- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Direct Plant Regeneration From Immature Male Inflorescence of Banana (Musa spp.)
Published: Vol 15, Iss 20, Oct 20, 2025 DOI: 10.21769/BioProtoc.5476 Views: 1438
Reviewed by: Diarmuid Seosamh Ó’MaoiléidighAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Novel Imaging Protocol for Investigating Arabidopsis thaliana Siliques and Seeds Using X-rays
Brylie A. Ritchie [...] Ansul Lokdarshi
Oct 5, 2023 2189 Views

Micrografting Technique of Hevea brasiliensis In Vitro Plantlets
Florence Dessailly [...] Julie Leclercq
Feb 20, 2025 1491 Views

Practical Guide to In Vitro Clonal Propagation of Nicotiana benthamiana Using Axillary Shoot Induction
Pradeep Chand Deo
Sep 20, 2025 1224 Views
Abstract
Banana (Musa spp.) is a globally important horticultural crop that faces significant challenges from pests and diseases, which threaten yield and long-term sustainability. The efficient production of clean, disease-free planting material is essential for both commercial plantations and small-holder systems. This paper presents a rapid and reproducible protocol for direct plant regeneration from immature male inflorescences of banana. The method involves surface sterilization of immature male flowers, longitudinal dissection, and culture on Murashige and Skoog (MS) medium supplemented with 6-benzylaminopurine (BAP), enabling direct shoot regeneration from floral meristems without an intermediate regenerable callus phase. This approach offers several advantages over traditional embryogenic cell suspension (ECS) methods, including simplified sterilization, high regeneration efficiency, and scalability. The protocol was successfully applied to multiple banana cultivars, including Cavendish (AAA) and Lady Finger (AAB), achieving 100% shoot regeneration efficiency with plantlet production within 6–8 months. This protocol provides a reliable and efficient alternative for rapid mass propagation of banana plants, supporting sustainable production and research applications.
Key features
• The protocol can be performed in a standard tissue culture lab without expensive instruments or complex setup, making it accessible for labs in resource-limited settings.
• Minimal contamination risk since immature male inflorescences enclosed within bracts are naturally protected, and the simplified sterilization procedure leads to consistently low contamination rates.
• Potential for high multiplication where each immature male flower produces 50–100 shoots under optimized conditions, reducing the number of subcultures needed for large-scale propagation.
• The method performed equally well in genetically distinct banana cultivars (AAA and AAB groups), suggesting broader applicability across diverse Musa genotypes.
Keywords: Musa spp.Graphical overview
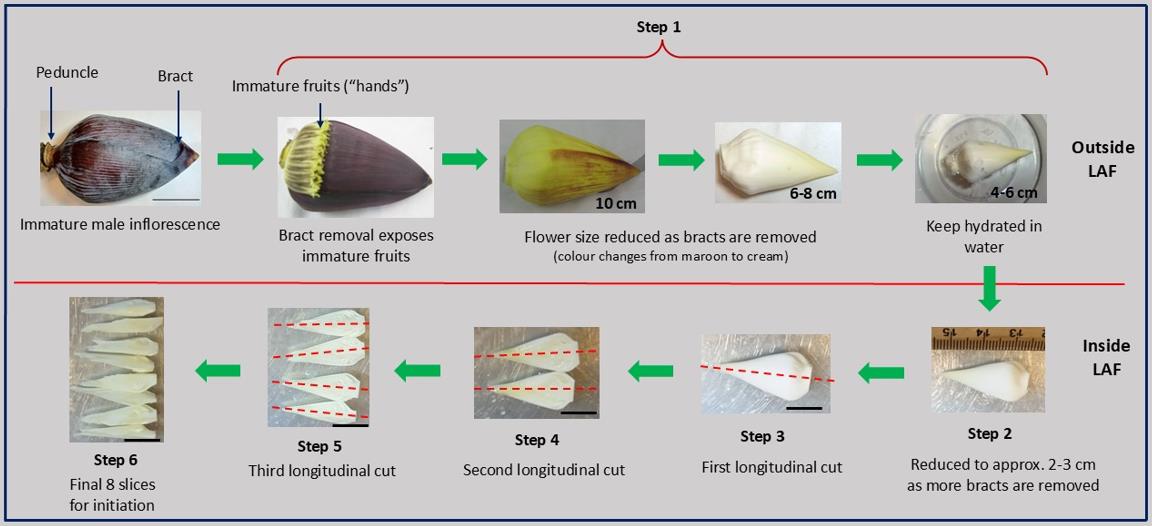
Stepwise longitudinal dissection of the banana bell for tissue culture. Bracts (maroon sheaths) are sequentially peeled until a cream-colored flower (~4–6 cm) is exposed and kept hydrated in sterile water in a small container (step 1). The bell is further reduced to approximately 2–3 cm under sterile conditions in a laminar air flow (LAF) hood (step 2), followed by a longitudinal cut along the red dashed line (step 3). Two subsequent longitudinal cuts (steps 4 and 5) produce eight thin slices (step 6): first cut → 2 slices, second → 4 slices, third → 8 slices. These final eight slices are used for culture. Scale bars: 5 cm (top panel) and 2 cm (bottom panel). Figure created by the author using original photographs of banana flowers taken by the author. All arrows, lines, and labels were added using Microsoft PowerPoint.
Background
Banana (Musa spp.) is a versatile horticultural crop grown globally for its sweet fruit, typically consumed fresh. In regions such as East Africa, bananas are also a staple when consumed for cooking as green bananas [1]. In addition to its subsistence role, the banana is a major commercial commodity with significant economic value in global markets [2,3].
Despite its importance, banana production is severely threatened by pests and diseases, including Fusarium wilt (Panama disease), Black Sigatoka, and Banana Bunchy Top Virus (BBTV), which collectively reduce yield and compromise crop sustainability [4,5]. As a result, there is an increasing demand for clean, disease-free planting materials to support both commercial plantations and smallholder farming systems [6].
Tissue culture has emerged as a vital tool for the rapid, large-scale production of uniform and disease-free banana plants. Currently, three main methodologies are used for banana micropropagation. The method of somatic embryogenesis via embryogenic cell suspension (ECS) begins with the induction of embryogenic calli from immature male inflorescences cultured on Murashige and Skoog (MS) medium [7] supplemented with plant growth regulators. These calli are transferred to liquid medium to establish ECS, which are later plated to develop somatic embryos. However, the efficiency of embryogenic callus induction is typically very low (~5%), and obtaining such calli from any single inflorescence is rare. In practice, multiple inflorescences must be dissected per cultivar to establish ECS cultures. An alternative ECS approach involves scalping, wherein in vitro plants are maintained on 6-Benzylaminopurine (BAP)-enriched media for 12–18 months to generate dense meristematic clumps (scalps) that are induced to form embryogenic calli [8] and references therein. However, this method is highly time-consuming, genotype-dependent, and resource-intensive.
The method of meristem culture from field-grown suckers or offshoots uses vegetative buds from suckers as explants. However, extensive surface sterilization is required, and initial cultures are often plagued by contamination. While shoot proliferation can be achieved using high levels of BAP and adenine hemisulfate, establishing contamination-free initial cultures remains a major bottleneck [9].
The method proposed here, direct regeneration from immature male inflorescence (our proposed method), starts with immature male flowers (commonly referred to as “bells”) being surface-sterilized using 70% ethanol, rinsed with sterile water, trimmed to approximately 4 cm, and longitudinally sectioned. The explants are cultured on MS medium supplemented with BAP, enabling floral meristems to directly reprogram into shoot meristems—bypassing the callus stage entirely. Generally, in most regeneration systems, when explants are placed on an appropriate medium containing an auxin and a cytokinin, the explants undergo dedifferentiation and form callus, which is a mass of undifferentiated cells. These calli are then reprogrammed by altering the concentrations of the hormones and media components to differentiate into a shoot and ultimately into a plantlet. However, in this direct regeneration, there is no formation of a regenerable calli. The shoots are formed by reprogramming the floral meristems into a shoot meristem.
This method offers several key advantages over existing protocols, namely simplified sterilization, as the enclosed nature of immature inflorescences minimizes contamination risk, requiring only basic surface sterilization procedures; high responsiveness, as nearly every flower can regenerate shoots, offering a major advantage over the low-efficiency ECS method; high yield per explant, since each flower has the capacity to produce large numbers of clonal plantlets, with reports of up to 80–130 under optimized conditions in other cultivars [10], reducing the number of subculture cycles required; and a scalable and genotype-flexible, as this method is suitable for both commercial-scale production and research, and has shown consistent performance across multiple Musa genotypes.
While similar methods have been briefly reported [10–12], these protocols lack sufficient detail for reliable replication. This method has been successfully developed and tested using Musa cultivars Cavendish (AAA) and Lady Finger (AAB), consistently achieving 100% shoot regeneration efficiency. The method was later adopted by others, who also achieved similar results—demonstrating its robustness and reproducibility.
In addition to micropropagation, this protocol may have broader applications in areas such as in vitro mutagenesis, transgenic line development, or virus indexing, where efficient plant regeneration is required from floral tissues.
Materials and reagents
Biological materials
1. Immature male inflorescences at least 10 from field plants
Reagents
1. Murashige and Skoog (MS) basal medium (Sigma, catalog number: M5524; contains MS macro and micro salts without vitamins)
2. Murashige and Skoog (MS) basal medium (Sigma, catalog number: M5519; contains MS macro, micro, and vitamins)
3. Biotin powder (Austratec, catalog number: B140)
4. 6-Benzylaminopurine (BAP) powder (Austratec, catalog number: B800)
5. 1-Naphthaleneacetic acid (NAA) powder (Austratec, catalog number: N600)
6. Indole-3-acetic acid (IAA) powder (Austratec, catalog number: I885)
7. Morel & Wetmore vitamin solution (100×) (Austratec, catalog number: M592)
8. Sucrose (PhytoTech Labs, catalog number: S391)
9. Phytagel (Sigma, catalog number: P8169)
10. Ethanol (70% and absolute) (Sigma, catalog number: 459844)
11. Sterile distilled or Milli-Q water
12. 1 M NaOH (Sigma, catalog number: 1091371000)
Solutions
1. Biotin stock (1 mg/mL) (see Recipes)
2. BAP stock (1 mg/mL) (see Recipes)
3. NAA stock (1 mg/mL) (see Recipes)
4. IAA stock (1 mg/mL) (see Recipes)
5. 2× Meristem formation media (see Recipes)
6. 2× Meristem proliferation media (see Recipes)
7. 2× Shoot differentiation media (see Recipes)
8. 2× Plant rooting and development media (see Recipes)
Recipes
1. Biotin stock (1 mg/mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Biotin | 1 mg/mL | 20 mg |
| Distilled water | To 20 mL | |
| Total | 20 mL |
Note: Biotin is only sparingly soluble in cold water. Warm the water to 40–50 °C before adding biotin to aid dissolution. Do not boil. Filter-sterilize the solution using a 0.22 μm filter. Dispense 1,000 μL aliquots into sterile 1.5 mL microfuge tubes. Store at -20 °C.
2. BAP stock (1 mg/mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| BAP (6-Benzylaminopurine) | 1 mg/mL | 20 mg |
| 1 M NaOH | 100 μL for dissolution | |
| Distilled water | To 20 mL | |
| Total | n/a | 20 mL |
Note: Dissolve BAP in a few drops of 1 N NaOH or ethanol before adding distilled water to make up 20 mL. Filter-sterilize using a 0.22 μm filter. Dispense 1,000 μL aliquots into sterile 1.5 mL microfuge tubes. Store at -20 °C.
3. NAA stock (1 mg/mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| NAA (naphthaleneacetic acid) | 1 mg/mL | 20 mg |
| Absolute ethanol (100%) | 14 mL | |
| Distilled water | To 20 mL (6 mL) | |
| Total | n/a | 20 mL |
Note: Dissolve NAA in 14 mL of absolute ethanol, then add 6 mL of distilled water to reach 20 mL (final ethanol concentration: 70%). Filter sterilize (0.22 μm), aliquot at 1,000 μL, and store at -20 °C.
4. IAA stock (1 mg/mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| IAA (Indole-3-acetic acid) | 1 mg/mL | 20 mg |
| Absolute ethanol (100%) | 14 mL | |
| Distilled water | To 20 mL (6 mL) | |
| Total | n/a | 20 mL |
Note: Dissolve IAA in 14 mL of absolute ethanol, then add 6 mL of distilled water to reach 20 mL (final ethanol concentration: 70%). Filter sterilize (0.22 μm), protect from light, aliquot at 1,000 μL, and store at -20 °C.
5. 2× Meristem formation media (1 L)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| MS powder (M5519) | 4.43 g/L | 4.43 g |
| Sucrose | 30 g/L | 30 g |
| Biotin stock (1 mg/mL) | 1 mg/L | 1 mL |
| BAP stock (1 mg/mL) | 5 mg/L and 7 mg/L | 5 mL and 7 mL (two batches) |
| Phytagel | 3 g/L | 3 g |
| Distilled water | n/a | Up to 1,000 mL |
| Total | 1,000 mL |
Note: When working with a new cultivar, it is recommended to try both 5 and 7 mg/L BAP concentrations to determine which is more effective for initiating meristem formation. Cytokinin responsiveness can vary between genotypes.
a. Dissolve MS powder (M5519) in 700 mL of sterile distilled water.
b. Add sucrose and mix well.
c. Add 1 mL of biotin (1 mg/mL stock) to achieve 1 mg/L final concentration.
d. Prepare two separate batches of media:
i. Add 5 mL of BAP stock (1 mg/mL) to one (for a 5 mg/L final concentration).
ii. Add 7 mL of BAP stock (1 mg/mL) to the second (for a 7 mg/L final concentration).
e. Adjust pH to 5.7 using 1 M NaOH (or HCl if needed).
f. Make up the volume to 1 L with distilled water.
g. Add 3 g of phytagel.
h. Autoclave at 121 °C, 15 psi for 15 min.
i. Cool to 50–60 °C and pour 25–30 mL per sterile 90 mm deep Petri plate under laminar airflow.
6. 2× Meristem proliferation media (1 L)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| MS powder (M5519) | 4.43 g/L | 4.43 g |
| Sucrose | 30 g/L | 30 g |
| Biotin stock (1 mg/mL) | 1 mg/L | 1 mL |
| NAA stock (1 mg/mL) | 1 mg/L | 1 mg |
| BAP stock (1mg/mL) | 5 mg/L and 7 mg/L | 5 mL and 7 mL (two batches) |
| Phytagel | 3 g/L | 3 g |
| Distilled water | n/a | Up to 1,000 mL |
| Total | 1,000 mL |
Note: As with meristem formation, when working with a new cultivar, it is recommended to try both 5 and 7 mg/L BAP concentrations, now in combination with 1 mg/L NAA, to determine the optimal hormonal balance for meristem proliferation. Auxin–cytokinin synergy can significantly influence shoot multiplication rates.
a. Dissolve MS powder (M5519) in ~700 mL of sterile distilled water.
b. Add sucrose and mix well.
c. Add 1 mL of biotin stock (1 mg/mL).
d. Prepare two separate media batches:
e. Add 5 mL of BAP stock (1 mg/mL) to one batch (for 5 mg/L BAP).
f. Add 7 mL of BAP stock (1 mg/mL) to the second batch (for 7 mg/L BAP).
g. Add 1 mL of NAA stock (1 mg/mL) to each batch.
h. Adjust pH to 5.8 using 1 M NaOH.
i. Bring volume to 1,000 mL with distilled water.
j. Add 3 g of phytagel per liter.
k. Autoclave at 121 °C, 15 psi, for 15 min.
l. Cool to 50–60 °C and pour 25–30 mL per sterile 90 mm deep Petri plate under laminar airflow.
7. 2× Shoot differentiation media (1 L)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| MS powder (M5519) | 4.43 g/L | 4.43 g |
| Sucrose | 30 g/L | 30 g |
| Biotin stock (1 mg/mL) | 1 mg/L | 1 mL |
| BAP stock (1mg/mL) | 2 mg/L | 2 mL |
| Phytagel | 3 g/L | 3 g |
| Distilled water | n/a | Up to 1,000 mL |
| Total | 1,000 mL |
Notes:
1. After successful meristem initiation and proliferation, the concentration of BAP is reduced from 5 or 7 mg/L to 2 mg/L in the shoot differentiation stage to encourage organized shoot development rather than excessive callus or shoot clustering.
2. High cytokinin levels (like 5–7 mg/L BAP) are beneficial for stimulating shoot bud formation, but prolonged exposure may inhibit elongation or result in vitrification. Reducing BAP supports shoot elongation and differentiation into distinct, transplantable shoots, particularly important for downstream rooting and acclimatization stages.
a. Dissolve 4.43 g of MS powder (M5519) in approximately 700 mL of sterile distilled water.
b. Add 30 g of sucrose and stir until fully dissolved.
c. Add 1 mL of biotin stock (1 mg/mL) to achieve a final concentration of 1 mg/L.
d. Add 2 mL of BAP stock (1 mg/mL) to reach a final concentration of 2 mg/L.
e. Adjust the pH of the solution to 5.8 using 1 M NaOH (or HCl if needed).
f. Make up the final volume to 1,000 mL with sterile distilled water.
g. Add 3 g of phytagel and mix well.
h. Autoclave the media at 121 °C, 15 psi, for 15 min.
i. Allow to cool to 50–60 °C.
j. Pour 25–30 mL of the media into sterile 90 mm deep Petri plates under laminar airflow.
8. 2× Plant rooting and development media (1 L)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| MS powder (M5524) | 4.43 g/L | 4.43 g |
| Morel & Wetmore vitamin | 1× | 10 mL |
| Sucrose | 30 g/L | 30 g |
| BAP stock (1 mg/mL) | 0.045 mg/L | 45 μL |
| IAA stock (1 mg/mL) | 0.2 mg/L | 200 μL |
| Phytagel | 3 g/L | 3 g |
| Distilled water | n/a | Up to 1,000 mL |
| Total | 1,000 mL |
Notes:
1. Root induction requires a hormonal environment with minimal cytokinin and low auxin, mimicking the natural physiological conditions needed for root initiation.
2. In this medium, BAP is reduced to 0.045 mg/L, and IAA is included at 0.2 mg/L, providing a hormonal balance conducive to healthy root development without excessive callusing or shoot regeneration.
3. The inclusion of Morel & Wetmore vitamins supports root formation by supplying essential cofactors. Notably, this mix contains calcium pantothenate (Vitamin B5), which enhances root development by promoting coenzyme A biosynthesis—a vital pathway for energy metabolism and cell division in developing root tissues.
4. Since IAA (Indole-3-acetic acid) is heat labile, it should indeed be added after autoclaving, once the medium has cooled to around 60 °C under sterile conditions. This helps preserve its biological activity and ensures consistent rooting results.
a. Dissolve MS powder and sucrose in approximately 700 mL of distilled water.
b. Add Morel & Wetmore Vitamin solution and BAP stock. Mix thoroughly.
c. Adjust the pH to 5.8 using 1 M NaOH or HCl.
d. Bring the volume up to 1,000 mL with distilled water.
e. Add phytagel and stir to disperse evenly.
f. Autoclave the media at 121 °C, 15 psi, for 15 min.
g. Allow the media to cool to 50–60 °C.
h. Under sterile conditions in a laminar airflow hood, add IAA stock (200 μL of 1 mg/mL) to the cooled media.
i. Mix gently and pour 70 mL per sterile 250 mL culture container under laminar airflow.
Laboratory supplies
1. Beakers (250 mL, 500 mL, 1 L)
2. Measuring cylinders (10 mL to 1 L)
3. Magnetic stirrer and stir bars (or glass rod for manual stirring)
4. Pipettes (adjustable micropipettes: 10–1,000 μL) and sterile tips
5. Syringe and 0.22 μm syringe filters (for sterilizing hormone stock solutions)
6. Sterile 250 mL culture containers or jars (with lids or breathable covers)
7. Forceps and scalpels (sterile, for explant transfer)
8. Glass or plastic media bottles (autoclavable, 1 L capacity)
9. Parafilm or sealing tape (for sealing culture vessels)
10. Lab marker pens (for labeling media and containers)
11. Autoclave-safe gloves and lab coat
12. Waste containers for biological and chemical waste
Equipment
1. Analytical balance for accurate weighing of media components
2. pH meter for adjusting medium pH
3. Autoclave for sterilizing media and reusable supplies
4. Laminar airflow hood (LAF cabinet) for aseptic operations
5. Hot plate with magnetic stirrer for dissolving components and mixing medium
6. Refrigerator/freezer (-20 °C) for storing stock solutions
7. Growth chamber or culture room for incubating cultures under controlled temperature and photoperiod
8. Water bath (optional) for gently warming stock solutions
9. Distilled or MilliQ water system for preparing media
10. Light meter (lux or PAR) (optional) to monitor light levels in culture room
Procedure
A. Explant preparation (see Graphical overview for details)
Note: Immature male inflorescences (bells) are the flowers located at the end of the banana bunch. Unlike the mature female flowers, which develop into banana fruits, the male inflorescence is a maroon-colored bell attached to the bunch by the peduncle. The optimal stage for harvest is when the bell is 10–15 cm beyond the last row of fruits. The size of the bells varies depending on the cultivar. For Cavendish (AAA) and Lady Finger (AAB), the immature bells used as explants were approximately 15–20 cm long when collected from field-grown plants. Only healthy, intact inflorescences without signs of necrosis or damage were selected. Minor cultivar-specific differences were noted: Cavendish inflorescences were slightly larger, while Lady Finger inflorescences were more compact.
1. Harvest immature male inflorescences (bells) from healthy field-grown banana plants.
2. Remove outer bracts and reduce the size to 4–6 cm.
3. Keep in water to keep the bells hydrated.
CAUTION: Henceforth, all procedures should be carried out under sterile conditions in a laminar air flow (LAF) hood.
4. Transfer the bells into a large beaker (500 mL or 1 L), add approximately 400–500 mL of 70% ethanol, and soak for 10 min.
5. Rinse 2–3 times in sterile distilled water under laminar airflow. Transfer individual bells into separate containers containing sterile water to keep them hydrated.
6. Further trim the explants to approximately 2–3 cm in size.
7. Dissect each explant longitudinally into eight equal segments as follows:
a. First, divide the bell lengthwise into two halves.
b. Then, slice each half into two sections.
c. Finally, divide each section longitudinally into two slices, resulting in eight equal segments per explant.
Optional: Instead of longitudinal sectioning, experienced researchers may dissect individual floral “hands” from immature male flowers under a stereo/dissecting microscope, allowing more precise explant isolation. For novices, longitudinal dissection of the bells is recommended, as it is simpler and minimizes the risk of damaging delicate tissues.
B. Culture initiation
In the development of this method, explants were cultured on MS medium supplemented with 5 or 7 mg/L BAP, with separate plates used for each concentration. Four explant sections were placed per plate. Both concentrations were evaluated to determine the optimum for achieving high-frequency shoot formation.
1. Culture four explant sections per plate on MS medium supplemented with either 5 or 7 mg/L BAP, using separate plates for each concentration.
Note: For new cultivars, testing both concentrations is advisable to determine the optimum for high-frequency shoot formation.
2. Incubate all cultures at 25 °C under a 16/8 h (light/dark) photoperiod.
3. After 3–4 weeks, transfer the explants to fresh (but similar) media and maintain for another 3–4 weeks at 25 ± 1 °C under a 16/8 h light/dark photoperiod.
Notes:
1. If using longitudinally sectioned explants, use two plates per banana flower (bell), with four explants per plate. The number of bells to be used will depend on availability.
2. If using individual hands dissected from the flowers, distribute them between 2–3 plates, with 7–10 hands per plate.
C. Meristem proliferation
1. After 3–4 weeks, transfer explants to meristem proliferation medium supplemented with BAP at 5 mg/L and NAA at 0.5 mg/L or 7 mg/L and NAA at 0.5 mg/L, depending on the treatment group.
2. Incubate for 2 months with monthly subculture on fresh medium for meristem proliferation at 25 ± 2 °C under a 16/8 h light/dark photoperiod.
3. Maintain cultures under optimal conditions (e.g., 25 ± 2 °C, 16 h photoperiod) and observe regularly for signs of active growth or stress.
Notes:
1. Curved bracts may be carefully removed during transfer to the proliferation medium. This enhances contact between the meristem and the medium and reduces the risk of tissue necrosis or contamination.
2. If phenolic exudation occurs (e.g., browning of the medium or explants), increase the subculture frequency to every 3 weeks. Frequent transfers help prevent the buildup of toxic compounds and promote healthier proliferation.
3. As meristematic clumps enlarge, they can be gently divided into smaller clumps during subculturing. This prevents overcrowding, promotes uniform proliferation, and increases the number of explants available for subsequent cultures.
D. Shoot differentiation
1. Transfer proliferating meristematic tissues to shoot differentiation medium containing a lower concentration of BAP (2 mg/L) to promote elongation and differentiation of individual shoots.
2. Maintain cultures under standard growth conditions (e.g., 25 ± 2 °C, 16 h photoperiod).
3. Subculture on fresh medium every 3–4 weeks to prevent nutrient depletion and accumulation of inhibitory metabolites until shoot formation.
4. Maintain cultures under standard growth conditions (e.g., 25 ± 2 °C, 16 h photoperiod).
Note: If shoot clusters enlarge excessively, gently divide them into smaller clumps during subculturing. This encourages uniform shoot development and prevents overcrowding, which can hinder shoot elongation or lead to vitrification.
E. Rooting and plant development
1. When in vitro shoots are >1 cm tall, gently separate them from the shoot cluster.
2. Transfer five shoots per container onto the prepared rooting medium consisting of full-strength MS macro and micronutrients, Morel & Wetmore vitamins, 0.045 mg/L BAP, 0.2 mg/L IAA, 3% sucrose, and 0.8% agar, with the pH adjusted to 5.8 prior to autoclaving.
3. Incubate under a 16 h photoperiod at 27 °C with light intensity of 20–30 μmol·m-2·s-1 (PPFD).
4. Observe for root emergence and shoot elongation over 1–2 months.
Notes:
1. Shoots typically root and elongate between 6 and 16 weeks, depending on cultivar.
2. If any vitrification, browning, or contamination is observed, transfer healthy shoots to fresh rooting medium.
3. Cultures can be maintained indefinitely on rooting medium by subculturing every 4 weeks.
4. This ensures sustained growth, avoids nutrient depletion, and preserves regeneration potential.
Validation of protocol
This protocol for direct regeneration from male inflorescence (bell) has been successfully applied to Musa spp. cv. Williams (AAA) and Lady Finger (AAB). The method was developed and optimized using Cavendish banana and is routinely used in our group for reliable and reproducible plantlet regeneration. For Cavendish, multiple meristem cultures were established on meristem formation media containing different concentrations of BAP, and the total number of bells used, total plants produced, and mean number of plants per bell (± SEM) were recorded (Table 1). All treatments produced high regeneration efficiency, with a mean regeneration rate of 100%. Although no quantitative data were collected for Lady Finger, the protocol successfully produced plants directly from field-derived material. The method consistently results in meristem proliferation, shoot differentiation, and root formation under standard in vitro conditions. Refer to Figure 1 for expected results.
Table 1. Effect of BAP concentration on meristem formation and plant production in Cavendish banana
| BAP concentration in meristem formation media | Total number of bells used | Total number of shoots produced | Mean number of shoots per bell ± SEM | Regeneration rate (%) |
|---|---|---|---|---|
| 5 mg/L | 9 | 227 | 25.22 ± 1.94a | 100 |
| 7 mg/L | 9 | 191 | 21.22 ± 1.93a | 100 |
Data represent mean ± SEM of shoots from nine bells per BAP treatment. Statistical analysis was performed using one-way ANOVA in Microsoft Excel (Windows 11).
Longitudinal sections of male flowers were cultured on MS medium supplemented with BAP at 5 or 7 mg/L in Petri dishes and incubated under light at 25 °C (Figure 1A). Approximately 23 shoots were obtained per bell (Table 1), with 100% regeneration observed at both BAP concentrations. The highest mean number of shoots (25 shoots) was obtained at 5 mg/L BAP, whereas 21 shoots were obtained at 7 mg/L BAP; however, the difference was not statistically significant (p > 0.05). Previous studies have reported lower shoot numbers, such as 12 shoots in the AA genotype banana cv. Pisang Mas [11], as well as higher yields, including up to 31 shoots in the AAB genotype cv. Poovan [12] and 80–130 shoots per male flower in the AAA genotype cv. Berangan [10]. These comparisons highlight that multiplication efficiency in banana is strongly cultivar-dependent, and the lower average observed in cv. Williams likely reflects inherent genotypic differences in regenerative capacity rather than a limitation of the protocol itself. After two weeks, the bracts at both BAP concentrations turned green and curved outward, exposing rudimentary flower hands, with some non-regenerable callus forming at the cut edges of the bracts and flower stem (Figure 1B). Following two months of subculture on the same medium supplemented with BAP (5 or 7 mg/L) and NAA (1 mg/L), the explants expanded and produced numerous globular structures (Figure 1C, D), some of which differentiated into shoot-like structures (Figure 1E). When meristems were transferred to medium containing a lower concentration of BAP (2 mg/L), numerous adventitious shoots formed (Figure 1F, G), which developed roots on rooting medium after six weeks (Figure 1H). Fully developed plantlets were acclimatized for four weeks (Figure 1I) and subsequently transplanted into soil, where they grew normally and appeared phenotypically healthy (Figure 1J).
While apical meristems from suckers produce only a single plant initially, requiring many multiplication cycles to obtain larger numbers, male inflorescences allow multiple shoots to be generated simultaneously, reducing the number of multiplication cycles needed. This demonstrates that using male inflorescences is an efficient strategy for rapid clonal propagation, supporting the robustness of the present protocol.
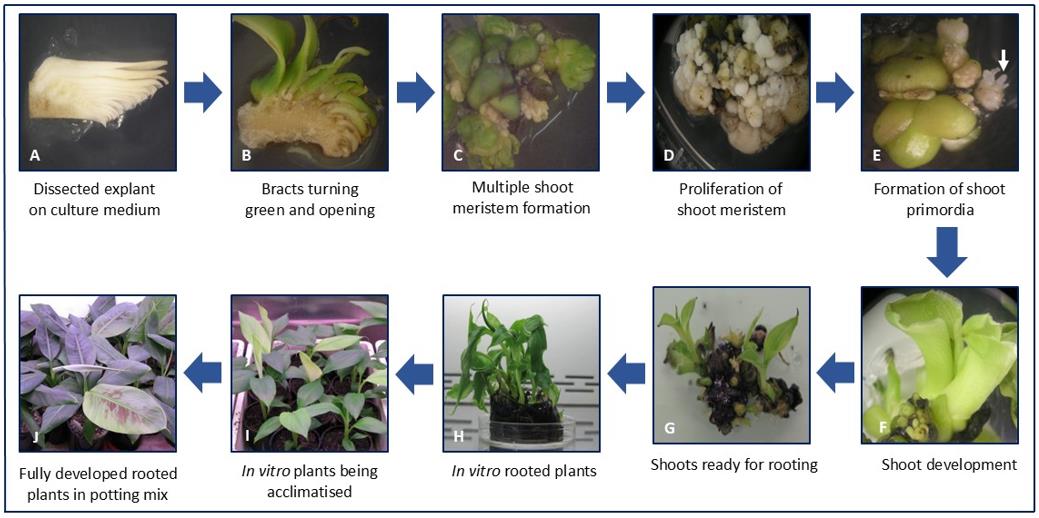
Figure 1. Direct regeneration from male inflorescence of banana (Musa spp. cv. Williams). (A) Longitudinal sections of male flowers cultured on MS medium supplemented with BAP (5 mg/L). (B) After two weeks, bracts turned green and curved outward, exposing rudimentary flowers, while non-regenerative watery callus formed on the cut surfaces. (C) Proliferation of the inflorescence on medium containing BAP (5 mg/L) and NAA (1 mg/L). (D) Repeated subculturing on the proliferation medium increased the frequency of meristem formation. (E) Globular meristematic structures differentiated into shoot primordia (white arrow). (F) Development of green shoots after transfer to shoot differentiation medium. (G) Clusters of shoots prepared for rooting. (H) Regenerated plantlets with numerous roots on rooting medium after three months. (I) Rooted plants acclimatized in potting mix. (J) Hardened plants successfully established in soil.
General notes and troubleshooting
General notes
1. Select the right developmental stage of explant: Use immature male flowers where the floral "hands" are just emerging but not fully developed. Overly mature or too small bells often give inconsistent or poor responses.
2. Dissect carefully to maintain tissue integrity: When preparing longitudinal sections, ensure clean and even cuts. Avoid crushing or damaging tissues, as this reduces viability and increases browning.
3. Keep explants hydrated at all times: From field collection to culture initiation, maintain hydration to reduce stress and improve survival. Always keep bells or segments in sterile water when not in use.
4. Use fresh media and precise hormone concentrations: Media should be freshly prepared and pH adjusted accurately. Even small variations in BAP or NAA concentrations can impact the type and extent of tissue response.
5. Observe explants regularly for contamination or browning: Check cultures weekly. Early signs of contamination should be addressed immediately by removing affected plates. Browning tissues should be subcultured earlier than scheduled.
6. Optimize light and temperature conditions: Maintain cultures at 25 °C with a 16/8 h light/dark cycle using fluorescent or LED light sources (30–40 μmol/m2/s). Avoid direct exposure to high-intensity light, which may stress the explants.
7. Remove outer bracts during the proliferation stage: Curved bracts can be trimmed off during subculture to allow better nutrient access and space for developing meristematic tissue.
8. Do not rush the shoot transfer to rooting medium: Wait until a good number of well-developed shoot clusters appear before transferring to rooting medium. Premature transfer may delay or reduce rooting efficiency.
9. Track performance by bell: Since results may vary depending on the source bell, label and monitor performance individually to identify high-responding material for future propagation.
10. Document morphological stages visually: Capturing images at each developmental stage (e.g., greening, callus formation, globular structure, shoot emergence) can help in protocol optimization and troubleshooting.
Troubleshooting
Problem 1: Bracts or flower tissues turn brown or necrotic within the first week.
Possible cause: Excessive tissue damage or phenolic oxidation during dissection or sterilization.
Solution: Use freshly harvested immature male flowers. Reduce the time in ethanol if tissues are sensitive. Include antioxidants, such as ascorbic acid (100 mg/L) or activated charcoal, in the medium. Subculture more frequently (every 2–3 weeks).
Problem 2: Explants do not survive or turn mushy after culture initiation.
Possible cause: Overhydration during sterilization or contamination.
Solution: Ensure proper draining after sterilization. Minimize water retention on explants before placing on medium. Maintain strict sterile techniques.
Problem 3: Medium does not solidify properly.
Possible cause: Incorrect phytagel concentration or pH.
Solution: Ensure pH is adjusted to 5.7 before autoclaving. Use 2.5–3 g/L phytagel if medium is too soft.
Problem 4: Bracts remain pale or do not curve outward.
Possible cause: Ineffective cytokinin concentration or poor light quality.
Solution: Confirm correct BAP concentration (5 or 7 mg/L). Check light intensity (30–40 μmol/m2/s) and photoperiod (16/8 h). Ensure explants are placed with the cut surface in contact with the medium.
Problem 5: No callus formation on cut edges.
Possible cause: Low explant responsiveness or incorrect BAP level.
Solution: Try using both BAP concentrations (5 and 7 mg/L) to determine the optimal response for the cultivar used. Test younger bells if the response is poor.
Problem 6: No globular structure formation or proliferation.
Possible cause: Subculture interval too long or auxin/cytokinin imbalance.
Solution: Subculture every 3–4 weeks to prevent tissue browning. Ensure correct NAA (1 mg/L) is included. Test a lower BAP:NAA ratio if over proliferation occurs.
Problem 7: Browning of tissues during the proliferation stage.
Possible cause: Accumulation of phenolics in the medium.
Solution: Include antioxidants. Subculture more frequently (every 2–3 weeks). Minimize tissue damage during transfers.
Problem 8: No shoot formation or very few shoots.
Possible cause: Prolonged use of high BAP concentration.
Solution: Reduce BAP to 2 mg/L for the differentiation stage. Confirm that tissues were fully proliferated before transfer.
Problem 9: Shoots form but do not root.
Possible cause: Inadequate auxin in the rooting medium.
Solution: Ensure the correct IAA concentration (0.2 mg/L) is used. Alternatively, test IBA (0.5–1 mg/L) for improved rooting. Consider a slight decrease in sucrose (to 20 g/L) for the rooting stage.
Acknowledgments
This work was supported by funding from the Bill & Melinda Gates Foundation and conducted at the Queensland University of Technology (QUT). The protocol was developed, and the manuscript was written solely by the author. Special thanks to Dr. Douglas Becker for his support and encouragement, and to Prof. James Dale for granting permission to carry out this work. The author acknowledges the valuable contributions of Harirah and Khalid [10], Wirakarnai et al. [11], and Nair et al. [12], whose published methods informed the development of the present protocol.
Competing interests
The author declares no conflicts of interest.
Ethical considerations
This protocol involves the use of banana inflorescence (“bells”) and does not involve human participants or animal subjects. Therefore, no ethical approval or informed consent was required.
References
- Chukwu, S. C., Awala, S. K., Angombe, S., Valombola, J. S., Nanhapo, P. I., Mberama, C., Rafii, M. Y., Oladosu, Y., Thomas, B., Okporie, E. O., et al. (2025). Recent progress in tissue culture techniques and biotechnological innovations for banana production (Musa spp.): a review. Discover Plants. 2(1): e1007/s44372–025–00099–2. https://doi.org/10.1007/s44372-025-00099-2
- Ploetz, R. C. (2007). Diseases of banana. In Plant Pathologist’s Pocketbook (pp. 59–71). CABI.
- FAO. (2020). Banana market review: Preliminary results for 2019. Food and Agriculture Organization of the United Nations. http://www.fao.org
- Ploetz, R. C. (2015). Fusarium wilt of banana. Phytopathology. 105(12): 1512–1521. https://doi.org/10.1094/PHYTO-04-15-0101-RVW
- Kumar, P. L., Selvarajan, R., Iskra-Caruana, M. L., Chabannes, M. and Hanna, R. (2021). Biology, etiology, and control of virus diseases of banana and plantain. Adv Virus Res. 110, 125–175. https://doi.org/10.1016/bs.aivir.2021.02.003
- Tripathi, J. N., Ntui, V. O., Tripathi, L. (2023). Precision genetics tools for genetic improvement of banana. Plant Genome. 17(2): e20416. https://doi.org/10.1002/tpg2.20416
- Murashige, T. and Skoog, F. (1962). A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol Plant. 15(3): 473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
- Strosse H., R. Domergue, B. Panis, J.V. Escalant and Côte, F. (2003). Banana and plantain embryogenic cell suspensions. In Vézina, A. and Picq, C. (Eds.). INIBAP Technical Guidelines 8. The International Network for the Improvement of Banana and Plantain, Montpellier, France.
- Sipen, P., Davey, M. R. (2012). Effects of N(6)-benzylaminopurine and Indole Acetic Acid on In Vitro Shoot Multiplication, Nodule-like Meristem Proliferation and Plant Regeneration of Malaysian Bananas (Musa spp.). Trop Life Sci Res. 23(2): 67–80. https://pubmed.ncbi.nlm.nih.gov/24575235/
- Harirah, A. A. and Khalid, N. (2006). Direct regeneration and RAPD assessment of male inflorescence derived plants of Musa acuminata cv. Berangan. Asia Pac J Mol Biol Biotechnol. 14(1): 11–17.
- Wirakarnai, S., Hossain, A. and Chandran, S. (2008). Plantlet Production through Development of Competent Multiple Meristem Cultures from Male Inflorescence of Banana, Musa acuminta cv. ‘Pisang Mas’ (AA). Am J Biochem Biotechnol. 4(4): 325–328. https://doi.org/10.3844/ajbbsp.2008.325.328
- Nair, A. R. G., Ravichandran, P. and Bejoy, M. (2018). Direct shoot regeneration from male immature flower buds of Musa paradisiaca Linn. cv. Poovan (AAB). Plant Sci Today. 5(4): 142–148. https://doi.org/10.14719/pst.2018.5.4.403
Article Information
Publication history
Received: Jun 27, 2025
Accepted: Sep 9, 2025
Available online: Sep 23, 2025
Published: Oct 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Deo, P. C. (2025). Direct Plant Regeneration From Immature Male Inflorescence of Banana (Musa spp.). Bio-protocol 15(20): e5476. DOI: 10.21769/BioProtoc.5476.
Category
Plant Science > Plant breeding > Micropropagation
Plant Science > Plant developmental biology > Morphogenesis
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








