- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Improved Immunohistochemistry of Mouse Eye Sections Using Davidson's Fixative and Melanin Bleaching
Published: Vol 15, Iss 22, Nov 20, 2025 DOI: 10.21769/BioProtoc.5510 Views: 1573
Reviewed by: Pilar Villacampa AlcubierreAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Visualization of Gap Junction–Mediated Astrocyte Coupling in Acute Mouse Brain Slices
Nine F. Kompier [...] Fritz G. Rathjen
Feb 20, 2025 2270 Views

A Novel Optimized Silver Nitrate Staining Method for Visualizing and Quantifying the Osteocyte Lacuno-Canalicular System (LCS)
Jinlian Wu [...] Libo Wang
Apr 20, 2025 1532 Views

Generation of Agarose-Based FFPE Cancer Organoids for Morphology Preservation
Mi Rim Lee [...] Yun-Hee Kim
Oct 5, 2025 1707 Views
Abstract
Immunohistochemistry (IHC) and immunofluorescence (IF) are fundamental molecular biology techniques to assess protein expression. However, the melanin present normally in the eye in the uveal tract (choroid, iris, and ciliary body) and the retinal pigment epithelium (RPE) poses a significant challenge for IHC and IF. This is because melanin interferes with both chromogenic and fluorescent detection methods. Additionally, formalin fixation, which is commonly used for IHC, can result in shrinkage and loss of cellular detail in the eye. This protocol provides an optimized approach using Davidson’s fixative with a hydrogen peroxide bleaching step to eliminate melanin interference in the mouse eye, improving the quality and interpretability of IHC analyses of the uveal tract and RPE. It is particularly useful for the analysis of uveal melanoma.
Key features
• Davidson’s fixative minimizes shrinkage of ocular tissues and preserves cell structure better than formalin.
• Hydrogen peroxide bleaching step removes all melanin, even in highly pigmented samples.
• The protocol is compatible with other tissues that require melanin removal.
Keywords: ImmunohistochemistryGraphical overview
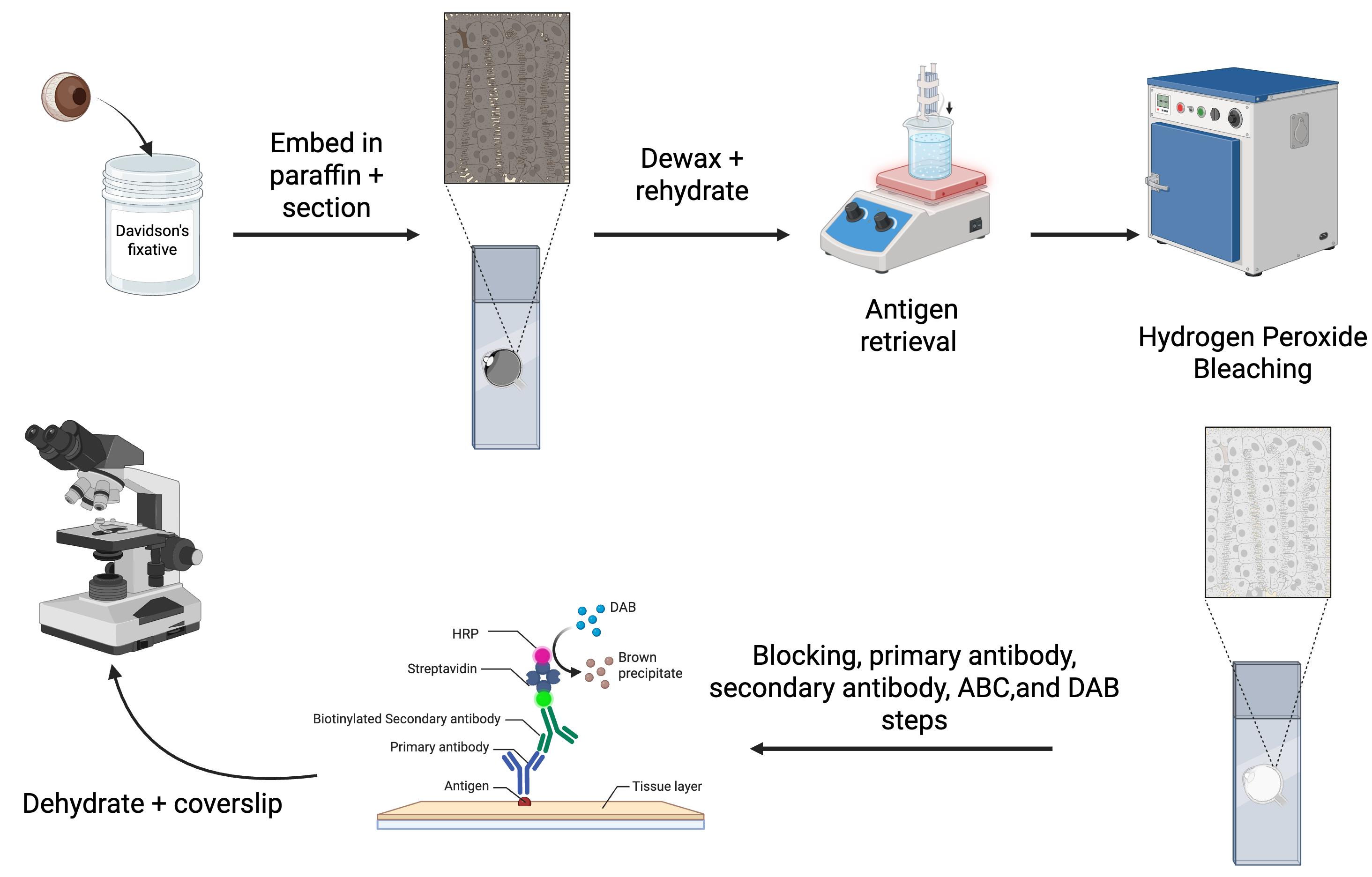
Overview of the immunohistochemistry protocol for eyes
Background
Immunohistochemistry (IHC) and immunofluorescence (IF) are essential techniques in molecular biology for assessing protein expression [1,2]. These methods, which rely on primary antibodies, can determine the presence and subcellular localization of proteins. This helps to identify processes such as cell division or activation of specific signaling pathways [2]. However, pigmentation in ocular tissues, particularly in the uveal tract (choroid, iris, ciliary body) and the retinal pigment epithelium (RPE), presents a significant challenge. Melanin interferes with both chromogenic and fluorescent detection methods, complicating the interpretation of IHC and IF results [3]. Additionally, distinguishing between the pigmented choroid and RPE, which are adjacent layers, can be difficult.
The most widely used chromogen for IHC is 3-3'-diaminobenzidine (DAB), which produces a brown precipitate that can be difficult to differentiate from melanin [3]. While azure B counterstaining can help distinguish between the DAB precipitate and melanin, its effectiveness is limited in highly pigmented tissues due to masking effects [3]. Alternatively, 3-amino-9-ethylcarbazole (AEC), a red chromogen, is an option, but its staining fades over time, making long-term storage of stained sections problematic [4]. Bleaching melanin before IHC has been explored, with methods such as potassium permanganate followed by oxalic acid, hydrogen peroxide, or trichloroisocyanuric acid (TCCA)[5]. However, these protocols have drawbacks, including reduced antigenicity and prolonged bleaching times, which can compromise tissue integrity [3].
In addition to pigment interference, the eye’s unique structure presents challenges in sectioning. Its hard crystalline lens and aqueous humor make it more difficult to slice than other tissues [1]. Formalin, the most commonly used fixative, can lead to cellular shrinkage, poor cellular detail preservation, and retinal detachment when used on ocular tissues [1]. Davidson’s fixative, a mixture of formaldehyde, ethanol, and acetic acid, is preferred for eye tissue IHC as it provides rapid tissue penetration and prevents excessive shrinkage [6].
In our lab, we study mouse models of uveal melanoma, which are highly pigmented tumors [7–13]. To overcome the challenges associated with pigment interference, we optimized an IHC protocol for mouse ocular tissues (Graphical overview). While not all primary antibodies are compatible with this method, we routinely use an S100b antibody to label melanocytes in the uveal tract and an RPE65 antibody for the RPE (Figure 1). In addition, we use a Ki67 antibody for uveal melanoma samples to label cells undergoing mitosis, and a CD68 antibody to identify melanophages. Melanophages are macrophages that have ingested melanin (Figure 2). In this paper, we present our optimized IHC protocol, which combines Davidson’s fixative with a hydrogen peroxide bleaching step to effectively eliminate melanin interference and improve protein detection in ocular tissues and uveal melanoma.
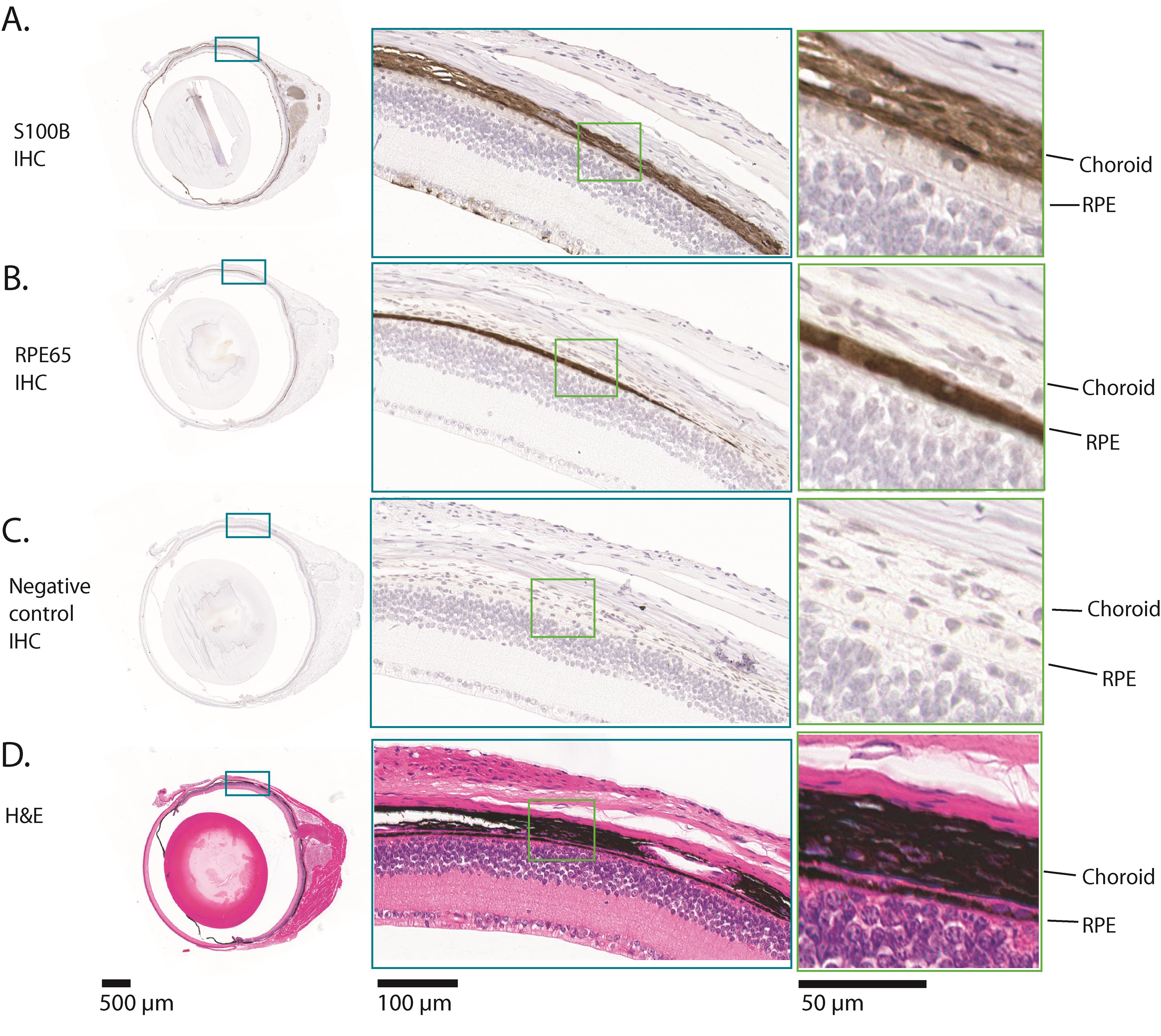
Figure 1. Example results of immunohistochemistry (IHC) for S100b and RPE65 on a normal adult mouse eye. The images shown are all serial sections from the same adult mouse eyeball. (A) This row shows the results of IHC for S100b. Melanocytes in the pigmented tissues, the choroid, ciliary body, and iris, express S100b, along with some cells in the innermost part of the neural retina and the optic nerve. The part of the eye that is enlarged shows S100b expression in the choroid. (B) This row shows the results of IHC for RPE65. RPE65 is expressed exclusively in the retinal pigment epithelium (RPE). The RPE is a monolayer of cuboidal cells. The part of the eye that is enlarged shows RPE65 expression. (C) This row shows the results of the negative control IHC, where no primary antibody was incubated with the section. There is no positive stain in any part of the eye. (D) This row shows a hematoxylin and eosin (H&E) stained section, in which there is abundant melanin in the choroid, iris, ciliary body, and RPE. Comparison of (D) with (C) shows that all melanin was removed during the hydrogen peroxide bleaching step. In each row, the teal and green boxes show progressively higher magnifications, from left to right.
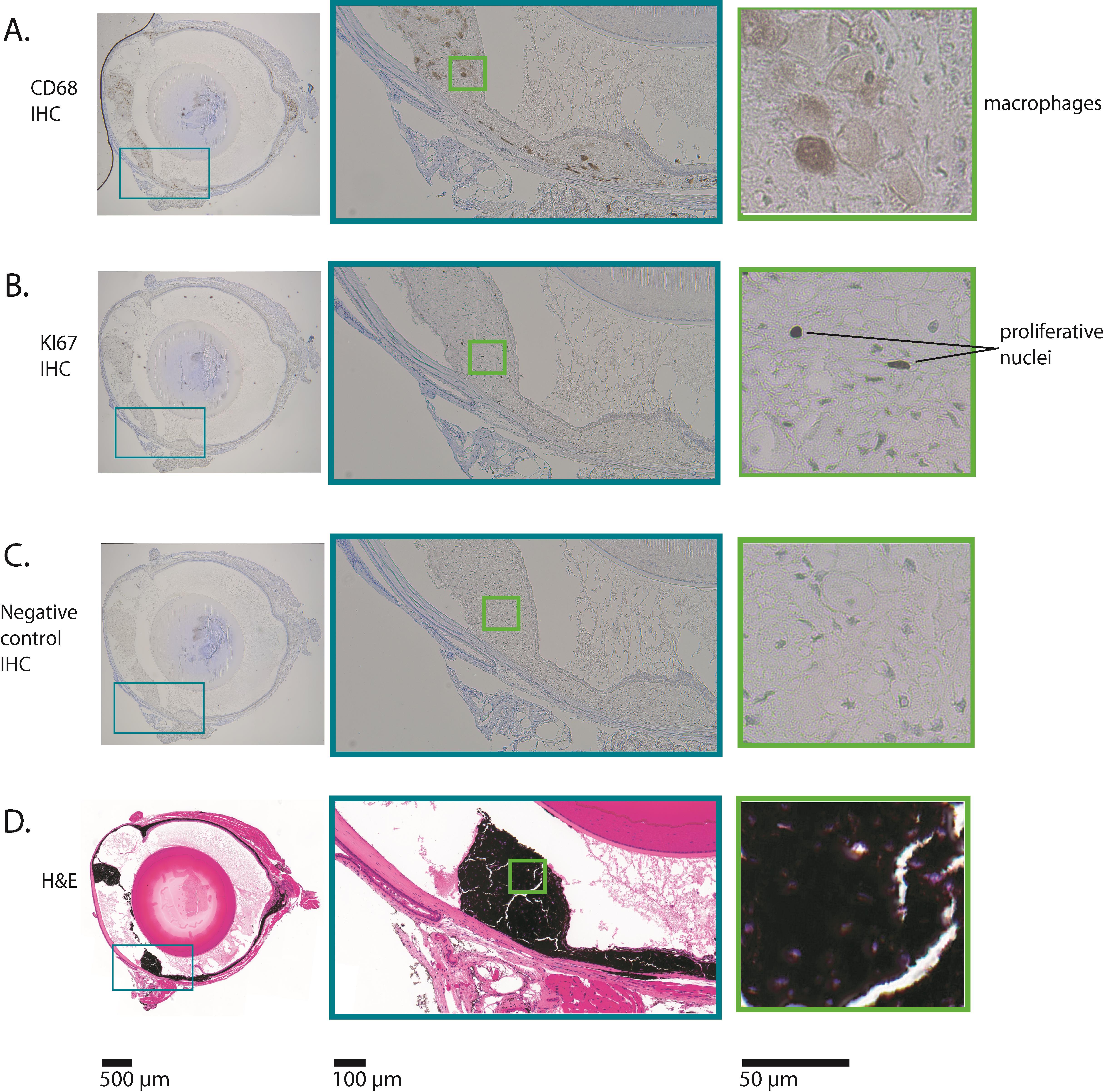
Figure 2. Example results of immunohistochemistry (IHC) for CD68 and Ki67 on a mouse eye with uveal melanoma. The images shown are all serial sections from the same adult mouse eyeball. This mouse expressed oncogenic GNAQQ209L via Mitf-cre (in melanocytes), which induced uveal melanoma. (A) This row shows the results of IHC for CD68. CD68 is expressed in macrophages. In this mouse uveal melanoma model, macrophages ingest melanin, becoming pigmented themselves. The macrophages in this section have abundant cytoplasm, consistent with active phagocytosis. The boxed area shows the part of the eye where the ciliary body and iris would have been located, but is now tumor tissue. (B) This row shows the results of IHC for Ki67. Ki67 is only expressed in nuclei that are undergoing mitosis. A small percentage of nuclei in this tumor are positive for Ki67 (indicated as "proliferative nuclei"), consistent with relatively slow growth. (C) This row shows the results of the negative control IHC, where no primary antibody was incubated with the section. There is no positive stain in any part of the eye. (D) This row shows a hematoxylin and eosin (H&E) stained section, in which there are very high levels of melanin in the thickened and abnormal iris, ciliary body, and choroid. Comparison of (D) with (C) shows that all melanin was removed during the hydrogen peroxide bleaching step. In each row, the teal and green boxes show progressively higher magnifications, from left to right.
Materials and reagents
Biological materials
1. Euthanized mouse
Note: This protocol has been successfully tested on a range of mouse ages, from newborn to adult.
Reagents
1. Paraffin (Fisherbrand, Histoplast PE, catalog number: 22900700)
2. VECTASTAIN® Elite® ABC-HRP Kit, peroxidase (rabbit IgG) (Vector Laboratories VECTPK6101), for use with anti-S100b, anti-CD68, and anti-Ki67 primary antibodies; this kit includes normal goat serum
3. VECTASTAIN® Elite® ABC-HRP Kit, peroxidase (mouse IgG) (Vector Laboratories VECTPK6102), for use with anti-RPE65 primary antibody; this kit includes normal horse serum
4. DAB Substrate kit peroxidase (with nickel) (Vector Laboratories, catalog number: SK4100)
5. Xylene histological grade (Fisher Chemical, catalog number: X3P-1GAL)
6. Hydrogen peroxide (H2O2) certified ACS 30% (Fisher Chemical, catalog number: H325-500), store at 4 °C
7. Triton X-100 (Sigma-Aldrich, catalog number: T8787-100ML)
8. Tween 20 (Sigma-Aldrich, catalog number: P9416-100ML)
9. Eukitt quick hardening mounting medium for microscopy (Sigma-Aldrich, catalog number: 03989-100ML)
10. Antigen unmasking solution, citric acid-based (100× stock citrate buffer) (Vector Laboratories, catalog number: H-3300)
11. Glacial acetic acid (Fisher Chemical, catalog number: A38-500)
12. Formaldehyde, 37% (Fisher Scientific, catalog number: BP531)
13. Distilled water
14. Ultrapure water (Purelab Flex 2; Elga Veolia)
15. 10× PBS (Sigma-Aldrich, catalog number: PPB006-20PAK)
16. Neutral buffered formalin, 10% (Simport, catalog number: M961-40FW)
17. Anti-S100b antibody (rabbit monoclonal antibody) (Abcam, catalog number: ab52642)
18. Anti-RPE65 antibody (mouse monoclonal antibody 401.8B11.3D9) (Thermo Fisher Scientific, catalog number: MA1-16578)
19. Anti-CD68 antibody (rabbit polyclonal antibody) (Abcam, catalog number: ab125212)
20. Anti-Ki67 antibody (rabbit polyclonal antibody) (Abcam, catalog number: ab15580)
21. Hematoxylin (Vector Laboratories, catalog number: H3401-500)
22. Ammonium hydroxide, ACS grade 30% (VWR, catalog number: BDH3014)
23. Hematoxylin (Vector Laboratories, catalog number: H3401-500)
Solutions
1. Davidson’s fixative (see Recipes)
2. Hydrogen peroxide bleaching solution (see Recipes)
3. 1× PBS/0.1% Tween 20 (see Recipes)
4. 1× PBS/0.3% Triton X-100 (see Recipes)
5. Blocking solution (see Recipes)
6. Primary antibody mix (anti-S100B or anti-KI67) (see Recipes)
7. Primary antibody mix (anti-RPE65) (see Recipes)
8. Primary antibody mix (anti-CD68) (see Recipes)
9. Secondary antibody mix (for S100b, CD68 and Ki67 assays) (see Recipes)
10. Secondary antibody mix (for RPE65 assays) (see Recipes)
11. ABC solution (see Recipes)
12. DAB-nickel solution (see Recipes)
13. Acid rinse solution (see Recipes)
14. Bluing solution (see Recipes)
Recipes
1. Davidson’s fixative
| Reagent | Final concentration | Volume (100 mL total) |
| Formaldehyde (37%) | 0.74% | 2 mL |
| Ethanol (100%) | 35% | 35 mL |
| Glacial acetic acid (100%) | 10% | 10 mL |
| Distilled water | n/a | 53 mL |
Note: Davidson’s fixative can be prepared ahead of time and stored in a glass bottle at room temperature.
2. Hydrogen peroxide bleaching solution
| Reagent | Final concentration | Volume (200 mL total) |
| 10× PBS | 1× | 20 mL |
| H2O2 (30%) at 4 °C | 10% | 66.6 mL |
| Ultrapure water | n/a | 113.4 mL |
3. 1× PBS/0.1% Tween 20
| Reagent | Final concentration | Volume (200 mL total) |
| 10× PBS | 1× | 20 mL |
| Tween 20 (100%) | 0.1% | 0.2 mL |
| Ultrapure water | n/a | 180 mL |
4. 1× PBS/0.3% Triton X-100
| Reagent | Final concentration | Volume (600 mL total) |
| 10× PBS | 1× | 60 mL |
| Triton X-100 (100%) | 0.3% | 1.8 mL |
| Ultrapure water | n/a | 538.2 mL |
5. Blocking solution
| Reagent | Final concentration | Volume (40 μL per eye section) |
| Normal serum (100%) (goat serum for S100b, CD68, and Ki67 antibody assays, and horse serum for RPE65 antibody assay) | 5% | 2 μL |
| 1× PBS/0.3% Triton X-100 | n/a | 38 μL |
Note: Normal serum is included in the VECTASTAIN Elite ABC kits.
6. Primary antibody mix (anti-S100B or anti-Ki67)
| Reagent | Final concentration | Volume (40 μL per eye section) |
| Normal goat serum (100%) | 5% | 2 μL |
| Primary antibody | 1:1,000 dilution | 0.04 μL |
| 1× PBS/0.3% Triton X-100 | n/a | 38 μL |
7. Primary antibody mix (anti-RPE65)
| Reagent | Final concentration | Volume (40 μL per eye section) |
| Normal horse serum (100%) | 5% | 2 μL |
| Primary antibody | 1:250 dilution | 0.16 μL |
| 1× PBS/0.3% Triton X-100 | n/a | 38 μL |
8. Primary antibody mix (anti-CD68)
| Reagent | Final concentration | Volume (40 μL per eye section) |
| Normal goat serum (100%) | 5% | 2 μL |
| Primary antibody | 1:500 dilution | 0.08 μL |
| 1× PBS/0.3% Triton X-100 | n/a | 38 μL |
9. Secondary antibody mix (for S100b, CD68 and Ki67 assays)
| Reagent | Final concentration | Volume (40 μL per eye section) |
| Normal goat serum (100%) | 1.5% | 0.6 μL |
| Biotinylated goat anti-rabbit secondary antibody, from kit VECTPK6101 | 1:200 dilution | 0.2 μL |
| 1× PBS/0.3% Triton X-100 | n/a | 39.2 μL |
10. Secondary antibody mix (for RPE65 assay)
| Reagent | Final concentration | Volume (40 μL per eye section) |
| Normal horse serum (100%) | 1.5% | 0.6 μL |
| Biotinylated horse anti-mouse secondary antibody, from kit VECTPK6102 | 1:200 dilution | 0.2 μL |
| 1× PBS/0.3% Triton X-100 | n/a | 39.2 μL |
11. ABC solution
| Reagent | Final concentration | Volume (40 μL per eye section) |
| VECTASTAIN® Elite® ABC-HRP Kit Reagent A (use kit corresponding to primary antibody choice) | 2% | 0.8 μL |
| VECTASTAIN® Elite® ABC-HRP Kit reagent B (use kit corresponding to primary antibody choice) | 2% | 0.8 μL |
| 1× PBS/0.3% Triton X-100 | n/a | 38.4 μL |
12. DAB-nickel solution
| Reagent | Final concentration | Volume (400 μL per slide) |
| DAB Substrate kit, reagent 1 | 1.7% | 6.8 μL |
| DAB Substrate kit, reagent 2 | 2% | 8 μL |
| DAB Substrate kit, reagent 3 | 1.6% | 6.4 μL |
| DAB Substrate kit, reagent 4 | 1.6% | 6.4 μL |
| Ultrapure water | n/a | 372.4 μL |
13. Acid rinse solution
| Reagent | Final concentration | Volume (200 mL total) |
| Glacial acetic acid (100%) | 2% | 4 mL |
| Distilled water | n/a | 196 mL |
14. Bluing solution
| Reagent | Final concentration | Volume (200 mL total) |
| Ammonium hydroxide (30%) | 0.09% | 600 μL |
| Tap water | n/a | 200 mL |
Laboratory supplies
1. Disposable gloves (Fisher Scientific, catalog number: 191301597C)
2. Peel-A-WayTM disposable embedding molds (Fisher Scientific, catalog number: 2219)
3. Tissue PathTM disposable embedding rings (Fisherbrand, catalog number: 22-038197)
4. Thermal gloves (Sigma-Aldrich, catalog number: Z108286)
5. Pasteur pipette (Sigma-Aldrich, catalog number: Z627992)
6. Pasteur pipette rubber bulbs (Sigma-Aldrich, catalog number: Z111597)
7. Kimwipes (Sigma-Aldrich, catalog number: Z671584)
8. 4 × 1.5 mL Eppendorf tubes (Thermo Scientific, catalog number: 3448PK)
9. ImmEdge hydrophobic barrier PAP pen (Vector Laboratories, catalog number: H-4000)
10. 15 mL Falcon tube (Fisher Scientific, catalog number: 14-959-53A)
11. Parafilm (Sigma-Aldrich, catalog number: HS234526C)
12. 20 mL disposable glass scintillation vials (VWR, catalog number: 100500-676)
13. Superfrost Plus microscope slides (VWR, catalog number: 48311-703)
Equipment
1. Curved micro-dissecting forceps (Sigma-Aldrich, catalog number: F4142-1EA)
2. Shandon Histocentre 3 (Fisher Scientific, catalog number: 8358-30-1022)
3. 1,000 μL micropipette (Gilson, catalog number: F123602)
4. 200 μL micropipette (Gilson, catalog number: F123601)
5. 20 μL micropipette (Gilson, catalog number: F123600)
6. Microwave
7. Wash-N-Dry slide rack (Electron Microscopy Sciences, VWR, catalog number: 102096-892)
8. Hot plate (Corning, catalog number: 679540)
9. Precision compact oven (Thermo Scientific, catalog number: PR305225M)
10. Thermometer (Fisherbrand, catalog number: 13201521)
11. 1,000 mL glass beaker (Fisherbrand, catalog number: FB1001000)
12. Humidity/slide moisture chamber (Fisher Scientific, catalog number: 50-192-6622)
13. Glass slide tray (WHEATON®, catalog number: 900304)
14. 23 × Glass jars with lids (WHEATON®, catalog number: 900200)
15. Water bath (Thermo Scientific, model: Precision 2825)
16. Microtome (Leica, model: RM2255)
Procedure
A. Tissue fixation and sectioning
1. Euthanize the mouse according to the animal care ethics regulations that govern the institute where the research is being conducted. For adult mice at the University of British Columbia, this is isoflurane anesthesia, followed by CO2, then cervical dislocation.
Note: Using an alternative method of euthanasia is not likely to affect the success of the experiment.
2. Enucleate the eyeball using curved micro dissecting forceps. Do not puncture the eyeball.
3. Place the eyeball directly into Davidson’s fixative in a glass scintillation vial and fix the eyeball with rocking at 4 °C for 3 h. For all steps in section A, fill the scintillation vial three-quarters full.
Note: For newborn mice to post-natal day 6 (P6), reduce the duration of step A3 to 1 h.
4. Rinse the eyeball briefly in 1× PBS.
5. Fix the eyeball in 10% neutral buffered formalin with rocking at 4 °C for 1 h.
6. Wash the eyeball in 1× PBS three times for 20 min with rocking at 4 °C.
7. Store the eyeball in 70% ethanol at 4 °C.
Pause point: The protocol can be resumed the following day, or up to 4 weeks later.
Note: Microtome sectioning is often described as an art. It requires significant skill, precision, and dexterity to produce consistently high-quality, thin slices of biological specimens that dry completely flat to a slide. Achieving success involves mastering the mechanical operation of the microtome. Eyes as a specimen type are very challenging. We usually use a local professional service, Wax-it Histology Services Inc., in Vancouver, Canada (https://waxitinc.com), to produce unstained mouse eyeball sections for our IHC. Wax-it's exact embedding protocol (from 70% ethanol to paraffin block) is proprietary information, but it consists of a basic series of ethanol, xylene, and paraffin solutions. In addition, the following embedding protocol works well for eyes in our laboratory. It is done manually.
8. Incubate the eyeball in new 70% ethanol for 20 min at 4 °C.
9. Incubate the eyeball in 85% ethanol for 20 min at 4 °C.
10. Incubate the eyeball in 90% ethanol for 20 min at 4 °C.
11. Incubate the eyeball in 95% ethanol for 20 min at 4 °C.
12. Incubate the eyeball in 100% ethanol for 3 × 20 min at 4 °C. Meanwhile, warm a scintillation vial filled with half xylene and half paraffin in a water bath set to 50 °C.
13. Place the eyeball into ice-cold 100% xylene and allow it to sit at room temperature for 10 min.
14. Incubate the eyeball in room-temperature 100% xylene for 10 min.
15. Transfer the eyeball into the prewarmed vial of 50% xylene/50% paraffin and leave the vial at 50 °C for 10 min.
16. Move the vial with the eyeball to the Histocentre 3 and incubate at 62 °C for 10 min, still in 50% xylene/50% paraffin.
Note: An oven can be used as an alternative to the Histocentre 3 embedding station.
17. Incubate the eyeball in paraffin for 3 × 1 h at 62 °C.
18. Fill one disposable embedding mold with paraffin.
19. Immediately move the eyeball into the mold and orient the eyeball with forceps such that it will be cross-sectioned.
20. Let the paraffin cool at room temperature.
Pause point: Store the paraffin blocks in an airtight container in the dark until ready to section.
21. Peel off the embedding mold and attach the paraffin block to an embedding ring.
22. Using the microtome, make a ribbon of two or three 5-μm-thick sections. We usually collect sections at the middle of the eye, where the optic nerve is present.
23. Holding on to a corner of the ribbon with forceps, place the ribbon on distilled water in a water bath set to 42 °C.
24. Float the ribbon for 30 s.
25. Lift the ribbon onto a Superfrost Plus microscope slide.
26. Dry the slide vertically overnight in a 37 °C warm room.
27. Store slides in an air-tight container in the dark until use.
Pause point: The slides can be stored for up to a year until ready to perform immunohistochemistry.
B. Dewax and rehydrate slides
1. Put slides into a glass tray.
2. Place the tray into a jar with 200 mL of 100% xylene for 10 min (in a fume hood).
3. Repeat step B2 with a second jar of fresh 100% xylene.
4. Transfer the tray into a jar with 200 mL of 100% ethanol for 5 min.
5. Repeat step B4 with a second jar of fresh 100% ethanol.
6. Transfer the tray into a jar with 200 mL of 95% ethanol for 2 min.
7. Transfer the tray into a jar with 200 mL of 90% ethanol for 2 min.
8. Transfer the tray into a jar with 200 mL of 80% ethanol for 2 min.
9. Transfer the tray into a jar with 200 mL of 70% ethanol for 2 min.
10. Transfer the tray into a jar with 200 mL of 50% ethanol for 2 min.
11. Transfer the tray into a jar with 200 mL of 30% ethanol for 2 min.
12. Transfer the tray into a jar with 200 mL of ultrapure water for 2 min.
13. Repeat step B12 with a second jar of fresh ultrapure water.
14. Transfer the tray into fresh 1× PBS for 5 min.
C. Antigen retrieval with citrate buffer
1. Warm up the hot plate to 320 °F/160 °C.
2. Shake the 100× stock citrate buffer hard.
3. Add 6 mL of stock citrate buffer to 594 mL of distilled water in a 1,000 mL beaker.
4. Microwave the beaker for 4.5 min (just until boiling) and put it on the hot plate.
Caution: The beaker will be hot. Use thermal gloves.
5. One at a time, transfer each slide to the Wash-N-Dry slide rack.
Critical: Work as quickly as you can to avoid slide drying.
6. Gently lower the Wash-N-Dry slide rack into the beaker. Leave for 10 min.
Note: You do not want very active boiling because that will damage the sections. You want little bubbles to be forming on the bottom of the beaker and Wash-N-Dry slide rack.
7. After 10 min, remove the beaker from the hotplate.
Caution: Use thermal gloves.
8. Leave the beaker at room temperature for 40 min.
9. Fill a glass jar with 200 mL of ultrapure water and add a tray.
10. Transfer each slide into the tray.
11. Once all slides are transferred, incubate for 3 × 5 min in ultrapure water.
12. Transfer the tray into 1× PBS for 5 min (you may reuse the solution from step B14.
D. Hydrogen peroxide bleaching
1. Make 200 mL of bleach solution just before use, using cold hydrogen peroxide (see Recipe 2).
2. Mix before adding to a glass jar.
3. Transfer the slide tray with the slides to be bleached into the bleach solution jar.
4. Cover the jar with its glass lid and seal the jar with parafilm.
5. Place the jar into the preheated 60 °C oven.
6. Leave for 2.5 h.
7. Transfer the tray into a jar of 1× PBS for 5 min (you may reuse the solution from step C12.
8. Transfer the tray into a second jar of fresh 1× PBS for 5 min.
9. Transfer the tray into a jar with 1× PBS/0.1% Tween 20 for 5 min (see Recipe 3).
E. Blocking
1. Prepare the humid chamber.
2. Shake the ImmEdge pen very hard.
3. Use a Kimwipe to dry the back of the slide and around the eye sections, leaving a 1.5 cm × 1.5 cm wet square around each eye section.
4. Using the ImmEdge pen, draw a 1.5 cm × 1.5 cm square around each section of eye.
Note: Do not get too close to the sections. Do not press the tip too hard against the slide, or too much will come out of the pen.
5. Lay the slide in the humid chamber.
6. Pipette 40 μL of blocking solution into the square you have created, completely covering the tissue section (see Recipe 5).
7. Repeat for the rest of the slides.
8. Cover the chamber and leave at room temperature on the benchtop for 1 h.
F. Primary antibody incubation and wash
1. Make the primary antibody solution 10 min before needed (see Recipes 6, 7, or 8).
2. Pick up a slide that needs a primary antibody solution from the humid chamber and pour off blocking solution onto a paper towel.
3. Dry the previous ImmEdge pen lines by gently blotting them with a Kimwipe to remove any liquid clinging there. Reinforce if necessary by redrawing the lines (not usually necessary).
Critical: Work quickly and do not let the tissue dry out.
4. Lay the slide back in the humid chamber. Pipette 40 μL of primary antibody solution into the square around each eye section.
5. Repeat for the rest of the slides that need primary antibody solution.
Reminder: Leave the negative control slide covered with blocking solution during this step.
6. Cover the chamber and leave at room temperature for 1 h and 20 min.
7. Pick up each slide and pour off the primary antibody solution or blocking solution onto a paper towel to dispose of it.
8. Transfer the slide to the glass tray sitting in a jar of 200 mL of 1× PBS/0.3% Triton X-100 (see Recipe 4).
9. When all slides are in the tray, incubate for 5 min.
G. Secondary antibody, ABC solution preparation, and wash
1. Make secondary antibody solution(s) 10 min before you need it (see Recipes 9 or 10).
2. Pick up a slide. Dry the back and edges of the slide with a Kimwipe. Dry the previous ImmEdge pen lines by gently blotting them with a Kimwipe to remove any liquid clinging there. Reinforce if necessary by redrawing the lines (not usually necessary).
Critical: Work quickly and do not let the tissue dry out.
3. Lay the slide in the humid chamber. Pipette 40 μL of secondary antibody solution into the square around each eye section.
4. Repeat the process for the rest of the slides.
5. Cover the chamber. Leave at room temperature for 30 min.
6. Make ABC solution 10 min after starting secondary antibody incubation (see Recipe 11).
Note: The ABC solution needs to sit for 15–30 min before use.
7. After the 30-min secondary antibody incubation, pick up each slide and pour off the secondary antibody solution onto a paper towel to dispose of it.
8. Transfer the slide to a glass tray sitting in a jar of 200 mL of fresh 1× PBS/0.3% Triton X-100. When all slides are in the tray, incubate for 5 min.
H. ABC step and wash
1. Pick up a slide. Dry the back and edges of the slide with a Kimwipe. Dry the previous ImmEdge pen lines by gently blotting them with a Kimwipe to remove any liquid clinging there. Reinforce if necessary by redrawing the lines (not usually necessary).
Critical: Work quickly and do not let the tissue dry out.
2. Lay the slide in a humid chamber.
3. Pipette 40 μL of the ABC solution into the square around each eye section.
5. Repeat the process for the rest of the slides.
6. Cover the chamber and leave at room temperature for 15 min.
7. After 15 min, pick up each slide and pour off ABC solution onto a paper towel.
8. Transfer the slide to a tray sitting in a jar of 200 mL of fresh 1× PBS/0.3% Triton X-100. When all slides are in the tray, incubate for 5 min.
I. DAB-nickel step and wash
1. Pick up a slide. Drain excess liquid off and lay flat on a paper towel.
2. Pipette 400 μL of DAB nickel solution onto the slide (see Recipe 12).
Caution: DAB is a carcinogen.
3. Allow color to develop for 20 s.
4. When time is up, immediately pour off the DAB solution into a beaker to dispose of it and place the slide in a tray sitting in a jar of tap water.
5. Repeat the process for the rest of the slides.
6. Transfer the tray into a second jar of fresh tap water for 5 min.
J. Counterstaining with hematoxylin
1. Transfer the tray into a jar with fresh tap water for 2 min.
2. Pick up a slide. Drain excess liquid off and lay flat on a paper towel.
3. Pipette 400 μL of hematoxylin onto the slide.
4. Leave the hematoxylin on for 30 s.
5. Pour off hematoxylin into a beaker to dispose of it and place the slide in a tray in a second jar of tap water.
6. Repeat for the remaining slides.
7. Once all slides are in the second jar of tap water, very gently swish the tray around to remove clinging hematoxylin.
8. Dip the tray 10 times in acid rinse solution (see Recipe 13).
9. Dip the tray 10 times in fresh tap water.
10. Dip the tray 5 times in bluing solution (see Recipe 14).
Caution: Bluing solution is toxic to aquatic life. Do not dispose of it down the sink.
11. Dip the tray 15 times in fresh tap water.
12. Immediately proceed to the dehydration process.
K. Dehydration
1. Transfer the tray into 30% ethanol for 2 min.
2. Transfer the tray into 50% ethanol for 2 min.
3. Transfer the tray into 70% ethanol for 2 min.
4. Transfer the tray into 80% ethanol for 2 min.
5. Transfer the tray into 90% ethanol for 2 min.
6. Transfer the tray into 95% ethanol for 2 min.
7. Transfer the tray into 100% ethanol for 5 min.
8. Repeat step K7 with a second jar of fresh 100% ethanol.
9. Transfer the tray into xylene for 10 min (use in a fume hood).
10. Repeat step K9 with a second jar of fresh xylene.
L. Coverslip
1. Working in a fume hood, remove one slide from the xylene using forceps and lay it flat on a paper towel.
2. Immediately add two drops of Eukitt mounting solution in one spot in the middle of the slide using a disposable glass pipette.
3. Gently lower a coverslip from one side of the slide to the other to direct the air bubbles to the edges.
Critical: Do not press down on the coverslip itself, even if there are air bubbles.
Note: To prevent bubbles from forming, do not drain off Xylene onto the paper towel. Instead, immediately add Eukitt directly to the eye section and lower the coverslip slowly, holding both sides of the coverslip until contact with Eukitt is made. Slowly release one side of the cover slip, and then the other side, which gives the Eukitt and any air bubbles time to spread to the edge of the slide.
4. Repeat with the rest of the slides.
5. Leave the slides flat to dry overnight in the fume hood.
Data analysis
The IHC signal was analyzed in images taken of slides using light microscopy. In Figure 1, we show example results imaged with a Histotech Slide Scanner, and in Figure 2, we show results imaged using a regular light microscope. In every experiment, a negative control slide was included that was incubated in the primary antibody dilution solution without any primary antibody. These negative control slides were always blank. Users should ensure that staining matches the expected results when reproducing this protocol. Positive Ki67 staining should be restricted to the nucleus (Figure 2). In a normal eye, RPE65 staining should be restricted to the RPE, while S100B staining should be abundant in the choroid (Figure 1). Note, S100B also stains the optic nerve and ganglion cells on the innermost layer of the neural retina.
We recommend using five biological replicates of each type of sample and genotype for qualitative IHC assessments. ImageJ can be used to quantify IHC results by determining the percent area of the sample that is positively stained or the percentage of nuclei that are labeled, in the case of anti-Ki67 IHC. Quantified results can be analyzed for significance using a student’s t-test or other statistical test, as appropriate to the dataset. We use Prism for graphing and statistical analysis.
Validation of protocol
This protocol has been used and validated in the following research articles:
• Longakit et al. [7]. Loss of NF1 Accelerates Uveal and Intradermal Melanoma Tumorigenesis, and Oncogenic GNAQ Transforms Schwann Cells. Cancer Research Communications. (Figure 5B; Figure 7C–E; Supplementary Figure 3A–D).
• Farag et al. [8]. BAP1 deficient human and mouse uveal melanomas up-regulate a shared EMT pathway. bioRxiv. (Figure 1A–D; Figure 5)
• Jain et al. [9]. Endothelin signaling promotes melanoma tumorigenesis driven by constitutively active GNAQ. Pigment Cell and Melanoma Research. (Figure 2F; Figure 5D; Figure 6D–E; Supplementary Figures 6–7)
General notes and troubleshooting
General notes
1. This is a long protocol (11 h). Despite the inconvenience, the entire protocol should be done as written, rather than incubating the primary antibody overnight. We found that overnight incubation with the primary antibody results in false-positive staining.
2. Keep the 10× PBS stock sterile to prevent the growth of microorganisms.
3. 10× PBS will precipitate at 4 °C. Store at room temperature.
4. Never let sections dry out at any time in this process.
5. We limit the number of slides per experiment to 6. This limits the difference in incubation times between the first and last slide processed at each step and reduces the chances of slides drying out during handling.
6. The squeeze bottles from the Vector kits can be opened to pipette out precise amounts. Hold the tip with a Kimwipe and pull it off (it will also snap back on afterward).
7. Do not wash the glass slide staining jars in a dishwasher. They will crack. To wash between uses, triple rinse the jar in clean water and turn it upside down to dry. Do not try to wash jars dedicated to xylene.
8. Keep kit reagents on ice when using and then return them to 4 °C for storage.
9. This protocol is compatible with other pigmented tissues (melanocytic lesions in the skin, for example). Perform the fixation step using just 10% buffered formalin and scale up the volume of solutions needed to cover the sections, depending upon their size.
10. To conserve resources, the solutions of xylene and ethanol for dehydration and rehydration can be reused for at least five experiments if they are stored in tightly closed 250 mL bottles between experiments. Top up as needed to replace solution lost to evaporation. Replace the solution if any discoloration or debris becomes visible in the bottle. Use a funnel to transfer solutions from the jar to the bottle.
11. It is best to have your negative control sections on a separate slide to avoid possible exposure to the primary antibody. Leave the negative control slide covered with blocking solution during the primary antibody incubation step. This is the correct negative control solution, because the primary antibodies are diluted in blocking solution.
12. Limit disturbance of sections during the IHC as much as possible. Fill jars with solution and then gently lower trays into them. Do not put trays into empty jars and then fill.
Troubleshooting
Problem 1: Slide label washes off in the xylene step.
Possible cause: Used a pen to label slides.
Solution: Use a pencil to label the slide, as it will resist xylene.
Problem 2: Nonspecific staining.
Possible causes: The section dried out during an incubation step, the primary incubation step was left overnight, or the DAB solution was left on the slide for too long.
Solution: Check slides to make sure that they are covered in solution and that the lines drawn with the ImmEdge pen are intact. Be very precise and consistent with the timing of DAB solution for every slide (20 s).
Problem 3: No staining.
Possible cause: A new primary antibody was tested with the protocol and was not compatible.
Solution: If you seek to use other antibodies, look for those that call for citrate buffer for antigen retrieval. Even then, not all primary antibodies/antigens will be compatible with the hydrogen peroxide bleaching step.
Problem 4: Hematoxylin counterstain is faint.
Possible cause: Solution has expired. Hematoxylin has a short shelf life.
Solution: Order fresh solution from the manufacturer.
Problem 5: Tissue comes off the slide during the procedure.
Possible causes: Slides were exposed to air/water, and the positive charge has been lost. The section did not dry completely flat to slide. Any tissue that is not dried flat can be lost and can pull adjacent tissues off the slide.
Solution: Order fresh Superfrost Plus slides and store in air-tight containers, taking care not to get slides wet before use. Dry sections thoroughly before using for IHC. Consider using a professional service for tissue sectioning.
Acknowledgments
Original Draft, Investigation, Visualization, Methodology, A.N.L.; Investigation, C.Z.; Investigation, C.H.; Conceptualization, Supervision, Funding acquisition, Project administration, Writing – review and editing, C.D.V.R. This protocol was used in Jain, F., Longakit, A., Huang, J. L. and Van Raamsdonk, C. D. (2020). Endothelin signaling promotes melanoma tumorigenesis driven by constitutively active GNAQ. Pigm Cell Melanoma R. 33(6): 834–849. https://doi.org/10.1111/pcmr.12900 [9]. This research was funded by a grant from the Canadian Institutes of Health Research, PJT-178178, to C.D. Van Raamsdonk.
Competing interests
The authors declare no conflicts of interest.
Ethical considerations
The research described in this article was conducted under the approval of the University of British Columbia (UBC) Animal Care Committee (UBC animal care protocol number A22-0051, C.V.R).
References
- McKay, J. S., Steele, S. J., Ahmed, G., Johnson, E. and Ratcliffe, K. (2009). An antibody panel for immunohistochemical analysis of the retina in Davidson's-fixed, paraffin-embedded eyes of rats. Exp Toxicol Pathol. 61(2): 91–100. https://doi.org/10.1016/j.etp.2008.06.005
- Lilyquist, J., White, K. A. M., Lee, R. J., Philips, G. K., Hughes, C. R. and Torres, S. M. (2017). Quantitative Analysis of Immunohistochemistry in Melanoma Tumors. Medicine (Madr). 96(15): e6432. https://doi.org/10.1097/md.0000000000006432
- Liu, C. H., Lin, C. H., Tsai, M. J., Chen, Y. H., Yang, S. F. and Tsai, K. B. (2018). Melanin Bleaching With Warm Hydrogen Peroxide and Integrated Immunohistochemical Analysis: An Automated Platform. Int J Surg Pathol. 26(5): 410–416. https://doi.org/10.1177/1066896918756998
- Shen, H. and Wu, W. (2015). Study of Melanin Bleaching After Immunohistochemistry of Melanin-containing Tissues. Appl Immunohistochem Mol Morphol. 23(4): 303–307. https://doi.org/10.1097/pai.0000000000000075
- Ugolini, F., Baroni, G., Nassini, R., De Logu, F. and Massi, D. (2021). A Fast and Automated Melanin-bleaching Method for Histopathologic Evaluation of Pigmented Melanoma Tissues. Appl Immunohistochem Mol Morphol. 30(4): 311–316. https://doi.org/10.1097/pai.0000000000001004
- Tokuda, K., Baron, B., Kuramitsu, Y., Kitagawa, T., Tokuda, N., Morishige, N., Kobayashi, M., Kimura, K., Nakamura, K., Sonoda, K. H., et al. (2018). Optimization of fixative solution for retinal morphology: a comparison with Davidson’s fixative and other fixation solutions. Jpn J Ophthalmol. 62(4): 481–490. https://doi.org/10.1007/s10384-018-0592-7
- Longakit, A. N., Urtatiz, O., Luty, A., Zhang, C., Hess, C., Yoo, A., Bourget, H. and Van Raamsdonk, C. D. (2025). Loss of NF1 Accelerates Uveal and Intradermal Melanoma Tumorigenesis, and Oncogenic GNAQ Transforms Schwann Cells. Cancer Res Commun. 5(2): 209–225. https://doi.org/10.1158/2767-9764.crc-24-0386
- Farag, R., Jain, F., Longakit, A. N., Luty, A. and Van Raamsdonk, C. D. (2023). BAP1 deficient human and mouse uveal melanomas up-regulate a shared EMT pathway. bioRxiv. e542173. https://doi.org/10.1101/2023.05.24.542173
- Jain, F., Longakit, A., Huang, J. L. and Van Raamsdonk, C. D. (2020). Endothelin signaling promotes melanoma tumorigenesis driven by constitutively active GNAQ. Pigm Cell Melanoma R. 33(6): 834–849. https://doi.org/10.1111/pcmr.12900
- Huang, J. Y., Urtatiz, O. and Van Raamsdonk, C. D. (2015). Oncogenic G Protein GNAQ Induces Uveal Melanoma and Intravasation in Mice. Cancer Res. 75(16): 3384–3397. https://doi.org/10.1158/0008-5472.can-14-3229
- Deo, M., Huang, J. Y. and Van Raamsdonk, C. D. (2013). Genetic Interactions between Neurofibromin and Endothelin Receptor B in Mice. PLoS One. 8(3): e59931. https://doi.org/10.1371/journal.pone.0059931
- Urtatiz, O., Haage, A., Tanentzapf, G. and Van Raamsdonk, C. D. (2021). Crosstalk with keratinocytes causes GNAQ oncogene specificity in melanoma. eLife. 10: e71825. https://doi.org/10.7554/elife.71825
- Urtatiz, O., Cook, C., Huang, J. L., Yeh, I. and Van Raamsdonk, C. D. (2019). GNAQQ209L expression initiated in multipotent neural crest cells drives aggressive melanoma of the central nervous system. Pigm Cell Melanoma R. 33(1): 96–111. https://doi.org/10.1111/pcmr.12843
Article Information
Publication history
Received: Jun 23, 2025
Accepted: Oct 9, 2025
Available online: Oct 23, 2025
Published: Nov 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Longakit, A. N., Hess, C., Zhang, C. and Van Raamsdonk, C. D. (2025). Improved Immunohistochemistry of Mouse Eye Sections Using Davidson's Fixative and Melanin Bleaching. Bio-protocol 15(22): e5510. DOI: 10.21769/BioProtoc.5510.
Category
Cancer Biology > General technique > Cell biology assays
Cell Biology > Cell staining > Whole cell
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link