- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models
Published: Vol 16, Iss 2, Jan 20, 2026 DOI: 10.21769/BioProtoc.5571 Views: 231
Reviewed by: Samantha HallerAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Differentiation, Maintenance, and Contraction Profiling of Human Induced Pluripotent Stem Cell–Derived Cardiomyocytes
Matthijs Snelders [...] Jeroen Essers
Mar 5, 2025 3938 Views

Isolation and Culture of Ferret Airway Stem Cells
Ziying Yan [...] Feng Yuan
Jul 20, 2025 2413 Views

Reprogramming White Fat Cells for Adipose Manipulation Transplantation (AMT) Therapy
Kelly An [...] Nadav Ahituv
Aug 5, 2025 2247 Views
Abstract
Adipogenic differentiation efficiency remains highly variable across laboratories and cellular models, underscoring a critical need for a robust and standardized protocol. Here, we describe an optimized and highly effective protocol for inducing adipogenesis in multiple models, including murine 3T3-L1 preadipocytes, stromal vascular fraction (SVF) from neonatal and adult mice, and human adipose-derived stem cells (hADSCs). Systematic optimization was performed on key parameters such as initial cell confluence, induction timing, inducer composition, and culture surface coating. We show that high cell density, rosiglitazone supplementation, and an extended primary induction phase combine to promote lipid accumulation. Notably, we introduce a crucial modification—prolonged low-dose insulin stimulation during the maintenance phase—that is essential for the efficient differentiation of adult SVF. Furthermore, when applied to hADSCs, the protocol consistently induced robust adipogenesis, confirming its cross-species applicability. Taken together, this comprehensive and reproducible protocol serves as a valuable tool for advancing in vitro adipogenesis research.
Key features
• Extend a robust, standardized adipogenic differentiation protocol from 3T3-L1 preadipocytes to clinically relevant models, including hADSCs and the heterogeneous SVF.
• Identify key optimized parameters—cell density, induction timing, and inducer composition—enabling highly reproducible differentiation across species.
Keywords: Adipogenic differentiationGraphical overview
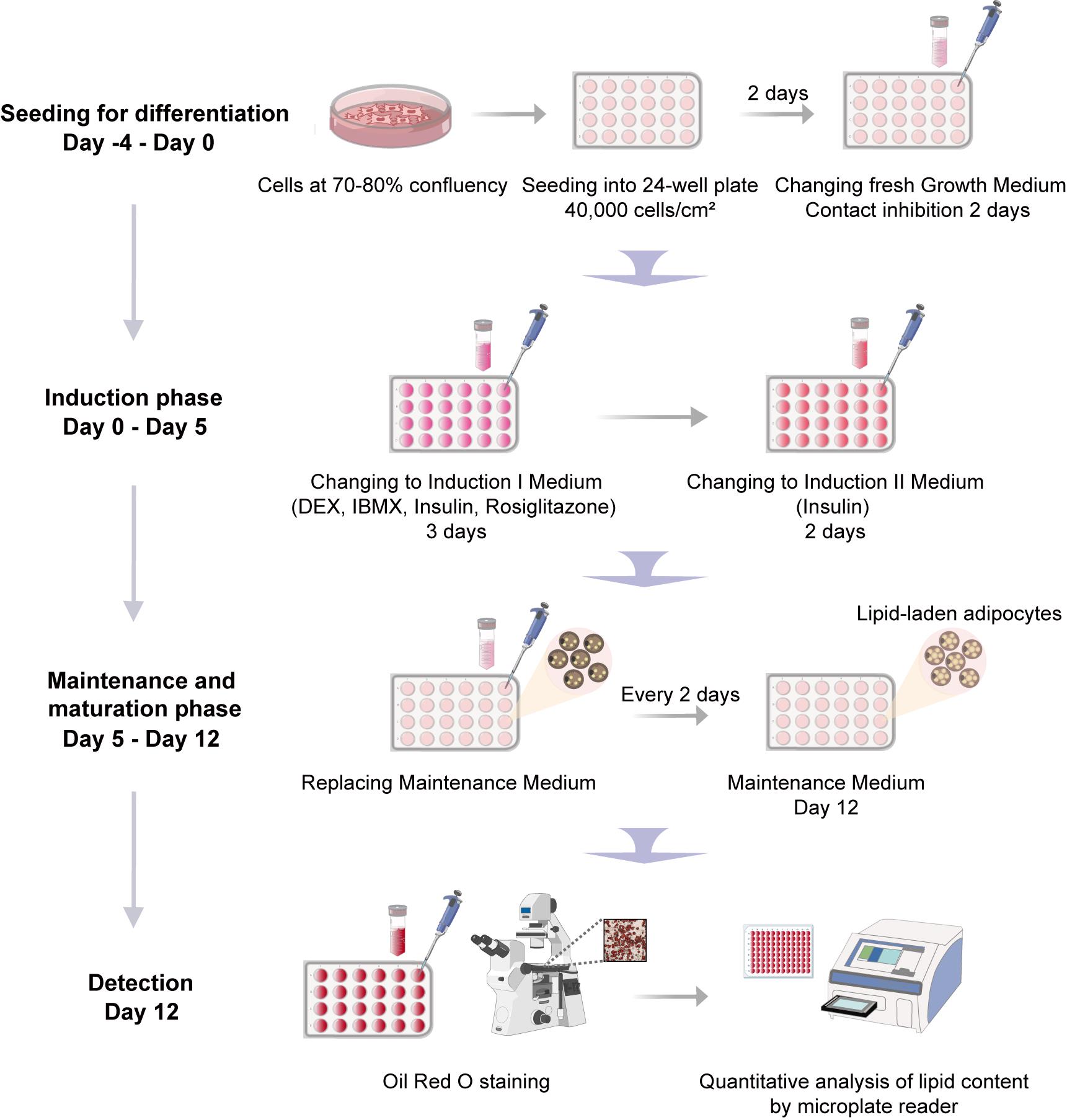
Schematic overview of the adipogenic differentiation protocol
Background
Adipogenic differentiation is a critical biological process with profound implications for metabolic health and regenerative medicine [1–3]. The escalating global burden of obesity and associated metabolic syndromes underscores the urgent need to elucidate the molecular mechanisms underlying adipocyte development and function [4]. However, progress in this field has been hampered by inconsistent differentiation outcomes, largely attributable to variations in cell sources, culture conditions, and induction protocols. These inconsistencies significantly compromise the reproducibility and translational relevance of adipogenesis research, highlighting the pressing demand for a standardized, efficient, and universally applicable differentiation methodology.
Adipogenesis, the process through which multipotent progenitor cells—including those of mesenchymal stromal and neural crest origin [5,6]—commit to preadipocytes and terminally differentiate into mature, lipid-laden adipocytes, is orchestrated by a well-defined transcriptional cascade [7,8]. Adipose-derived stem cells (ASCs) serve as a major cellular source of committed preadipocytes in adipose tissue [3]. This transformation is primarily governed by the master regulators peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer binding protein (C/EBP) family members [9]. The conventional adipogenic induction cocktail—composed of dexamethasone (DEX), 3-isobutyl-1-methylxanthine (IBMX), and insulin—has been widely employed as the standard methodology across cellular models [10,11]. Building upon this foundation, substantial efforts have been dedicated to protocol refinement. The recognition of PPARγ as the central regulator of adipogenesis prompted the incorporation of potent agonists such as rosiglitazone. As a thiazolidinedione-class antidiabetic drug, rosiglitazone improves glycemic control via insulin sensitization, while its ability to stimulate adipocyte differentiation and lipid accumulation accounts for the weight gain observed clinically [12,13]. This dual activity enables rosiglitazone to significantly enhance differentiation efficiency in both cell lines and primary preadipocytes [14,15]. Further strategies include prolonged IBMX exposure and the use of alternative PPARγ agonists like mifepristone [16,17]. Remarkably, single-agent mifepristone treatment is capable of inducing adipogenesis in 3T3-L1 preadipocytes [18].
Despite these advances, adipogenesis research continues to face substantial challenges in protocol standardization. Considerable variability exists in inducer concentrations across laboratories, with studies reporting different optimal levels of DEX, IBMX, and insulin, particularly when working with out-of-thaw or high-passage cells [17,19]. Additionally, differences in induction timing, serum lots, and culture conditions further contribute to inconsistent differentiation outcomes across cell sources and species [20,21]. The absence of a standardized, universally applicable protocol remains a major limitation in the field, hindering both reproducibility and translational applications.
In response to these challenges, we present a comprehensively optimized adipogenic differentiation protocol developed through systematic parameter optimization. Our method builds upon established approaches while introducing critical refinements to achieve superior performance across multiple cellular models, including 3T3-L1 preadipocytes, primary stromal vascular fraction (SVF) from neonatal and adult mice, and human adipose-derived stem cells (hADSCs). It should be noted that the inherent heterogeneity of SVF, which contains a mixture of fibroblasts, leukocytes, endothelial cells, and stromal cells, may contribute to variations in differentiation efficiency between replicates, donors, and laboratories. Nevertheless, this protocol offers a valuable standardized tool for investigating adipogenesis in diverse experimental settings.
Materials and reagents
Biological materials
1. 3T3-L1 preadipocytes (Cell Resource Center, Peking Union Medical College; NSTI-BMCR, catalog number: 1101MOU-PUMC000155)
2. Human adipose-derived stem cells (hADSCs) (Procell, catalog number: CP-H202), provided as a cultured cell line
3. Primary mouse stromal vascular fraction (SVF), isolated from the inguinal white adipose tissue (iWAT) of two distinct age groups: postnatal day 8 (P8) pups and 8-week-old (8W) adult mice (purchased from Beijing SPF Biotechnology); cells from passages 1 or 2 were used for subsequent differentiation experiments
Reagents
1. Dulbecco's modified Eagle medium (DMEM), high glucose (Gibco, catalog number: 11995065)
2. Dulbecco's modified Eagle medium/Nutrient mixture F-12 (DMEM/F12) (Gibco, catalog number: 11330032)
3. Mesenchymal stem cell medium (MSCM) kit (ScienCell, catalog number: 7501, formulated with phenol red)
4. Newborn calf serum (NBCS) (Every Green, catalog number: 22011-8612)
5. Fetal bovine serum (FBS), heat-inactivated (Hyclone, catalog number: SH30406.05)
6. Penicillin-streptomycin (P/S) solution (Gibco, catalog number: 15140122)
7. GlutaMAX supplement (Gibco, catalog number: 35050061)
8. Phosphate-buffered saline (PBS), without Ca2+ and Mg2+ (Biosharp, catalog number: BL302A)
9. 0.25% Trypsin-EDTA solution (Gibco, catalog number: 25200056)
10. Dimethyl sulfoxide (DMSO), cell culture grade (Sigma-Aldrich, catalog number: D2650)
11. Dexamethasone (DEX) (Sigma-Aldrich, catalog number: D4902, suitable for cell culture)
12. 3-Isobutyl-1-methylxanthine (IBMX) (Sigma-Aldrich, catalog number: I7018)
13. 5 mg/mL insulin (Macgene, catalog number: CC101)
14. 50 mM rosiglitazone (Macgene, catalog number: CH004)
15. 10 mM indomethacin (Solarbio, catalog number: II0100)
16. Ethanol (Thermo Fisher Scientific, catalog number: BP2818-500)
17. Isopropanol (Sigma-Aldrich, catalog number: 190764)
18. Oil Red O powder (Solarbio, catalog number: O8010)
19. 4% paraformaldehyde (PFA) solution (Solarbio, catalog number: P1110)
20. Collagenase I (Sigma-Aldrich, catalog number: C2674)
21. 0.1% Gelatin (OriCell, catalog number: GLT-11301)
22. Poly-D-Lysine (PDL) (Sigma-Aldrich, catalog number: P7280)
Solutions
1. Growth medium for 3T3-L1 preadipocytes (see Recipes)
2. Growth medium for hADSC cells (see Recipes)
3. Growth medium for primary mouse SVF (see Recipes)
4. 1 mM DEX solution (see Recipes)
5. 50 mM IBMX solution (see Recipes)
6. Adipogenic induction I medium for 3T3-L1 and hADSC cells (see Recipes)
7. Adipogenic induction I medium for primary mouse SVF (see Recipes)
8. Adipogenic induction II medium for 3T3-L1 and hADSC cells (see Recipes)
9. Adipogenic induction II medium for primary mouse SVF (see Recipes)
10. Adipogenic maintenance medium for 3T3-L1 and hADSC cells (see Recipes)
11. Adipogenic maintenance medium for primary mouse P8 SVF (see Recipes)
12. Adipogenic maintenance medium for primary mouse 8W SVF (see Recipes)
13. 0.3% Oil Red O stock solution (see Recipes)
14. Oil Red O working solution (see Recipes)
15. 1 mg/mL Collagenase I solution (see Recipes)
16. 1 mg/mL PDL solution (see Recipes)
17. 20 μg/mL PDL working solution (see Recipes)
18. Washing solution (see Recipes)
Recipes
1. Growth medium for 3T3-L1 preadipocytes
| Reagent | Final concentration | Volume |
|---|---|---|
| DMEM | 89% | 44.5 mL |
| NBCS | 10% | 5 mL |
| P/S | 1% | 0.5 mL |
| Total | 100% | 50 mL |
Note: To prevent spontaneous differentiation during the maintenance phase, it is recommended to use newborn calf serum (NBCS) instead of fetal bovine serum (FBS). NBCS provides a lower baseline level of adipogenic-promoting factors, which helps maintain 3T3-L1 preadipocytes in an undifferentiated state during expansion, in accordance with the standard protocol for this cell line. For optimal stability, the prepared growth medium can be stored at 4 °C for up to 4 weeks.
2. Growth medium for hADSC cells
| Reagent | Final concentration | Volume |
|---|---|---|
| MSCM | 93% | 46.5 mL |
| FBS | 5% | 2.5 mL |
| P/S | 1% | 0.5 mL |
| Growth supplement | 1% | 0.5 mL |
| Total | 100% | 50 mL |
Note: All medium components are sourced from the Mesenchymal Stem Cell Medium kit and used per the manufacturer's protocol. For optimal stability, the prepared growth medium can be stored at 4 °C for up to 4 weeks.
3. Growth medium for primary mouse SVF
| Reagent | Final concentration | Volume |
|---|---|---|
| DMEM/F12 | 88% | 44 mL |
| FBS | 10% | 5 mL |
| P/S | 1% | 0.5 mL |
| GlutaMAX | 1% | 0.5 mL |
| Total | 100% | 50 mL |
Note: For optimal stability, the prepared growth medium can be stored at 4 °C for up to 4 weeks.
4. 1 mM DEX (1,000× stock solution)
| Reagent | Stock concentration | Quantity |
|---|---|---|
| Ethanol | 2.54 mL | |
| DEX | 1 mM | 1 mg |
F.W: 392.4 g/mol.
Note: Dexamethasone stock solution is prepared in molecular biology–grade ethanol (≥99.5% purity). For optimal stability, sterilize the stock solution through a 0.22-μm filter before aliquoting. Store aliquots at -80 °C to avoid repeated freeze-thaw cycles.
5. 50 mM IBMX (100× stock solution)
| Reagent | Stock concentration | Quantity |
|---|---|---|
| DMSO | 1 mL | |
| IBMX | 50 mM | 11.5 mg |
F.W: 222.2 g/mol.
Note: For optimal stability, sterilize the stock solution through a 0.22-μm filter before aliquoting. Store aliquots at -80 °C to avoid repeated freeze-thaw cycles. If crystallization is observed during storage or handling, briefly warm the aliquot to 37 °C and vortex thoroughly until complete redissolution prior to use.
6. Adipogenic induction I medium for 3T3-L1 and hADSC cells
| Reagent | Final concentration | Volume |
|---|---|---|
| DMEM | 43.85 mL | |
| FBS | 10% | 5 mL |
| P/S | 1% | 0.5 mL |
| 1 mM DEX | 1 μM | 0.05 mL |
| 50 mM IBMX | 0.5 mM | 0.5 mL |
| 5 mg/mL insulin | 10 μg/mL | 0.1 mL |
| 50 mM rosiglitazone | 2.5 μM | 2.5 μL |
| Total | 50 mL |
Note: The switch from NBCS to FBS is critical for the initiation of adipogenic differentiation in 3T3-L1 preadipocytes. For optimal activity, prepare the adipogenic induction I medium fresh on the day of use by aseptic addition of all components to DMEM.
7. Adipogenic induction I medium for primary mouse SVF
| Reagent | Final concentration | Volume |
|---|---|---|
| DMEM/F12 | 43.35 mL | |
| FBS | 10% | 5 mL |
| P/S | 1% | 0.5 mL |
| GlutaMAX | 1% | 0.5 mL |
| 1 mM DEX | 1 μM | 0.05 mL |
| 50 mM IBMX | 0.5 mM | 0.5 mL |
| 5 mg/mL insulin | 10 μg/mL | 0.1 mL |
| 50 mM rosiglitazone | 2.5 μM | 2.5 μL |
| Total | 50 mL |
Note: For optimal activity, prepare the adipogenic induction I medium fresh on the day of use by aseptic addition of all components to DMEM/F12.
8. Adipogenic induction II medium for 3T3-L1 and hADSC cells
| Reagent | Final concentration | Volume |
|---|---|---|
| DMEM | 44.4 mL | |
| FBS | 10% | 5 mL |
| P/S | 1% | 0.5 mL |
| 5 mg/mL insulin | 10 μg/mL | 0.1 mL |
| Total | 50 mL |
Note: For optimal activity, prepare the adipogenic induction II medium fresh on the day of use by aseptic addition of all components to DMEM.
9. Adipogenic induction II medium for primary mouse SVF
| Reagent | Final concentration | Volume |
|---|---|---|
| DMEM/F12 | 43.9 mL | |
| FBS | 10% | 5 mL |
| P/S | 1% | 0.5 mL |
| GlutaMAX | 1% | 0.5 mL |
| 5 mg/mL insulin | 10 μg/mL | 0.1 mL |
| Total | 50 mL |
Note: For optimal activity, prepare the adipogenic induction II medium fresh on the day of use by aseptic addition of all components to DMEM/F12.
10. Adipogenic maintenance medium for 3T3-L1 and hADSC cells
| Reagent | Final concentration | Volume |
|---|---|---|
| DMEM | 89% | 44.5 mL |
| FBS | 10% | 5 mL |
| P/S | 1% | 0.5 mL |
| Total | 50 mL |
Note: For optimal stability, the prepared adipogenic maintenance medium can be stored at 4 °C for up to 4 weeks.
11. Adipogenic maintenance medium for primary mouse P8 SVF
| Reagent | Final concentration | Volume |
|---|---|---|
| DMEM/F12 | 88% | 44 mL |
| FBS | 10% | 5 mL |
| P/S | 1% | 0.5 mL |
| GlutaMAX | 1% | 0.5 mL |
| Total | 50 mL |
Note: For optimal stability, the prepared adipogenic maintenance medium can be stored at 4 °C for up to 4 weeks.
12. Adipogenic maintenance medium for primary mouse 8W SVF
| Reagent | Final concentration | Volume |
|---|---|---|
| DMEM/F12 | 43.99 mL | |
| FBS | 10% | 5 mL |
| P/S | 1% | 0.5 mL |
| GlutaMAX | 1% | 0.5 mL |
| 5 mg/mL insulin | 1 μg/mL | 0.01 mL |
| Total | 50 mL |
Notes: For optimal activity, prepare the adipogenic maintenance medium fresh on the day of use by aseptic addition of all components to the DMEM/F12.
13. 0.3% Oil Red O stock solution
| Reagent | Stock concentration | Quantity |
|---|---|---|
| Isopropanol | 100 mL | |
| Oil Red O | 0.3% | 0.3 g |
Note: To prepare the 0.3% Oil Red O stock solution, dissolve 0.3 g of powder in 100 mL of 100% isopropanol and stir the mixture overnight at room temperature to ensure complete dissolution. For optimal stability, store the solution at room temperature in a sealed container for up to 1 year.
14. Oil Red O working solution
| Reagent | Final concentration | Volume |
|---|---|---|
| ddH2O | 1 mL | |
| 0.3% Oil Red O | 0.2% | 2 mL |
Note: The Oil Red O working solution is prepared immediately before use by a 2:1 (v/v) dilution of the 0.3% stock solution with distilled water (ddH2O). The solution is then filtered twice through Whatman filter paper to remove crystalline aggregates and used within 2 h of preparation.
15. 1 mg/mL collagenase I solution
| Reagent | Final concentration | Volume |
|---|---|---|
| 1× PBS | 40 mL | |
| Collagenase I | 1 mg/mL | 50 mg |
| FBS | 20% | 10 mL |
| Total | 50 mL |
Note: All procedures use 1× calcium- and magnesium-free PBS. The Collagenase I used has been sourced from Clostridium histolyticum. To preserve stability, aliquot the stock solution to avoid repeated freeze-thaw cycles and store at -20 °C for up to 6 months.
16. 1 mg/mL PDL solution
| Reagent | Final concentration | Quantity |
|---|---|---|
| ddH2O | 5 mL | |
| PDL | 1 mg/mL | 5 mg |
Note: To preserve stability, aliquot the stock solution to avoid repeated freeze-thaw cycles and store at -20 °C for up to 6 months.
17. 20 μg/mL PDL working solution
| Reagent | Final concentration | Volume |
|---|---|---|
| ddH2O | 9.8 mL | |
| 1 mg/mL PDL | 20 μg/mL | 0.2 mL |
Note: For optimal cell attachment, the working solution should be prepared freshly before use and filter-sterilized. It can be stored at 4 °C for up to 1 month.
18. Washing solution
| Reagent | Final concentration | Volume |
|---|---|---|
| 1× PBS | 44.5 mL | |
| FBS | 10% | 5 mL |
| P/S | 1% | 0.5 mL |
| Total | 50 mL |
Note: The washing solution should be filter-sterilized (0.22 μm) and may be stored at 4 °C for up to 3 months.
Laboratory supplies
1. Cell culture dish, 60 mm × 15 mm (Corning, catalog number: 430196, tissue culture treated)
2. Cell culture dish, 100 mm × 20 mm (Corning, catalog number: 430167, tissue culture treated)
3. 6-well cell culture plate (Corning, catalog number: 3516, tissue culture treated)
4. 24-well cell culture plate (Corning, catalog number: 3524, tissue culture treated)
5. 96-well cell culture plate (Corning, catalog number: 3599, tissue culture treated)
6. 1.5 mL microtubes (Axygen, catalog number: MCT-150-C)
7. 15 mL centrifuge tube (Corning, catalog number: 430790)
8. 50 mL centrifuge tube (Corning, catalog number: 430828)
9. 10 mL serological pipettes (Corning, catalog number: 4488)
10. Cryogenic vials (Corning, catalog number: 430659)
11. 70 μm sterile cell strainers (Falcon, catalog number: 352350)
12. Parafilm (Amcor, catalog number: PM-996)
13. Micropipette tips, 0.5–10 μL (Axygen, catalog number: T-300)
14. Micropipette tips, 20–200 μL (Axygen, catalog number: T-200Y)
15. Micropipette tips, 100–1,000 μL (KIRGEN, catalog number: KG1313)
16. 0.22 μm syringe filter unit (Merck, catalog number: SLGPR33RB)
17. 10 mL Luer-LokTM syringe (BD, catalog number: 302149)
18. Isopropanol freezing container (Nalgene, catalog number: 5100-0001)
19. Whatman filter paper (Cytiva, catalog number: 1825-025)
Equipment
1. Inverted fluorescence microscope (Thermo Fisher Scientific, model: EVOS FL Auto 2)
2. Biosafety cabinet (BAKER, model: SG403A-HE-INT)
3. Laminar flow cabinet (ESCO, model: ACB-4A1)
4. Cell culture incubator (37 °C, 5% CO2) (Thermo Fisher Scientific, model: 371)
5. Centrifuge (Eppendorf, model: 5810R)
6. Water bath (Shanghai Yiheng, model: HWS-24)
7. Hemocytometer (Jiangsu, model: Improved Neubauer)
8. Inverted microscope (Leica, model: DMi8)
9. Surgical tools (scissors: 9.5 cm, straight, from Zhuoyue Medical Devices; forceps: 11 cm, straight or straight with a hook, from Zhuoyue Medical Devices)
10. Single-channel variable pipette, 0.1–2 μL (Rainin, model: Pipet-Lite XLS, catalog number: B735596768)
11. Single-channel variable pipette, 0.5–10 μL (Rainin, model: Pipet-Lite XLS, catalog number: B736643566)
12. Single-channel variable pipette, 20–200 μL (Rainin, model: Pipet-Lite XLS, catalog number: B735593478)
13. Single-channel variable pipette, 100–1,000 μL (Rainin, model: Pipet-Lite XLS, catalog number: B726316711)
14. -20 °C freezer (Haier, model: DW-40L508)
15. -80 °C freezer (Panasonic, model: MDF-682)
16. Vortex mixer (Kylin-Bell, model: VORTEX-5)
17. Analytic balance (Kunshan Youkeweite, model: CN-LQC5003)
18. LN2 dewar/storage system (DONGYA, model: YDS-110-290F)
19. Microplate reader (TECAN, model: Spark)
Software and datasets
1. ImageJ software (Fiji, version 1.53c, publicly available at https://fiji.sc/)
2. GraphPad Prism software (version 8.0.1 for Windows; GraphPad Software, Boston, Massachusetts, USA; https://www.graphpad.com/)
Procedure
A. Adipogenic differentiation of 3T3-L1 and hADSC cells
A1. Cell culture and seeding for differentiation
1. Cell maintenance: Maintain 3T3-L1/hADSC cells in growth medium (refer to Recipe 1 for 3T3-L1 or Recipe 2 for hADSCs). Culture and passage cells routinely at 70%–80% confluence to prevent spontaneous differentiation.
2. Day -4 (seeding):
a. Coating: Add 500 μL of 20 μg/mL PDL or 0.1% gelatin solution to each well of a 24-well plate to ensure complete coverage of the growth surface. Incubate the plate at room temperature for at least 1 h. After incubation, aspirate the solution and rinse each well three times with 500 μL of sterile ddH2O for 2–3 min per rinse. For UV sterilization, place the uncovered plate inside the biosafety cabinet at a distance of approximately 20 cm from the UV lamp and irradiate for 30 min. To ensure safety, the UV sterilization step must be performed only when the cabinet is closed. After sterilization, air-dry the plate inside the biosafety cabinet with the lid off for 30 min before cell seeding.
b. Single-cell suspension preparation and counting: For cells grown in a 60 mm × 15 mm culture dish, aspirate the medium and rinse with 2 mL of prewarmed 1× PBS. Then, digest using 1 mL of 0.25% Trypsin-EDTA (for 3T3-L1) or 0.05% Trypsin-EDTA (for hADSCs) at 37 °C for 2–3 min. Neutralize with double the volume of complete growth medium, gently pipette to dissociate clusters, and centrifuge at 300× g for 5 min. Resuspend the pellet in 1 mL of fresh medium and determine cell concentration with a hemocytometer.
c. Seed the cells at a density of 40,000 cells/cm2 into a 24-well plate in 500 μL of prewarmed growth medium (refer to Recipe 1 for 3T3-L1 or Recipe 2 for hADSCs).
d. Gently rock the plate back-and-forth and side-to-side in a cross-like motion to ensure even distribution of cells across the well surface.
3. Day -2 (medium change): After 2 days of incubation, carefully aspirate the medium and add 500 μL of prewarmed growth medium (refer to Recipe 1 for 3T3-L1 or Recipe 2 for hADSCs).
4. Day 0 (induction initiation):
a. After an additional 2 days of incubation (for a total of 4 days post-seeding), the cells should have reached 100% confluence and undergone contact inhibition, a state where cell proliferation ceases due to dense cell-to-cell contact.
Note: Cells must reach 100% confluence and undergo a 2-day post-confluence period before induction. If not confluent, continue culture in growth medium until this state is achieved.
b. Carefully and completely aspirate the growth medium.
c. Designate this day as day 0 of the differentiation protocol.
A2. Adipogenic induction and maintenance
1. Days 0–3 (induction I phase): Add 700 μL of adipogenic induction I medium (see Recipe 6) and incubate for 3 days. This phase initiates the differentiation program.
2. Days 3–5 (induction II phase):
a. After 3 days, carefully aspirate the adipogenic induction I medium.
b. Add 500 μL of prewarmed adipogenic induction II medium (see Recipe 8) and incubate for 2 days. This phase promotes terminal differentiation and lipid accumulation.
3. Days 5–7 (early maintenance phase):
a. After 2 days, carefully aspirate the adipogenic induction II medium.
b. Add 500 μL of prewarmed adipogenic maintenance medium (see Recipe 10) and incubate for 2 days. This phase allows for lipid maturation.
4. Day 7 onward (continued maintenance and maturation phase):
a. Replace the medium every 2 days with 500 μL of prewarmed adipogenic maintenance medium (see Recipe 10).
b. Monitoring: Lipid droplets become visible under a microscope from days 3–4 onward and continue to enlarge and coalesce thereafter. The maximal level of lipid accumulation, which is suitable for subsequent analysis, is usually achieved by days 10–12.
B. Adipogenic differentiation of primary mouse SVF
B1. Isolation of primary mouse SVF
1. Euthanize mice by cervical dislocation following anesthesia with 3% isoflurane. As shown in Figure 1A and B, the inguinal white adipose tissue (iWAT) is excised, and associated lymph nodes are carefully removed [22]. Perform dissection procedures as follows:
a. Exposure: Make a ventral skin incision and reflect the skin to expose the iWAT depot.
b. Dissection: Gently dissect the iWAT fat pad free from the underlying connective tissue.
c. Lymph node removal: Identify and carefully remove the opaque, whitish lymph nodes embedded within the iWAT using fine forceps and scissors.
Note: The average iWAT yield was approximately 0.15–0.25 g per depot from 8-week-old mice (body weight: 20–25 g) and 0.05–0.1 g per depot from P8 mice (body weight: 4–6 g). All animal procedures were conducted in accordance with the ethical principles of animal welfare of the Beijing Institute of Neurosurgery.
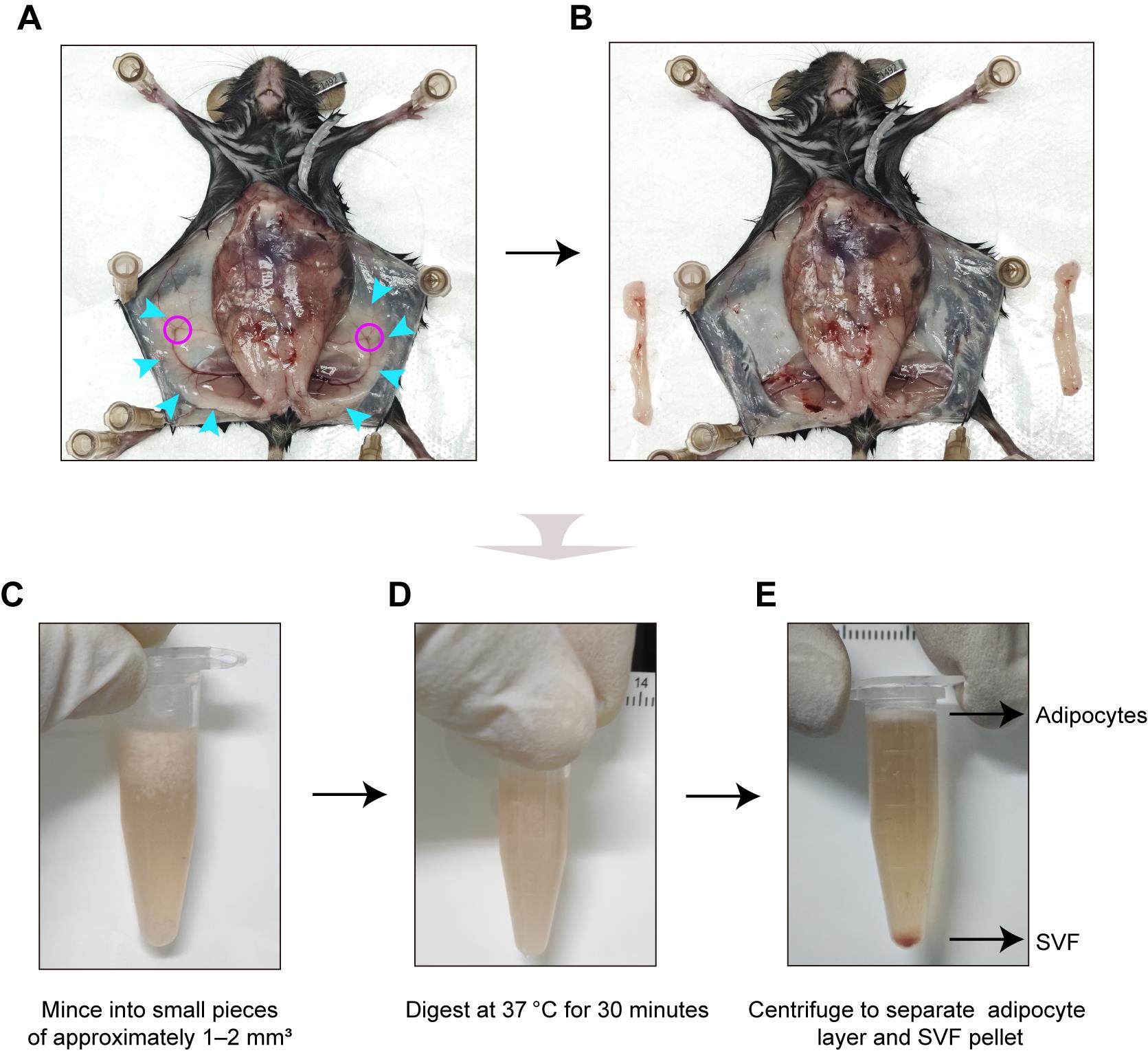
Figure 1. Isolation of stromal vascular fraction (SVF) from mouse inguinal white adipose tissue. (A, B) Representative images of the dissection of inguinal white adipose tissue (iWAT) from 8-week-old mice, showing the tissue site before (A) and after (B) iWAT removal. Blue arrows, iWAT; rosy red circles, lymph nodes. (C, D) Representative images of minced tissue fragments before (C) and after (D) collagenase digestion at 37 °C for 30 min. (E) Representative images of phase separation after centrifugation: upper adipocyte layer and pelleted SVF.
2. Rinse the isolated iWAT three times in a 6-well plate with 2 mL of washing solution (see Recipe 18). Gently agitate for 1–2 min per rinse to eliminate blood cells and contaminants.
3. Transfer the tissue into a sterile 1.5 mL microcentrifuge tube and record its weight.
4. Add 1 mg/mL collagenase I solution (50 μL per 10 mg tissue; see Recipe 15) and mince the tissue into small pieces of approximately 1–2 mm3 using sterile surgical scissors (Figure 1C).
5. Incubate the digestion mixture at 37 °C for 30 min (Figure 1D).
6. During incubation, shake the mixture vigorously every 10 min to promote complete digestion and tissue dissociation.
7. After 30 min, terminate the digestion by adding an equal volume of growth medium and vigorously shaking the mixture approximately 20 times.
8. Centrifuge the tube at 300× g for 5 min at 4 °C to separate the mixture into distinct layers (Figure 1E).
9. Carefully aspirate and discard the upper layer containing floating adipocytes and lipids. Collect the pellet at the bottom of the tube, which contains the SVF cells.
10. Resuspend the SVF pellet in 1 mL of PBS containing 2% FBS.
11. Centrifuge the suspension at 300× g for 5 min and carefully aspirate the supernatant.
12. Resuspend the pellet in 1 mL of PBS with 2% FBS and filter the cell suspension through a sterile 70 μm cell strainer to remove any undigested tissue fragments or large aggregates.
13. After a final centrifugation at 300× g for 5 min, resuspend the purified SVF pellet in 1 mL of growth medium (see Recipe 3).
14. Plate the cell suspension onto a 60 mm culture dish pre-coated with 0.1% gelatin, then add an additional 4 mL of growth medium (see Recipe 3). Incubate the dish in a humidified cell culture incubator at 37 °C with 5% CO2.
15. Approximately 24 h after plating, aspirate the initial medium using a 100–1,000 μL single-channel adjustable micropipette to remove non-adherent cells and debris.
16. Gently add 5 mL of prewarmed growth medium (see Recipe 3).
17. Continue culturing the primary SVF and refreshing the growth medium every 2–3 days.
18. Once reaching 70%–80% confluence—typically within 5–7 days and characterized by cells covering most of the culture surface, with small, visible intercellular gaps—passage the P0 SVF for subsequent experiments.
B2. Cell culture and seeding for differentiation
1. Cell culture: Primary SVF are harvested and passaged upon reaching 70%–80% confluence. For differentiation experiments, cells between passage 1 (P1) and P2 are used to ensure optimal differentiation potential.
2. Day -4 (seeding):
a. Coating: Coat the plates according to the detailed protocol provided in step A1.2a.
b. Single-cell suspension preparation and counting: Prepare a single-cell suspension and perform cell count as described in step A1.2b, using 0.25% Trypsin-EDTA with a digestion time of 2–3 min at 37 °C.
c. Seed the SVF at a density of 40,000 cells/cm2 into a 24-well plate in 500 μL of prewarmed growth medium (see Recipe 3).
d. Gently rock the plate back-and-forth and side-to-side in a cross-like motion to ensure even distribution of cells across the well surface.
3. Day -2 (medium change): After 2 days of incubation, carefully aspirate the medium and add 500 μL of prewarmed growth medium (see Recipe 3).
4. Day 0 (induction initiation):
a. After an additional 2 days of incubation (for a total of 4 days post-seeding), the SVF should have reached 100% confluence and undergone contact inhibition.
b. Carefully and completely aspirate the growth medium.
c. Designate this day as day 0 of the differentiation protocol.
B2. Adipogenic induction and maintenance
1. Days 0–3 (induction I phase): Add 700 μL of adipogenic induction I medium (see Recipe 7) and incubate for 3 days. This phase initiates the differentiation program.
2. Days 3–5 (induction II phase):
a. After 3 days, carefully aspirate the adipogenic induction I medium.
b. Add 500 μL of prewarmed adipogenic induction II medium (see Recipe 9) and incubate for 2 days. This phase promotes terminal differentiation and lipid accumulation.
3. Days 5–7 (early maintenance phase):
a. After 2 days, carefully aspirate the adipogenic induction II medium.
b. Add 500 μL of prewarmed adipogenic maintenance medium (refer to Recipe 11 for P8 SVF or Recipe 12 for 8W SVF) and incubate for 2 days to promote lipid maturation.
Note: For adult SVF, the addition of 1 μg/mL insulin in adipogenic maintenance medium facilitates lipid droplet formation.
4. Day 7 onward (continued maintenance and maturation):
a. Replace the medium every 2 days with 500 μL of prewarmed adipogenic maintenance medium (refer to Recipe 11 for P8 SVF or Recipe 12 for 8W SVF).
b. Monitoring: Lipid accumulation is monitored as described in step A2.4b.
C. Oil Red O Staining
1. Fixation
a. Following adipogenic induction, aspirate the culture medium and gently rinse the cells twice with 500 μL of 1× PBS for 1–2 min per rinse.
b. Fix the cells by adding commercially available 4% PFA solution (e.g., 300 μL per well for a 24-well plate) for 20 min at room temperature.
c. Following fixation, carefully aspirate the 4% PFA and gently wash the cells three times with 500 μL of 1× PBS (10 min per wash) to ensure complete removal of residual fixative.
2. Staining
a. After the final wash, add freshly prepared Oil Red O working solution to cover the fixed cells (e.g., 500 μL per well of a 24-well plate) for staining.
b. Incubate for 20 min at room temperature, protected from light.
Note: Prolonged incubation may lead to overstaining and darkening of lipid droplets.
3. Washing and imaging
a. Following aspiration of the Oil Red O working solution, gently wash the stained cells three times with 500 μL of ddH2O (5 min per wash) to remove nonspecifically bound dye and minimize the background.
Note: To preserve adipocyte integrity throughout the washing and fixation procedures, all solutions are gently added and aspirated slowly along the side of each well to minimize shear stress and prevent detachment of the lipid-laden cells.
b. Proceed to acquire brightfield images of the stained lipid droplets using an inverted microscope.
4. Quantitative analysis of lipid content
a. For quantitative analysis of lipid content, solubilize the stained Oil Red O by adding 100% isopropanol to each well (e.g., 500 μL/well for a 24-well plate).
b. Shake gently for 10 min at room temperature to ensure complete extraction.
c. Transfer 200 μL of the extracted dye solution to a clear 96-well plate and measure the absorbance at a wavelength of 570 nm using a microplate reader. The measured optical density (OD) value is directly proportional to the lipid content in the sample.
Data analysis
All values represent the mean ± S.E.M. of three independent biological replicates. Statistical significance between two groups was determined by an unpaired two-tailed Student’s t-test. For comparisons between more than two groups involving one variable, one-way ANOVA with Bonferroni post hoc correction was applied. All statistical tests and graphing were conducted in GraphPad Prism software (version 8.0), and differences were considered significant at P < 0.05.
Validation of protocol
Here, we report a well-defined and highly effective adipogenic differentiation protocol optimized for multiple cellular models, including murine 3T3-L1 preadipocytes, SVF from neonatal and adult mice, and hADSCs. Through systematic optimization of critical parameters—such as cell density, induction timing, surface coating, and inducer composition—we establish a robust and reproducible system suitable for diverse adipogenesis research.
Combined enhancement of adipogenesis by high cell density, rosiglitazone, and prolonged induction in 3T3-L1 preadipocytes
As shown in Figure 2, adipogenesis was more pronounced at 100% confluence than at 70% and was significantly enhanced by the addition of rosiglitazone in induction I medium compared to the rosiglitazone-free control. Moreover, extending the induction I period from 2 to 3 days led to a marked increase in lipid content across all conditions (Figure 3). These results confirm that high cell density (100% confluence), rosiglitazone supplementation, and prolonged induction I period produce a combined enhancement of lipid accumulation in 3T3-L1 preadipocytes, as previously established in our validated differentiation protocol [23].
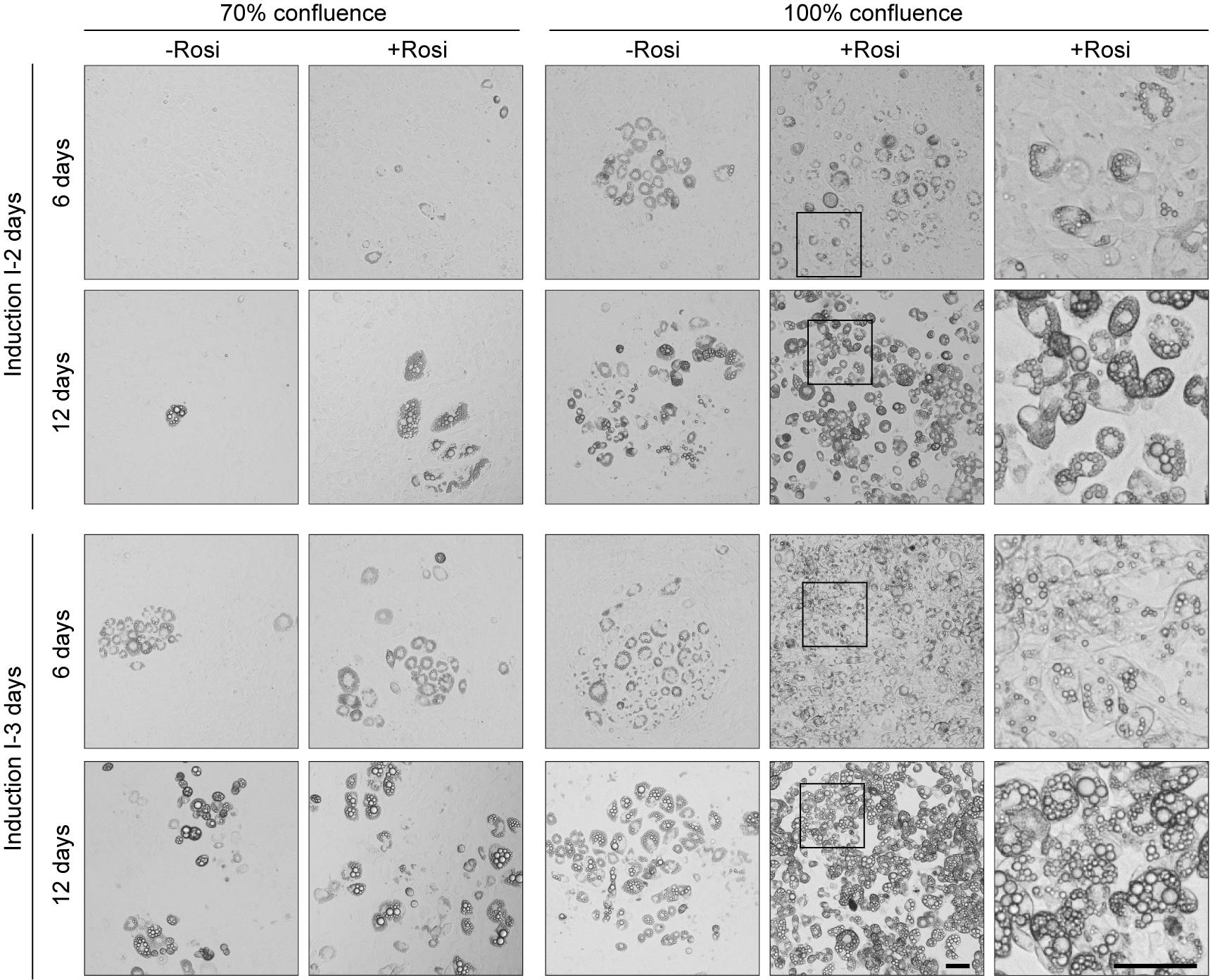
Figure 2. High confluence, rosiglitazone, and extended induction combined to enhance adipogenic differentiation in 3T3-L1 preadipocytes. Representative images of 3T3-L1 preadipocytes following 6 and 12 days of differentiation under the indicated conditions of initial confluence (70% or 100%), with or without rosiglitazone, and induction I medium durations (2 or 3 days). Rosi, rosiglitazone. The furthest right panel shows a magnified view of the area boxed in the 100%+Rosi condition. Scale bar, 50 μm.
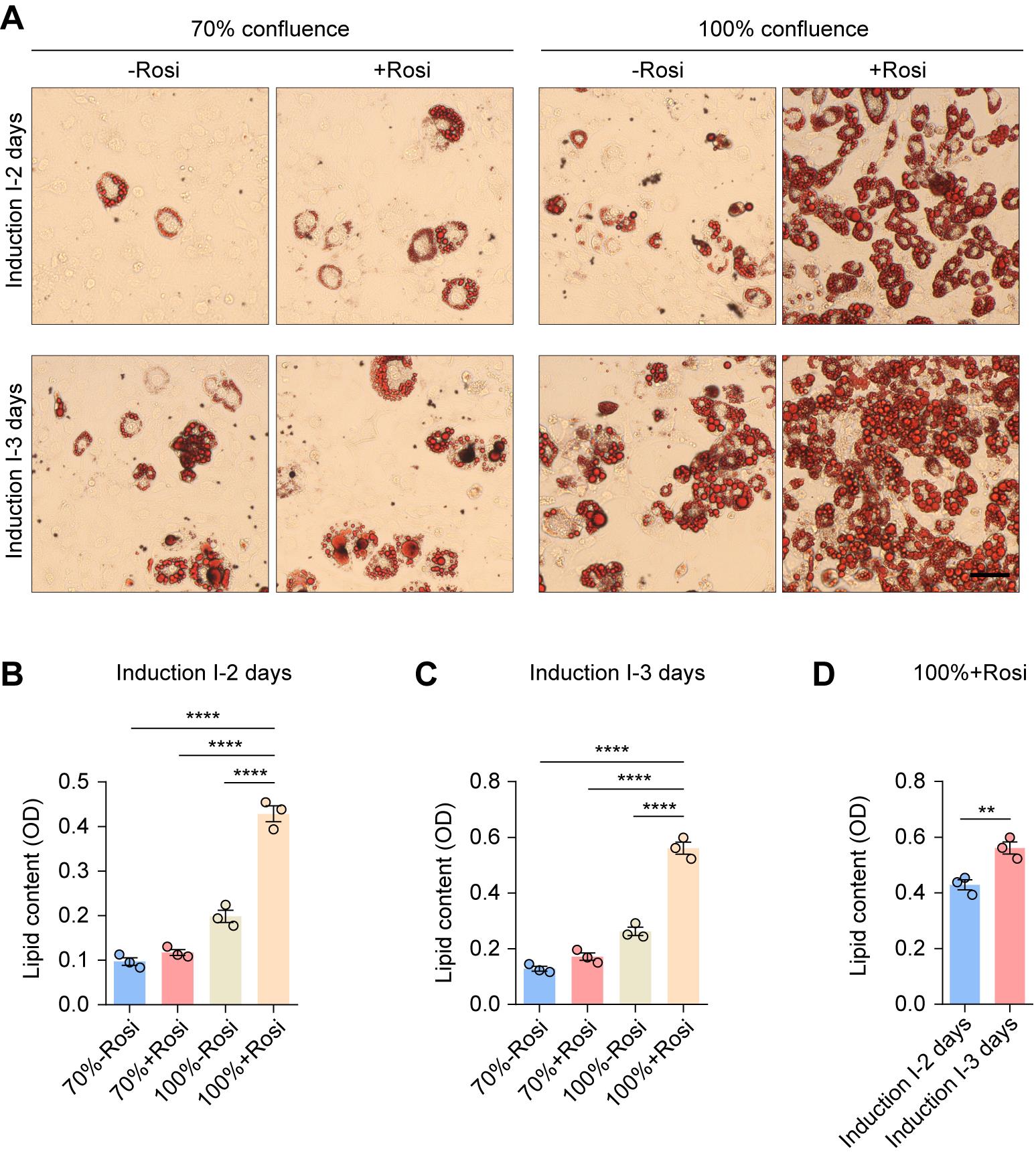
Figure 3. Representative Oil Red O staining and quantitative analysis of lipid content. (A) Representative Oil Red O staining images of 3T3-L1 preadipocytes following 12 days of differentiation under the indicated conditions of initial confluence (70% or 100%), with or without rosiglitazone, and induction I medium durations (2 or 3 days). Rosi, rosiglitazone. Scale bar, 50 μm. (B–D) Quantitative analysis of lipid content. OD, optical density. Values are presented as mean ± S.E.M. from three independent biological experiments. **P < 0.01, ****P < 0.0001. One-way ANOVA followed by Bonferroni post hoc test (B, C) and unpaired two-tailed Student’s t-test (D).
Surface coating prevents cell detachment without affecting adipogenic efficiency
Having defined the key chemical parameters for optimal differentiation, we next evaluated the impact of surface coating on adipogenic efficiency and cell adhesion. Lipid accumulation was comparable among uncoated, PDL-, and 0.1% gelatin-coated surfaces, indicating that coating type does not alter adipogenic potential under the optimized protocol (Figure 4). Nonetheless, uncoated surfaces were highly susceptible to cell detachment in later induction stages. Therefore, while not affecting efficiency, the use of PDL or 0.1% gelatin coating is essential to maintain monolayer integrity and prevent cell loss throughout the process.
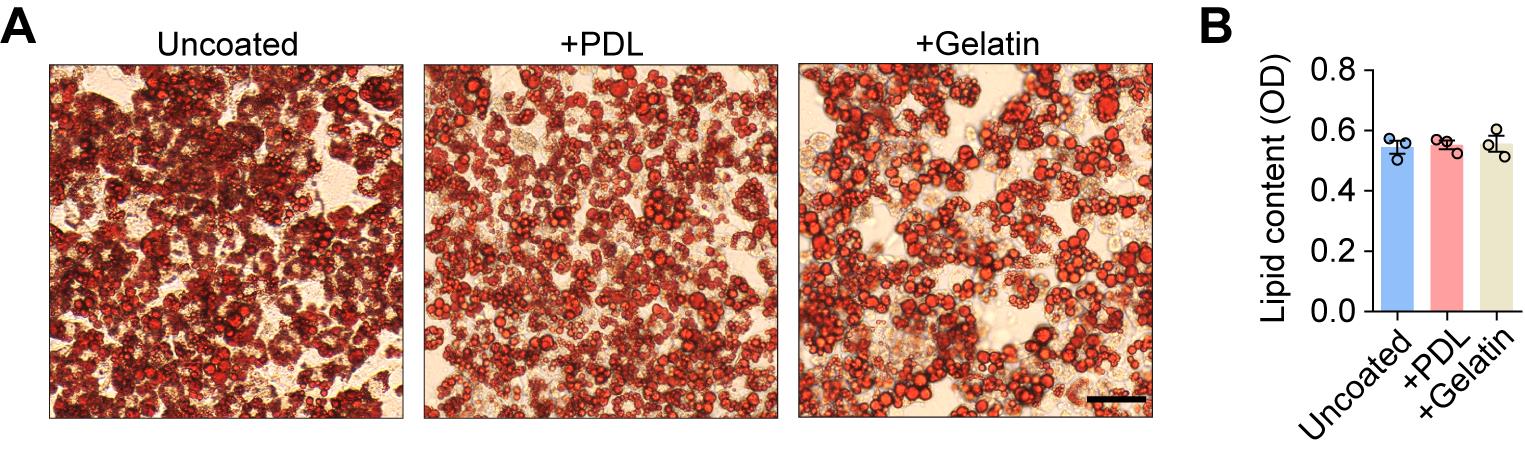
Figure 4. Adipogenic differentiation is similar on uncoated and poly-D-Lysine (PDL)- or gelatin-coated surfaces. (A) Representative Oil Red O staining images of 3T3-L1 preadipocytes following 12 days of differentiation on uncoated and PDL- or 0.1% gelatin-coated surfaces. Scale bar, 50 μm. (B) Quantitative analysis of lipid content. OD, optical density. Values are presented as mean ± S.E.M. from three independent biological experiments.
Adult SVF requires prolonged insulin stimulation for optimal differentiation
The initial protocol (10 μg/mL insulin in induction I medium) effectively differentiated neonatal (P8) mouse SVF but proved suboptimal for adult (8-week) SVF (Figure 5A). Intriguingly, continuous supplementation of the maintenance medium with 1 μg/mL insulin markedly enhanced lipid accumulation in adult SVF (Figure 5B). In contrast, reducing the insulin concentration to 1 μg/mL during the induction phase diminished lipid accumulation, confirming the necessity of a 10 μg/mL dose at the induction phase. Therefore, while the standard protocol is adequate for neonatal SVF, optimal differentiation of adult SVF critically depends on prolonged low-dose insulin stimulation during the maintenance phase.
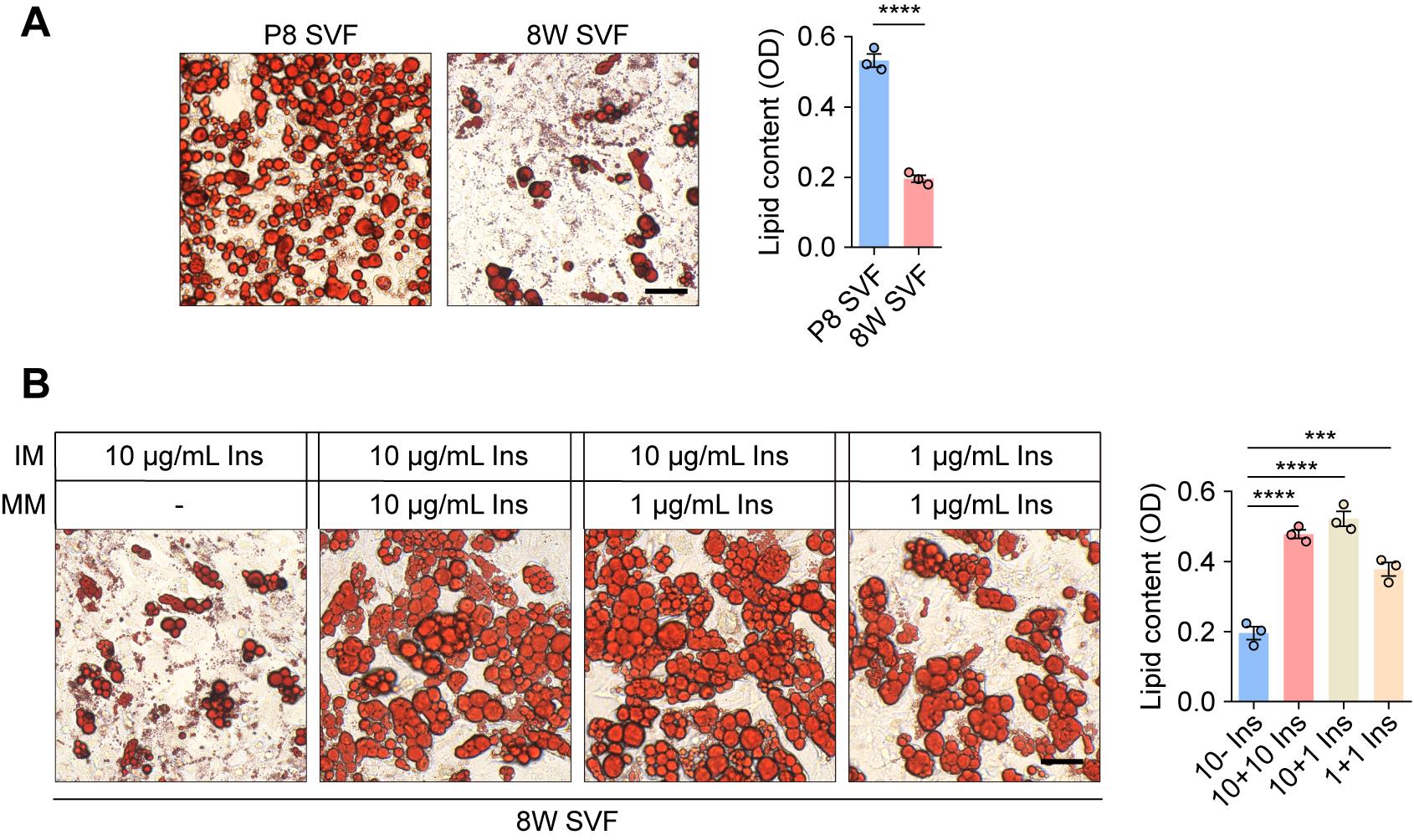
Figure 5. Optimization of insulin concentration for adult stromal vascular fraction (SVF). (A) Representative Oil Red O staining images of primary P8 and 8W SVF following 12 days of differentiation. Quantitative analysis of lipid content is shown on the right. Scale bar, 50 μm. (B) Representative Oil Red O staining images of primary 8W SVF following 12 days of differentiation under varying insulin concentrations in the induction and maintenance media. Quantitative analysis of lipid content is shown on the right. Scale bar, 50 μm. Ins, insulin; IM, induction medium; MM, maintenance medium. OD, optical density. Values are presented as mean ± S.E.M. from three independent biological experiments. ***P < 0.001, ****P < 0.0001. Unpaired two-tailed Student’s t-test (A) and one-way ANOVA followed by Bonferroni post hoc test (B).
Efficient induction of adipogenesis in hADSCs using the optimized protocol
To evaluate the broader applicability of our optimized protocol, we assessed its performance on human adipose-derived stem cells (hADSCs). The protocol successfully induced robust adipogenesis, confirming its cross-species functionality. Importantly, in our hands and under the standardized 12-day differentiation timeline, our formulation—containing DEX, IBMX, insulin, and rosiglitazone—yielded significantly greater lipid accumulation than a previously reported protocol (DEX, IBMX, insulin, and indomethacin) [11,24], as quantified by Oil Red O staining (Figure 6). These results validate the efficacy of our differentiation strategy.
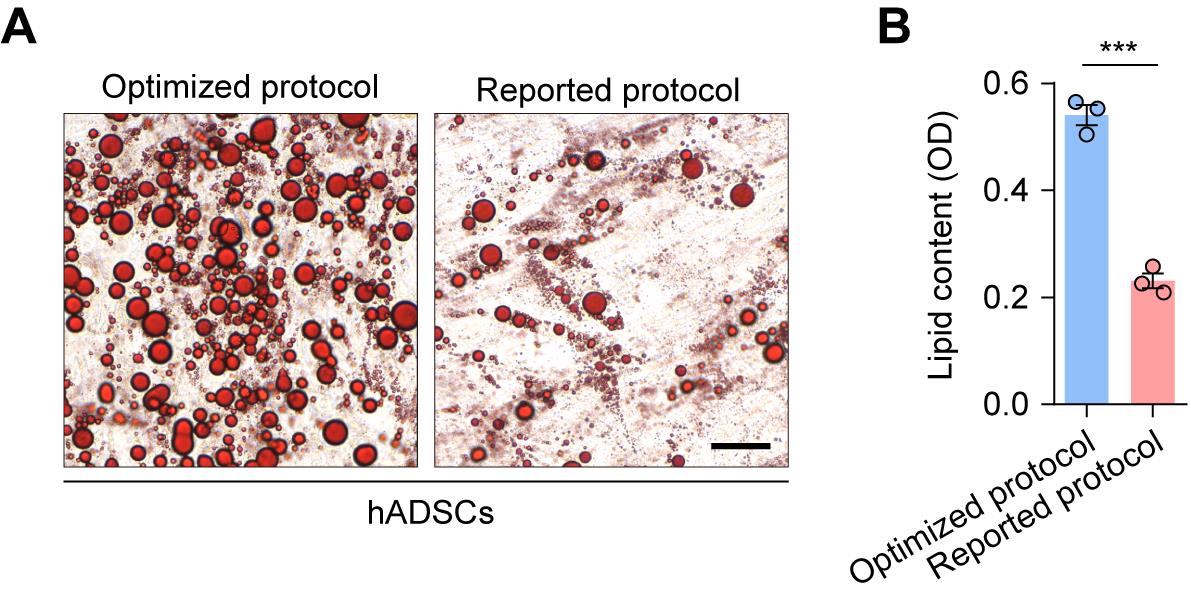
Figure 6. Comparison of adipogenic differentiation in human adipose-derived stem cells (hADSCs) using the optimized and a previously reported protocol. (A) Representative Oil Red O staining images of hADSCs following 12 days of adipogenic differentiation using the optimized protocol or the previously reported protocol (DEX, IBMX, insulin, and 200 μM indomethacin). Scale bar, 50 μm. (B) Quantitative analysis of lipid content. OD, optical density. Values are presented as mean ± S.E.M. from three independent biological experiments. ***P < 0.001. Unpaired two-tailed Student’s t-test.
In conclusion, our findings establish a highly efficient and adaptable adipogenic differentiation protocol suitable for multiple cell types. The systematic optimization of induction conditions, surface coatings, and age-specific insulin regimens ensures high reproducibility and performance, providing a valuable tool for future in vitro studies of adipogenesis.
General notes and troubleshooting
General notes
1. Reagent preparation and storage: Stock solutions should be freshly prepared and aliquoted immediately. Single-use aliquots are recommended and must be stored at -20 or -80 °C. Avoid repeated freeze-thaw cycles to preserve reagent stability.
2. Isolation of primary SVF: Due to their high susceptibility to microbial contamination, maintaining strict aseptic technique is critical throughout all steps of primary SVF isolation. The use of sterile reagents and equipment is mandatory.
3. Cell passage number: The differentiation potential of cells, including the 3T3-L1 preadipocytes, primary SVF, and hADSCs, is well-documented to decline with increasing passage number. To ensure optimal results, it is critical to use cells at low passages (e.g., 3T3-L1 before P10, mouse SVF at P1–P2, and hADSCs at P3–P6).
4. Cell confluence: For efficient adipogenesis, cells must reach 100% confluence and subsequently undergo an additional 2-day period of contact inhibition prior to induction. Sub-confluent cultures at induction will result in poor differentiation efficiency.
5. Post-induction handling: Following the induction of differentiation, the medium becomes highly viscous, and the cell layer turns unstable. During medium changes, fresh prewarmed medium should be added gently along the wall of the plate to avoid disruption of the cell layer.
Troubleshooting
Problem 1: Cell detachment and death during induction.
Possible causes:
1) Insufficient or degraded plate coating.
2) Excessive mechanical stress during medium changes.
Solutions:
1) Optimize coating: Coat plates immediately before cell seeding and avoid long-term storage of coated plates.
2) Refine medium change: Prewarm all media to 37 °C before use. Gently add and remove medium along the side of the well, ensuring the stream does not directly impact the cell monolayer.
Problem 2: Lipid droplets appear black or darkened in Oil Red O staining.
Possible causes:
1) Over-staining or excessive incubation with Oil Red O working solution.
2) Inadequate washing steps after staining, leading to precipitate formation.
3) Use of an overly concentrated Oil Red O stock or working solution.
Solutions:
1) Optimize staining duration: Reduce the incubation time with Oil Red O working solution to 15–20 min.
2) Ensure thorough washing: Rinse gently but thoroughly with distilled water at least three times after staining.
3) Prepare working solution correctly: Dilute the Oil Red O stock solution appropriately according to the protocol and filter it before use to remove undissolved particles.
Problem 3: Inconsistent differentiation results between replicates or batches.
Possible causes:
1) Variability between different lots of FBS.
2) Minor deviations in cell passage number or seeding density at the time of induction.
Solutions:
1) Utilize a single, qualified batch of FBS for the entire set of related experiments.
2) Strictly standardize the acceptable cell passage range and ensure consistent seeding densities.
Acknowledgments
Author contributions: Conceptualization, J.F. and W.H.; Funding acquisition, W.H.; Investigation, L.Z. and Y.G.; Methodology, R.S.; Writing–original draft, J.F. and W.H.; Writing–review & editing, J.F., Y.W., and W.H.
Funding source: This work was supported by grants from the National Natural Science Foundation of China (82371353).
Original research paper for this protocol: Fan et al. [23].
Competing interests
The authors declare no competing interests.
Ethical considerations
The animal study was approved by the Ethics Committee of Beijing Institute of Neurosurgery (Approval No. 201902005; valid from 1 February 2019 to 31 December 2025).
References
- Ghaben, A. L. and Scherer, P. E. (2019). Adipogenesis and metabolic health. Nat Rev Mol Cell Biol. 20(4): 242–258. https://doi.org/10.1038/s41580-018-0093-z
- Bunnell, B., Flaat, M., Gagliardi, C., Patel, B. and Ripoll, C. (2008). Adipose-derived stem cells: Isolation, expansion and differentiation. Methods. 45(2): 115–120. https://doi.org/10.1016/j.ymeth.2008.03.006
- Cawthorn, W. P., Scheller, E. L. and MacDougald, O. A. (2012). Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 53(2): 227–246. https://doi.org/10.1194/jlr.r021089
- Phelps, N. H., Singleton, R. K., Zhou, B., Heap, R. A., Mishra, A., Bennett, J. E., Paciorek, C. J., Lhoste, V. P., Carrillo-Larco, R. M., Stevens, G. A., et al. (2024). Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet. 403(10431): 1027–1050. https://doi.org/10.1016/s0140-6736(23)02750-2
- Billon, N., Iannarelli, P., Monteiro, M. C., Glavieux-Pardanaud, C., Richardson, W. D., Kessaris, N., Dani, C. and Dupin, E. (2007). The generation of adipocytes by the neural crest. Development. 134(12): 2283–2292. https://doi.org/10.1242/dev.002642
- Sanchez-Gurmaches, J. and Guertin, D. A. (2014). Adipocyte lineages: Tracing back the origins of fat. Biochim Biophys Acta. 1842(3): 340–351. https://doi.org/10.1016/j.bbadis.2013.05.027
- Walther, T. C., Chung, J. and Farese, R. V. (2017). Lipid Droplet Biogenesis. Annu Rev Cell Dev Biol. 33(1): 491–510. https://doi.org/10.1146/annurev-cellbio-100616-060608
- Farmer, S. R. (2006). Transcriptional control of adipocyte formation. Cell Metab. 4(4): 263–273. https://doi.org/10.1016/j.cmet.2006.07.001
- de Sá, P. M., Richard, A. J., Hang, H. and Stephens, J. M. (2017). Transcriptional Regulation of Adipogenesis. Compr Physiol. 7: 635–674. https://doi.org/10.1002/cphy.c160022
- Zebisch, K., Voigt, V., Wabitsch, M. and Brandsch, M. (2012). Protocol for effective differentiation of 3T3-L1 cells to adipocytes. Anal Biochem. 425(1): 88–90. https://doi.org/10.1016/j.ab.2012.03.005
- Scott, M. A., Nguyen, V. T., Levi, B. and James, A. W. (2011). Current Methods of Adipogenic Differentiation of Mesenchymal Stem Cells. Stem Cells Dev. 20(10): 1793–1804. https://doi.org/10.1089/scd.2011.0040
- Yki-Järvinen, H. (2004). Thiazolidinediones. N Engl J Med. 351(11): 1106–1118. https://doi.org/10.1056/NEJMra041001
- Tontonoz, P. and Spiegelman, B. M. (2008). Fat and Beyond: The Diverse Biology of PPARγ. Annu Rev Biochem. 77(1): 289–312. https://doi.org/10.1146/annurev.biochem.77.061307.091829
- Pu, Y. and Veiga-Lopez, A. (2017). PPARγ agonist through the terminal differentiation phase is essential for adipogenic differentiation of fetal ovine preadipocytes. Cell Mol Biol Lett. 22(1): 6. https://doi.org/10.1186/s11658-017-0037-1
- Schouwink, M., Öner-Sieben, S. and Ensenauer, R. (2025). Longitudinal expression profiles of key markers during stages of adipogenic differentiation of 3T3-L1 cells using the PPARG agonist rosiglitazone. Biochem Biophys Res Commun. 770: 151850. https://doi.org/10.1016/j.bbrc.2025.151850
- Hua, Y., Ke, S., Wang, Y., Irwin, D. M., Zhang, S. and Wang, Z. (2016). Prolonged treatment with 3-isobutyl-1-methylxanthine improves the efficiency of differentiating 3T3-L1 cells into adipocytes. Anal Biochem. 507: 18–20. https://doi.org/10.1016/j.ab.2016.05.007
- Zhao, X., Hu, H., Wang, C., Bai, L., Wang, Y., Wang, W. and Wang, J. (2019). A comparison of methods for effective differentiation of the frozen-thawed 3T3-L1 cells. Anal Biochem. 568: 57–64. https://doi.org/10.1016/j.ab.2018.12.020
- Hashimoto, T. and Hirano, K. (2024). Effects of mifepristone on adipocyte differentiation in mouse 3T3-L1 cells. Cell Mol Biol Lett. 29(1): 45. https://doi.org/10.1186/s11658-024-00559-9
- Khanmohammadi, M., Khanjani, S., Edalatkhah, H., Zarnani, A. H., Heidari-Vala, H., Soleimani, M., Alimoghaddam, K. and Kazemnejad, S. (2014). Modified protocol for improvement of differentiation potential of menstrual blood-derived stem cells into adipogenic lineage. Cell Prolif. 47(6): 615–623. https://doi.org/10.1111/cpr.12133
- Mehra, A., Macdonald, I. and Pillay, T. S. (2007). Variability in 3T3-L1 adipocyte differentiation depending on cell culture dish. Anal Biochem. 362(2): 281–283. https://doi.org/10.1016/j.ab.2006.12.016
- Heimann, M., Elashry, M. I., Klymiuk, M. C., Eldaey, A., Wenisch, S. and Arnhold, S. (2023). Optimizing the Adipogenic Induction Protocol Using Rosiglitazone Improves the Physiological Parameters and Differentiation Capacity of Adipose Tissue-Derived Mesenchymal Stem Cells for Horses, Sheep, Dogs, Murines, and Humans. Animals. 13(20): 3224. https://doi.org/10.3390/ani13203224
- Bowles, A. C., Tucker, A., Bunnell, B. A. (2018). Isolation and Flow Cytometric Analysis of the Stromal Vascular Fraction Isolated from Mouse Adipose Tissue. Methods Mol Biol. 1773: 1–9. https://doi.org/10.1007/978-1-4939-7799-4_1
- Fan, J., Ma, T., Ren, X., Chang, Z., Zhang, D., Wang, Y., Cheng, S., Qi, X., Liu, K., Wang, Y., et al. (2025). cTAGE5 is essential for adipogenesis and adipose tissue development. Nat Commun. 16(1): 5457. https://doi.org/10.1038/s41467-025-60698-1
- Adam, L. T., Pitere, R. R., Ambele, M. A. and Pepper, M. S. (2025). Adipogenic Differentiation of Human Adipose-Derived Mesenchymal Stromal/Stem Cells (ASCs). Methods Mol Biol. 2938: 63–67. https://doi.org/10.1007/978-1-0716-4607-6_7
Article Information
Publication history
Received: Oct 22, 2025
Accepted: Dec 10, 2025
Available online: Dec 25, 2025
Published: Jan 20, 2026
Copyright
© 2026 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Fan, J., Zhou, L., Geng, Y., Song, R., Wang, Y. and He, W. (2026). Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models. Bio-protocol 16(2): e5571. DOI: 10.21769/BioProtoc.5571.
Category
Stem Cell > Adult stem cell > Adipose Stem Cell
Cell Biology > Cell isolation and culture > Cell differentiation
Stem Cell > Adult stem cell > Maintenance and differentiation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








