback, gently push a small volume of saline and observe resistance to determine if it is in the vein.
Acknowledgments
Author contributions: Conceptualization, Jie Wang, Jun Fang, Yunshuang Ye; Investigation, Xiaohang Fu, Yunshuang Ye; Writing—original draft, Yunshuang Ye, Xiaohang Fu; Writing—review and editing, Jie Wang, Jun Fang, Yunshuang Ye; Funding acquisition, Jie Wang; Supervision, Jun Fang.
This work was supported by the Biosecurity Research Project (Grant no. 23SWAQ24), the National Natural Science Foundation of China General Program (Grant no. 82471504 and 32271148), and the Research Grant of Key Laboratory of Anesthesiology and Resuscitation (Huazhong University of Science and Technology), Ministry of Education (no. 2025MZFS002) and the Shanghai Songjiang District Key Medical Discipline Construction Project (Project No. 24SJYXZDB01).
This protocol was developed and validated in the following research articles: Wang et al. [18], Yang et al. [19], Chen et al. [20], Liu et al. [21], and Guo et al. [22]. This protocol is based on prior tail vein catheterization techniques and was modified for the specific needs of awake mouse research.
Competing interests
The authors declare no competing interests.
Ethical considerations
Ethical approval: All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Songjiang Hospital/Songjiang Research Institute Affiliated to Shanghai Jiao Tong University School of Medicine (Protocol number: ACE-005-2025) and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
All procedures aimed at minimizing animal stress and discomfort. Mice were continuously monitored during and after procedures for any signs of distress.
References
- Dalsgaard, T., Cecchi, C. R., Askou, A. L., Bak, R. O., Andersen, P. O., Hougaard, D., Jensen, T. G., Dagnæs-Hansen, F., Mikkelsen, J. G., Corydon, T. J., et al. (2018). Improved Lentiviral Gene Delivery to Mouse Liver by Hydrodynamic Vector Injection through Tail Vein. Mol Ther Nucleic Acids. 12: 672–683. https://doi.org/10.1016/j.omtn.2018.07.005
- Song, S., Wei, Q., Wang, K., Yang, Q., Wang, Y., Ji, A. and Chen, G. (2022). Fluorescent Labeling of Polymannuronic Acid and Its Distribution in Mice by Tail Vein Injection. Mar Drugs. 20(5): 289. https://doi.org/10.3390/md20050289
- Nozohouri, E., Ahn, Y., Zoubi, S., Patel, D., Archie, S. R., Akter, K. A., Siddique, M. B., Huang, J., Abbruscato, T. J., Bickel, U., et al. (2024). The Acute Impact of Propofol on Blood–Brain Barrier Integrity in Mice. Pharm Res. 41(8): 1599–1611. https://doi.org/10.1007/s11095-024-03735-w
- Shilo, M., Motiei, M., Hana, P. and Popovtzer, R. (2014). Transport of nanoparticles through the blood–brain barrier for imaging and therapeutic applications. Nanoscale. 6(4): 2146–2152. https://doi.org/10.1039/c3nr04878k
- Wu, Q., Xing, Z., Luo, S., Chen, B., Yu, X., Tao, R., Bao, N. and Zhao, J. (2021). Assembly and operation of an easy-to-make portable device for facilitating mouse lateral tail-vein injection. Lab Anim. 51(1): 11–21. https://doi.org/10.1038/s41684-021-00889-7
- Watanabe, M., Takimoto, H. R., Hashimoto, K., Ishii, Y. and Sasaki, N. (2025). Effectively simplified Adriamycin‐induced chronic kidney disease mouse model: Retro‐orbital vein injection versus tail‐vein injection. Anim Models Exp Med. 8(3): 568–572. https://doi.org/10.1002/ame2.12553
- Luo, D., Chen, S. and Zhang, Y. (2022). Effects of different injection methods of propofol anesthesia on the behavior and electroencephalography recording in mice. Ibrain. 8(1): 109–116. https://doi.org/10.1002/ibra.12030
- Wong, K. P., Sha, W., Zhang, X. and Huang, S. C. (2011). Effects of Administration Route, Dietary Condition, and Blood Glucose Level on Kinetics and Uptake of18F-FDG in Mice. J Nucl Med. 52(5): 800–807. https://doi.org/10.2967/jnumed.110.085092
- Bagchi, S., Nozohouri, E., Ahn, Y., Patel, D., Bickel, U. and Karamyan, V. T. (2023). Systemic and Brain Pharmacokinetics of Milnacipran in Mice: Comparison of Intraperitoneal and Intravenous Administration. Pharmaceutics. 16(1): 53. https://doi.org/10.3390/pharmaceutics16010053
- Du Preez, A., Law, T., Onorato, D., Lim, Y. M., Eiben, P., Musaelyan, K., Egeland, M., Hye, A., Zunszain, P. A., Thuret, S., et al. (2020). The type of stress matters: repeated injection and permanent social isolation stress in male mice have a differential effect on anxiety- and depressive-like behaviours, and associated biological alterations. Transl Psychiatry. 10(1): e1038/s41398–020–01000–3. https://doi.org/10.1038/s41398-020-01000-3
- Swan, J., Boyer, S., Westlund, K., Bengtsson, C., Nordahl, G. and Törnqvist, E. (2023). Decreased levels of discomfort in repeatedly handled mice during experimental procedures, assessed by facial expressions. Front Behav Neurosci. 17: e1109886. https://doi.org/10.3389/fnbeh.2023.1109886
- Song, K., Ge, X., Engelbach, J. A., Thio, L. L., Neil, J. J., Ackerman, J. J. H. and Garbow, J. R. (2023). Subcutaneous deuterated substrate administration in mice: An alternative to tail vein infusion. Magn Reson Med. 91(2): 681–686. https://doi.org/10.1002/mrm.29888
- Wilde, E., Aubdool, A. A., Thakore, P., Baldissera, L., Alawi, K. M., Keeble, J., Nandi, M. and Brain, S. D. (2017). Tail‐Cuff Technique and Its Influence on Central Blood Pressure in the Mouse. J Am Heart Assoc. 6(6): e005204. https://doi.org/10.1161/jaha.116.005204
- Assenmacher, C. A., Lanza, M., Tarrant, J. C., Gardiner, K. L., Blankemeyer, E. and Radaelli, E. (2022). Post Mortem Study on the Effects of Routine Handling and Manipulation of Laboratory Mice. Animals. 12(23): 3234. https://doi.org/10.3390/ani12233234
- David, J. M., Duarte Vogel, S., Longo, K., Sanchez, D. and Lawson, G. (2014). The use of eutectic mixture of lidocaine and prilocaine in mice (Mus musculus) for tail vein injections. Vet Anaesth Analg. 41(6): 654–659. https://doi.org/10.1111/vaa.12177
- Srinageshwar, B., Dils, A., Sturgis, J., Wedster, A., Kathirvelu, B., Baiyasi, S., Swanson, D., Sharma, A., Dunbar, G. L., Rossignol, J., et al. (2019). Surface-Modified G4 PAMAM Dendrimers Cross the Blood–Brain Barrier Following Multiple Tail-Vein Injections in C57BL/6J Mice. ACS Chem Neurosci. 10(9): 4145–4150. https://doi.org/10.1021/acschemneuro.9b00347
- Wang, Y. L., Wang, L., Xu, W., He, M., Dong, H., Shi, H. Y., Chen, Y. Q. and Huang, Z. L. (2023). Paraventricular thalamus controls consciousness transitions during propofol anaesthesia in mice. Br J Anaesth. 130(6): 698–708. https://doi.org/10.1016/j.bja.2023.01.016
- Wang, J., Du, H., Jiang, L., Ma, X., de Graaf, R. A., Behar, K. L. and Mason, G. F. (2013). Oxidation of ethanol in the rat brain and effects associated with chronic ethanol exposure. Proc Natl Acad Sci USA. 110(35): 14444–14449. https://doi.org/10.1073/pnas.1306011110
- Yang, D., Nie, B. B., He, J. G., Lv, Z. Q., Mo, F. F., Ouyang, S. Y., Wang, J., Chen, J., et al. (2024). Exploring cerebral structural and functional abnormalities in a mouse model of post-traumatic headache induced by mild traumatic brain injury. Zool Res. 45(3): 648–662. https://doi.org/10.24272/j.issn.2095-8137.2023.323
- Chen, L., Wu, K., He, J., Hou, J., Zhang, Y., Liu, L., Wang, J. and Xia, Z. (2024). Circadian Regulation of the Lactate Metabolic Kinetics in Mice Using the [1H-13C]-NMR Technique. Mol Neurobiol. 61(8): 5802–5813. https://doi.org/10.1007/s12035-024-03927-w
- Liu, T., Li, Z., He, J., Yang, N., Han, D., Li, Y., Tian, X., Liu, H., Manyande, A., Xiang, H., et al. (2020). Regional Metabolic Patterns of Abnormal Postoperative Behavioral Performance in Aged Mice Assessed by 1H-NMR Dynamic Mapping Method. Neurosci Bull. 36(1): 25–38. https://doi.org/10.1007/s12264-019-00414-4
- Guo, M., Fang, Y., Zhu, J., Chen, C., Zhang, Z., Tian, X., Xiang, H., Manyande, A., Ehsanifar, M., Jafari, A. J., et al. (2021). Investigation of metabolic kinetics in different brain regions of awake rats using the [1H-13C]-NMR technique. J Pharm Biomed Anal. 204: 114240. https://doi.org/10.1016/j.jpba.2021.114240
Article Information
Publication history
Received: Nov 1, 2025
Accepted: Dec 29, 2025
Available online: Jan 13, 2026
Published: Feb 5, 2026
Copyright
© 2026 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Ye, Y., Fu, X., Wang, J. and Fang, J. (2026). A Low-Stress, Long-Duration Stable Tail Vein Catheterization and Precise Drug Delivery Protocol for Awake, Freely Moving Mice. Bio-protocol 16(3): e5585. DOI: 10.21769/BioProtoc.5585.
Category
Neuroscience > Behavioral neuroscience > Animal model
Biological Sciences > Biological techniques
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








