- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In Vitro Screening of Microbial Extracts Against the Oomycetes Phytophthora capsici and Pythium ultimum
Published: Vol 15, Iss 18, Sep 20, 2025 DOI: 10.21769/BioProtoc.5451 Views: 1265
Reviewed by: Shweta PanchalAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Simplifying Barley Leaf Rust Research: An Easy and Reproducible Infection Protocol for Puccinia hordei on a Small Laboratory Scale
Caroline I. Skoppek and Jana Streubel
Jul 20, 2023 1705 Views

Silencing Arbuscular Mycorrhizal Fungal Gene Using Chitosan Nanoparticle-Mediated dsRNA Delivery System
Chumei Yan [...] Xianan Xie
Jun 5, 2025 2645 Views

A Reliable In Planta Inoculation and Antifungal Screening Protocol for Rhizoctonia solani-Induced Sheath Blight in Rice
Alinaj Yasin [...] Palash Deb Nath
Nov 5, 2025 1582 Views
Abstract
Oomycetes are a predominantly plant-pathogenic group of organisms often considered and managed as fungi; however, due to their evolutionary divergence from true fungi, many conventional fungicides are ineffective against them. Their unique physiological characteristics make them challenging to work with, highlighting the need for a standardized and reproducible procedure for anti-oomycete assays. Previous studies describe methods to obtain sporulation forms in the laboratory, but there remains a disconnect between spore production and the subsequent screening process for potential biological pesticides based on microbial organic extracts. This protocol bridges that gap by providing a complete and reliable workflow from spore production to screening. In this study, we present an efficient in vitro protocol to identify microbial extracts with activity against Phytophthora capsici and Pythium ultimum. The protocol includes a method for obtaining zoospores of P. capsici and oospores of P. ultimum, followed by a simple and rapid screening assay to detect microbial extracts that inhibit the growth of these pathogens. The extracts are dispensed onto plates in two concentrations and allowed to dry. This facilitates pauses in the protocol and allows for storage of the plates until the biological material is ready for the assay. The protocol’s effectiveness has been validated with these two oomycetes, resulting in the identification of active extracts in both cases. Moreover, it can be adapted to other pathogens.
Key features
• This protocol is designed for in vitro susceptibility testing of oomycetes, using Phytophthora zoospores and Pythium oospores as inoculum sources.
• This protocol can be easily used for the identification of natural compounds with anti-oomycete activity.
Keywords: OomycetesGraphical overview
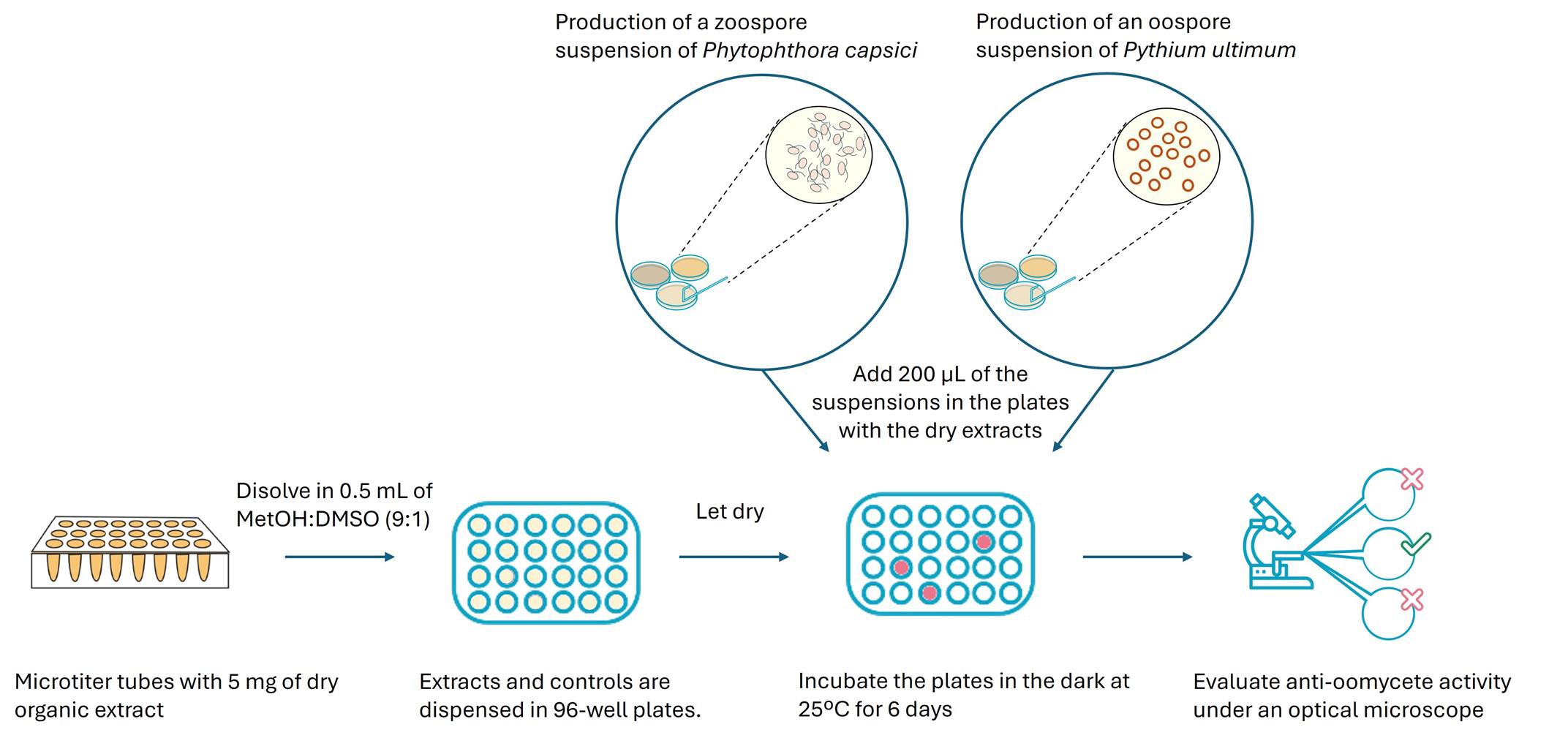
Graphical summary of a screening method for identifying microbial extracts with anti-oomycete activity
Background
It is estimated that globally, between 10% and 40% of crop yields are lost due to pests and diseases [1]. Fungi and oomycetes both significantly impact agriculture. Oomycetes exhibit a morphology similar to true fungi, yet they are phylogenetically distinct [2]. They constitute a predominantly pathogenic group, with approximately 60% of known species infecting plants. Moreover, global climate change is expected to shift their geographic distribution toward higher latitudes, potentially increasing their agricultural impact [3]. Phytophthora capsici is a highly destructive oomycete plant pathogen that infects numerous plant species, including many agriculturally important crops such as solanaceous, cucurbitaceous, and leguminous plants [4,5]. Globally, diseases caused by P. capsici have resulted in annual economic losses exceeding 100 million USD [6]. Another major oomycete pathogen is Pythium ultimum, which causes damping-off and root rot in more than 300 host species, including maize, soybean, wheat, and ornamental plants, also leading to significant economic losses [4,7]. These pathogens can rapidly develop resistance to conventional fungicides, significantly reducing their effectiveness. Furthermore, physiological and biochemical differences between oomycetes and true fungi limit the efficacy of many existing fungicides against diseases caused by Phytophthora and Pythium species [8,9]. Therefore, the development of new agents with specific anti-oomycete activity is imperative.
Experimental research requires the use of valid and effective protocols that allow for the rapid selection of extracts or compounds with activity against the target pathogen. Specifically, in the case of oomycetes, the screening techniques based on methods used for fungi are often employed [10]. Originally, in vitro screenings were based on clinical microbiology protocols for antifungal susceptibility testing [11]. These guidelines were later adapted for other similar organisms and for phytopathogenic microorganisms for use in agricultural research [12]. Recently, similar systems have been validated specifically for oomycetes, but using mycelium instead of oospores in the case of Pythium, due to the difficulty of obtaining them [13], or zoosporangia in the case of Phytophthora [14].
This protocol offers some key advantages over existing methodologies. First, it is designed to use spores, which represent the most desirable source of inoculum. This ensures high sensitivity and reproducibility. Second, it provides a high-throughput assay model that can be adapted for use with other pathogens. It can even be adapted to other types of samples, such as plant extracts or pure compounds, by adjusting the assay concentration accordingly. However, it also has limitations, such as the need for further optimization of spore production for different oomycete and fungal species. Moreover, the spore developmental stage is critical for the proper performance of the assay. This protocol fills a critical methodological gap, enabling the exploration of new anti-oomycete and antifungal products and contributing to the development of effective strategies for managing plant diseases in agriculture.
Materials and reagents
Biological materials
1. Phytophthora capsici
Note: The isolate used in this study was kindly provided by Dr. Venkat Gangavarapu (Valent Bioscience, Illinois, USA).
2. Pythium ultimum (CECT 20902)
Reagents
1. Distilled water
2. Aluminum foil (Albal)
3. Rye seeds (Biográ)
4. Sucrose (PanReac AppliChem, catalog number: 131621)
5. American bacteriological agar (Condalab, catalog number: 1802)
6. Tomato juice (Granini)
7. Calcium carbonate (CaCO3) (Eurominerals, catalog number: Calprec PA E-170)
8. Yeast extract (Condalab, catalog number: 1702)
9. Potassium phosphate monobasic (KH2PO4) (PanReac AppliChem, catalog number: 131509)
10. Magnesium sulfate heptahydrate (MgSO4·7H2O) (PanReac AppliChem, catalog number: 131404)
11. L-Asparagine monohydrate (Sigma-Aldrich, catalog number: A8381)
12. D(+)-Glucose anhydrous (PanReac AppliChem, catalog number: 141341)
13. Potato dextrose broth (PDB) (Condalab, catalog number: 1261)
14. Casitone Bacto (Gibco, catalog number: 225930)
15. Sodium chloride (NaCl) (PanReac AppliChem, catalog number: 131659)
26. Methanol (MeOH) (PanReac AppliChem, catalog number: 361091.1612)
17. Dimethyl sulfoxide (DMSO) (Thermo Fisher Scientific, catalog number: 032434.M1)
18. Cycloheximide (Biomar Microbial Technologies, catalog number: CH0019)
19. Lactophenol blue solution (Sigma-Aldrich, catalog number: 61335)
Solutions
1. Rye A medium (see Recipes)
2. CaCO3 solution (see Recipes)
3. UA medium (see Recipes)
4. K medium (see Recipes)
5. Potato Dextrose Broth medium (PDB) (see Recipes)
6. Luria Bertani (LB) (see Recipes)
7. Solvent mixture (see Recipes)
8. Cycloheximide solution (see Recipes)
Recipes
1. Rye A medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Rye seeds | 60 g/L | 30 g |
| Sucrose | 20 g/L | 10 g |
| Agar | 15 g/L | 7.5 g |
| Distilled water | 1,000 mL | 500 mL |
| Total | 1,000 mL | 500 mL |
Place 30 g of rye seeds in a 500 mL beaker and cover them with 200 mL of distilled water. Let it rest for 16 h covered with aluminum foil at room temperature. After 16 h, remove the water but keep it. Add another 200 mL of water to the rye and blend for 2 min. Heat the mixture in a water bath at 68 °C for 1 h. Filter the mixture through gauze and discard the grain. Add the previously collected water and make up to 500 mL. Then add the remaining compounds and dissolve them using a magnetic stir bar placed on a magnetic stirrer. The medium is sterilized by autoclaving at 121 °C for 20 min in a 1 L Pyrex bottle. Pour 20 mL into sterile Petri plates, let the plates completely solidify, and store them at room temperature in a clean plastic bag.
2. CaCO3 solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| CaCO3 | 40 g/L | 2 g |
| Distilled water | 1,000 mL | 50 mL |
| Total | 1,000 mL | 50 mL |
Dissolve 2 g of CaCO3 in 50 mL of distilled water using a magnetic stir bar placed on a magnetic stirrer. This solution is reserved for the preparation of UA medium. Store at room temperature in a 50 mL polypropylene tube.
3. UA medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tomato juice | 20 mL/L | 4 mL |
| CaCO3 solution | 7.5 mL/L | 1.5 mL |
| Sucrose | 0.5 g/L | 0.1g |
| Yeast extract | 0.1 g/L | 0.02 g |
| Distilled water | 972.5 mL | 194.5 mL |
| Total | 1,000 mL | 200 mL |
Filter 4 mL of tomato juice through gauze. Transfer it to a graduated cylinder and add the CaCO3 solution. Adjust the volume to the mark (200 mL) with distilled water. Transfer the mixture to a 500 mL beaker, add the remaining components, and dissolve them using a magnetic stir bar on a magnetic stirrer. Sterilize the medium by autoclaving at 121 °C for 20 min in a 500 mL Pyrex bottle. Store at 4 °C in the dark for up to 5 days.
4. K medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| KH2PO4 | 0.5 g/L | 0.1 g |
| MgSO4·7H2O | 0.25 g/L | 0.05 g |
| Asparagine | 1 g/L | 0.2 g |
| Yeast extract | 0.5 g/L | 0.1 g |
| Glucose | 25 g/L | 5 g |
| Distilled water | 1,000 mL | 200 mL |
| Total | 1,000 mL | 200 mL |
Prepare 200 mL of the medium by dissolving the components in distilled water using a magnetic stir bar on a magnetic stirrer. Sterilize by autoclaving at 121 °C for 20 min in a 500 mL Pyrex bottle. Store at room temperature for up to 14 days.
5. Potato Dextrose Broth medium (PDB)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| PDB | 13.25 g/L | 2.65 g |
| Distilled water | 1,000 mL | 200 mL |
| Total | 1,000 mL | 200 mL |
Dissolve 2.65 g of PDB in 200 mL of distilled water in a 500 mL Pyrex bottle using a magnetic stir bar on a magnetic stirrer. Sterilize the medium by autoclaving at 121 °C for 15 min. Store at room temperature for up to 14 days.
6. Luria Bertani (LB)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Casitone | 10 g/L | 5 g |
| NaCl | 10 g/L | 5 g |
| Yeast extract | 5 g/L | 2.5 g |
| Agar | 18 g/L | 9 g |
| Distilled water | 1,000 mL | 500 mL |
| Total | 1,000 mL | 500 mL |
Prepare the medium by dissolving the components in distilled water using a magnetic stir bar on a magnetic stirrer. The medium is sterilized by autoclaving at 121 °C for 20 min in a 1 L Pyrex bottle. Pour 20 mL into sterile Petri plates, let the plates completely solidify, and store them at room temperature in a clean plastic bag.
7. Solvent mixture
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| MeOH | 90% | 90 mL |
| DMSO | 10% | 10 mL |
| Total | 100% | 100 mL |
Prepare only the volume needed for immediate use, as MeOH evaporates quickly and the solution cannot be stored. Mix the two components and gently invert the tube to mix.
8. Cycloheximide solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Cycloheximide | 2 mg/mL | 4 mg |
| Sterile distilled water | 1 mL | 2 mL |
| Total | 1 mL | 2 mL |
Dissolve 4 mg of cycloheximide in 2 mL of sterile distilled water using a magnetic stir bar on a magnetic stirrer. Starting from this 2,000 µg/mL solution, prepare serial 1:10 dilutions at 200 µg/mL, 20 µg/mL, and 2 µg/mL in sterile distilled water. Store at 4 °C.
Caution: This substance is highly toxic for humans; take exhaustive precautions when handling.
Laboratory supplies
1. 90 × 14 mm Petri dish (Deltalab, catalog number: 200200)
2. 96-well sterile microplates (Avantor, catalog number: 734-2327)
3. Sterile inoculating loops (Avantor, catalog number: 612-7277)
4. 15 mL sterile polypropylene conical tube (Fisherbrand, catalog number: 07-200-886)
5. 50 mL sterile polypropylene conical tube (Fisherbrand, catalog number: 05-539-13)
6. Sterile gauze pads (4 layers, 20 thread/cm2) (Indas, catalog number: 482174)
7. 3 mL sterile transfer pipette (Falcon, catalog number: 357575)
8. 0.1–10 µL non-filter pipette tips (Fisherbrand, catalog number: 02-707-455)
9. 5–300 µL non-filter pipette tips (Fisherbrand, catalog number: 02-707-447)
10. 100–1,250 µL non-filter pipette tips (Fisherbrand, catalog number: 02-707-400)
11. 1.5 mL Eppendorf-type microtube (Deltalab, catalog number: 200400P)
12. 1.2 mL round-bottom microtubes (Deltalab, catalog number: 409009)
13. 1,000 mL borosilicate bottles (Fisher Scientific, Pyrex, catalog number: 11912629)
14. 500 mL borosilicate bottles (Fisher Scientific, Pyrex, catalog number: 11902629)
15. Beaker polypropylene 500 mL (Kartell LABWARE, catalog number: 1806)
16. 500 mL graduated cylinder (BRAND, catalog number: 35054)
17. Magnetic stirring bar (Kartell LABWARE, catalog number: 764)
Equipment
1. Water bath (J.P. Selecta, catalog number: 6000137)
2. Autoclave (Matachana, series S1000, model: 1006v-1)
3. Measuring scale (Kern, model: ARJ 120-4M)
4. 0.2–2 µL micropipette (Gilson, model: Pipetman F144801)
5. 2–20 µL micropipette (Gilson, model: Pipetman F123600)
6. 20–200 µL micropipette (Gilson, model: Pipetman F123601)
7. 200–1,000 µL micropipette (Gilson, model: Pipetman, F123602)
8. 2–200 µL multichannel micropipette (Gilson, model Pipetman: FA10011)
9. Ultrasonic bath (Branson, model: CPX2800-E)
10. Vibrating shaker vortex (J.P. Selecta, catalog number: 7001725)
11. Neubauer counting chamber (BRAND, catalog number: 717805)
12. Microscope (Nikon Eclipse E200) equipped with E Plan 4× 0.10 Nikon lens
13. Magnetic stirrer (Heidolph, model: MR Hei Mix D)
14. Biosafety cabinet (Telstar, model: Bio II A)
15. Blender (Oster, model: Classic 3 speed)
16. Centrifuge (Sigma, model: 4-16S)
17. Incubation room with controlled temperature of 25 °C
Procedure
All manipulations are performed under aseptic conditions in a vertical laminar flow biological safety cabinet.
A. Distribution of extracts on plates
1. Prepare extracts from microorganism fermentations [15] in round-bottom microtubes, each containing 5 mg of dry organic extract.
2. Dissolve the 5 mg of extract in 0.5 mL of a solvent mixture of MetOH:DMSO (9:1). Use an ultrasonic bath and vortex for a few seconds, if necessary, to fully dissolve the extracts to a final concentration of 10 mg/mL.
3. Take 10 μL (corresponding to 100 μg) of this extract solution and place it in well A3 of a 96-well sterile microplate (Figure 1).
4. Place 1 μL (10 μg) of the same dissolved extract in well B3 (Figure 1).
5. Place the following extract in wells A4 and B4, and so on sequentially. Following this procedure, 40 extracts can be tested at two final concentrations of 500 μg/mL (ppm) and 50 μg/mL (ppm) in one microtiter plate (Figure 1).
6. Let the extracts dry in the well.
Note: DMSO will not evaporate, but it will help to solubilize extract components when the oomycete spore suspension is added. The volume of DMSO present in each well is 1 or 0.1 μL, depending on the extract concentration. Plates can be handled as if the DMSO is completely dry.
Pause point: Use the dissolved extracts to prepare all the necessary microplates. Plates with dried extract can be stored at -20 °C for extended storage before use.
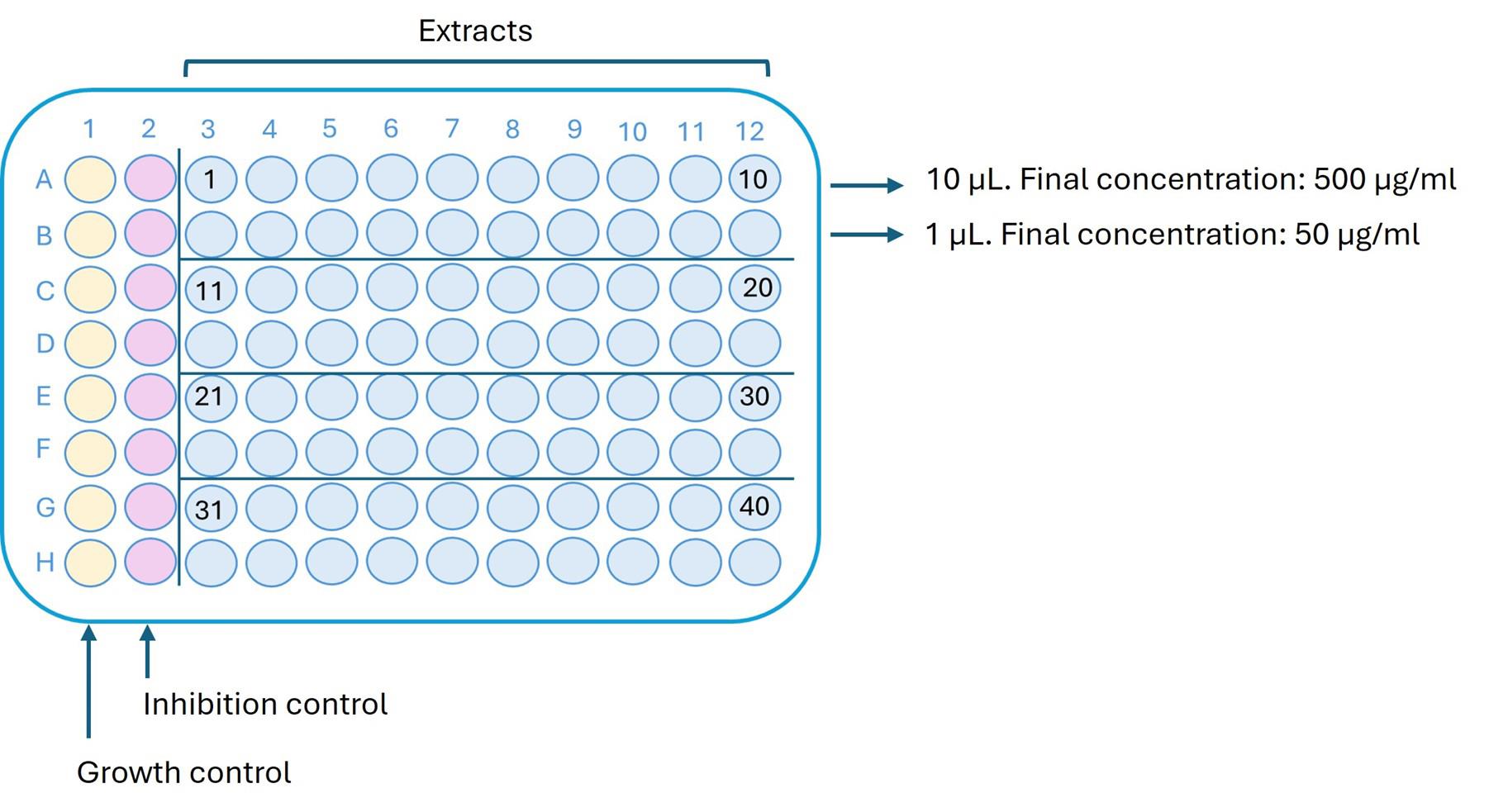
Figure 1. Arrangement of extracts and controls in a 96-well plate. Column 1 contains the growth control prepared with the corresponding zoospore/oospore suspension. Column 2 contains the dose-response inhibition control. Extracts are dispensed in the remaining columns at two test concentrations.
B. Growth control
Assign the first column to the growth control, in which the pathogen is added to verify normal growth under the assay conditions (Figure 1).
Note: For the strains used in this protocol, the amount of DMSO present in each well is not toxic. However, it is recommended to previously test that the vehicle used for extract distribution is not toxic to your particular strain.
C. Inhibition control: cycloheximide
In the second column, place the inhibition control, where the pathogen exhibits varying responses to the control agent. Use cycloheximide as the inhibition control at eight concentrations. These concentrations vary depending on the target pathogen: 2.5, 1, 0.75, 0.5, 0.25, 0.1, 0.05, and 0.01 µg/mL for P. capsici and 60, 40, 20, 10, 5, 2.5, 1, and 0.75 µg/mL for P. ultimum.
Prepare working solutions from stock solutions of 2,000 µg/mL, 200 µg/mL, 20 µg/mL, and 2 µg/mL, as described in Table 1. Then, add 10 µL of each corresponding working solution to the wells to achieve the final assay concentrations after adding the spore suspension.
Begin by adding 10 µL of the highest concentration solution to well A2, and continue sequentially down the column, ending with the lowest concentration in well H2 (Figure 1).
Table 1. Stock solution concentrations and corresponding volumes to achieve final assay concentrations of cycloheximide
| Stock 2,000 µg/mL (µL) | Stock 200 µg/mL (µL) | Stock 20 µg/mL (µL) | Stock 2 µg/mL (µL) | Sterile distilled water (µL) | Working solutions concentration (µg/mL) | Assay working solutions (µL) | Assay cycloheximide concentration (µg/mL) |
|---|---|---|---|---|---|---|---|
| 30 | 20 | 1,200 | 10 | 60 | |||
| 20 | 30 | 800 | 10 | 40 | |||
| 10 | 40 | 400 | 10 | 20 | |||
| 50 | 200 | 10 | 10 | ||||
| 25 | 25 | 100 | 10 | 5 | |||
| 12.5 | 37.5 | 50 | 10 | 2.5 | |||
| 5 | 45 | 20 | 10 | 1 | |||
| 37.5 | 12.5 | 15 | 10 | 0.75 | |||
| 25 | 25 | 10 | 10 | 0.5 | |||
| 12.5 | 37.5 | 5 | 10 | 0.25 | |||
| 5 | 45 | 2 | 10 | 0.1 | |||
| 25 | 25 | 1 | 10 | 0.05 | |||
| 5 | 45 | 0.2 | 10 | 0.01 |
D. Phytophthora capsici zoospore production
1. Grow P. capsici in Rye A for 15–20 days at 25 °C in the dark (inoculate by transferring a small mycelial fragment to the center of the plate using a sterile inoculating loop).
2. Check for the presence of sporangia on the plates under the microscope. Add 20 mL of sterile distilled water to a Rye A plate and gently scrape the surface using a sterile inoculating loop.
3. Use 0.5 mL of the suspension prepared in step D2 to inoculate a plate containing 10 mL of UA liquid medium, which supports subsequent zoospore release.
Note: Use transfer pipettes instead of standard 1,000µL micropipette tips, as their wider openings help avoid clogging caused by clumping of the suspension.
4. Incubate the plate at 25 °C in the dark for 4 days (Figure 2A).
5. Transfer the plate to 4 °C for 1 h and then warm to room temperature for 20 min to allow zoospores to be released from the sporangia into the medium (Figure 2B) (see Troubleshooting 1).
6. Remove the spore suspension from the plate using a sterile transfer pipette and filter it immediately through sterile gauze to eliminate mycelia debris. Collect the clean zoospore suspension in a 50 mL sterile polypropylene tube.
Note: Place 10 µL of the suspension onto an LB plate and spread it using a sterile inoculating loop to check for sterility. After 24 h, inspect the plate to confirm the absence of bacterial growth.
7. To count the zoospore suspension, mix 20 μL of the suspension with 20 μL of lactophenol blue in a sterile Eppendorf-type tube to immobilize the zoospores and facilitate counting.
8. Count the zoospores using a Neubauer counting chamber under the microscope.
Note: Note that the suspension is diluted by half due to mixing with lactophenol blue. Multiply the count by two to obtain the final concentration of the zoospore suspension.
9. Prepare the suspension for the assay at a final concentration of 1.3 × 103 zoospores/mL in K medium.
Critical: The suspension must be prepared and used immediately.
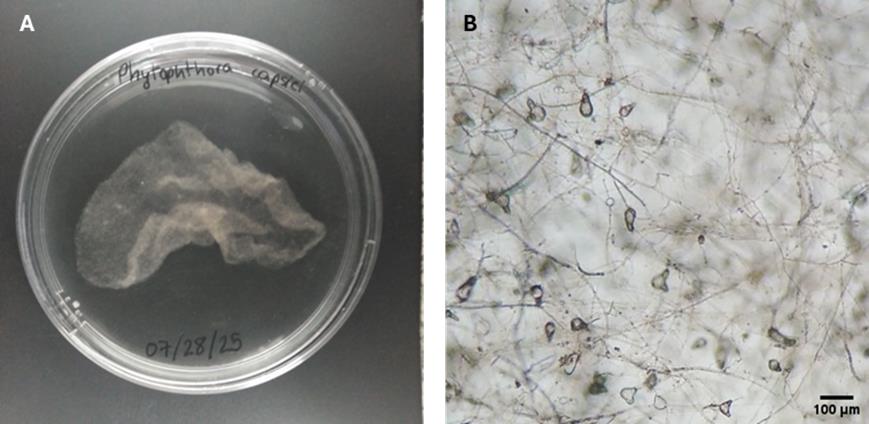
Figure 2. Phytophthora capsici morphology after 4 days of cultivation in UA medium. (A) Macroscopic growth. (B) Mycelial growth and sporangia (40× magnification).
E. Pythium ultimum oospore production
1. Grow P. ultimum in Rye A for 25–30 days at 25 °C in the dark (Figure 3A).
Note: Normally, at least 25 days are needed for the oospore to form; however, always check under the optical microscope that they have formed before moving on to step E2 (Figure 3B).
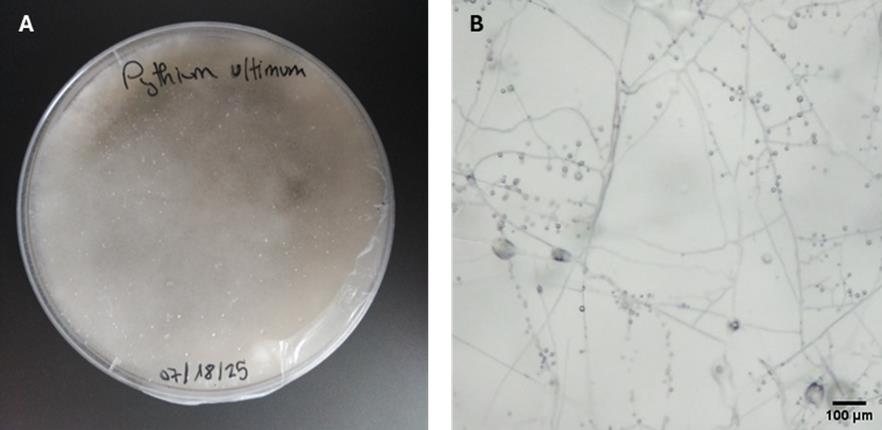
Figure 3. Pythium ultimum morphology after 25 days of cultivation in Rye A medium. (A) Macroscopic growth. (B) Mycelial growth and oospores (40× magnification).
2. Add 15 mL of sterile distilled water to a Rye A plate and gently scrape the surface using a sterile inoculating loop.
3. Remove the oospore suspension from the plate using a sterile transfer pipette and transfer it to a 50 mL polypropylene tube.
4. Sonicate the suspension for 5 min in an ultrasonic bath (40 kHz) to allow the oospores to separate from the mycelia.
5. Filter the suspension through sterile gauze to remove mycelial debris. Collect the clean oospore suspension in a sterile 50 mL polypropylene tube.
Note: Place 10 µL of the suspension onto an LB plate and spread it using a sterile inoculating loop to check for sterility. After 24 h, inspect the plate to confirm the absence of bacterial growth.
6. Centrifuge at 1,831× g for 5 min. Discard the supernatant, leaving approximately 5 mL to obtain a concentrated suspension.
7. Count the oospores using a Neubauer counting chamber under the microscope.
8. Prepare the suspension for the assay at a final concentration of 1 × 103 oospores/mL in PDB medium.
Critical: The suspension must be prepared and used on the same day.
F. Performing the anti-oomycete assay
1. Dispense 200 μL of the spore suspension into each well of the plate containing the dry extracts and the growth control column using a multichannel pipette (Figure 4).
2. Dispense 190 μL of the spore suspension into the inhibition control column using a multichannel pipette.
3. Incubate the assay plates in a chamber at 25 °C in darkness for 6 days.
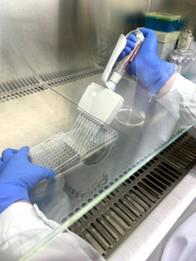
Figure 4. Performing the anti-oomycete assay. Dispense the spore suspension into a 96-well microplate.
G. Evaluation of the anti-oomycete activity
1. Perform a visual inspection of the wells.
2. Reevaluate any wells showing an apparent lack of growth under the E Plan 4× 0.10 NA objective lens of an optical microscope.
3. Classify the level of growth inhibition based on visual and microscopic observations, assigning the appropriate category as follows (Figure 5):
(-) No inhibition: Mycelial growth comparable to the control without inhibitor.
(+) Intermediate inhibition: Approximately 50% reduction in mycelial growth is observed.
(++) Apparent total inhibition with residual viability: No visible growth is observed; however, microscopic examination reveals initial germination that is not progressing.
(+++) Total inhibition: Complete suppression of spore germination and mycelial growth.
Record any additional alterations in hyphal growth or development as comments.
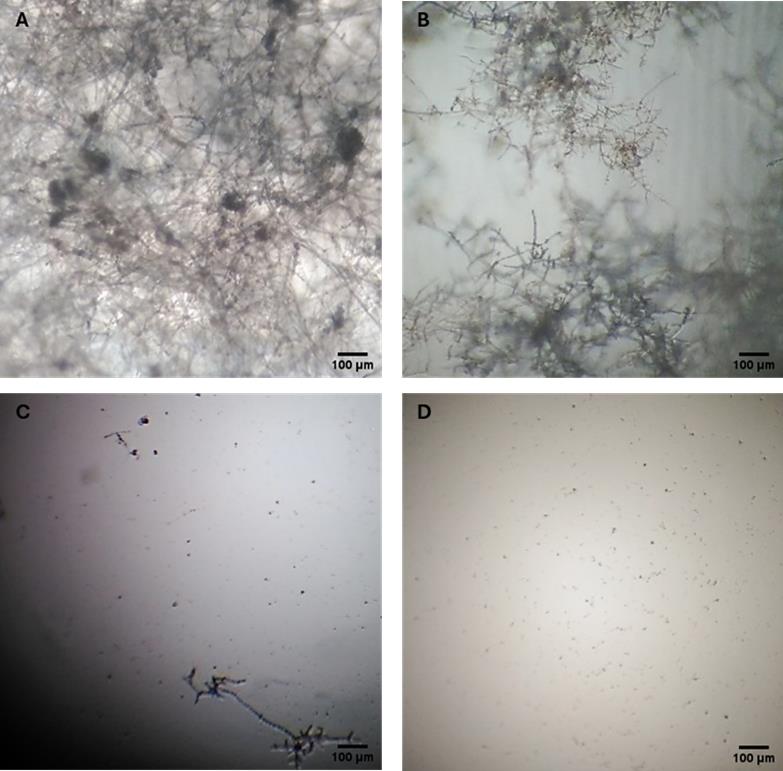
Figure 5. Microscopic evaluation of anti-oomycete activity. (A) (-) No inhibition. (B) (+) Intermediate inhibition. (C) (++) Apparent total inhibition with residual viability (D) (+++) Total inhibition (40× magnification).
Data analysis
For accurate interpretation of the results, always begin by verifying that both the growth control and the inhibition control are functioning correctly. The growth control should show abundant and homogeneous growth. In the inhibition control, a minimum inhibitory concentration of cycloheximide at 2.5 µg/mL is consistently effective against P. capsici. Complete inhibition of P. ultimum requires a concentration of 20 µg/mL.
To be considered active, a microbial extract must exhibit at least 50% inhibition (+) at a concentration of 50 µg/mL in two independent replicates.
Greater experience with the morphology of oomycetes and fungi contributes to a more reliable interpretation of the results.
Validation of protocol
This protocol has been validated for reproducibility and robustness through multiple independent replicates and appropriate experimental controls.
Based on the company’s experience, over 40,000 extracts have been tested against P. capsici and 1,300 against P. ultimum. Using the criteria described in the data analysis, we obtained approximately 1% active extracts against P. capsici and 0.5% against P. ultimum.
General notes and troubleshooting
General notes
1. It is very important to maintain sterile conditions throughout the entire process, which includes working in a biosafety cabinet and using sterilized materials and tools. All solutions and media should be autoclaved or filter-sterilized, as appropriate.
2. This protocol is optimized for P. capsici and P. ultimum but can be adapted to other pathogenic oomycetes or filamentous fungi with similar growth characteristics (e.g., Phytophthora infestans or Botrytis cinerea). For species-specific adaptations, optimize conidia concentration and incubation times.
3. To minimize variability, use spores from the same batch of plates and maintain consistent experimental conditions, including temperature, media, and incubation times.
4. Responses may vary depending on the oomycete strain. Perform preliminary experiments to standardize conditions and ensure uniform spore growth in the assay and sensitivity to extracts.
5. Small fluctuations in environmental conditions can affect strain growth. Maintain standardized conditions in all experiments.
6. Although this protocol is designed for microbial extracts, the assay method can be applied to study other types of extracts (e.g., plant extracts) or synthetic and natural compounds.
7. Cycloheximide is hazardous. Always follow institutional safety guidelines for its handling, use, and disposal. Consider alternative translation inhibitors if safety concerns arise.
Troubleshooting
Problem 1: After placing the 4-day UA medium plates at 4 °C and then allowing them to warm to room temperature, zoospores of P. capsici are not released.
Possible cause: The sporangia have not matured sufficiently, and zoospores have not formed.
Solution: Prepare batches of UA medium plates. Use the batch with 5 days of growth, as one additional day is usually sufficient for sporangia to mature and produce zoospores.
Problem 2: Excessive growth in the P. ultimum control; variable results from plate to plate in the inhibition control.
Possible cause: Mycelium fragments are present in the oospore suspension (Figure 6).
Solution: Examine the oospore working suspension and filter it through gauze again (double-folded if necessary) to ensure a clean oospore suspension (Figure 6).
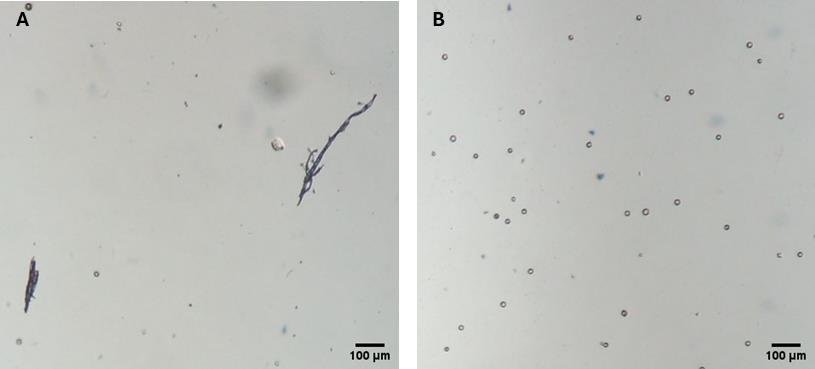
Figure 6. Microscopic images of P. ultimum oospore suspension. (A) Oospore suspension with mycelium fragments. (B) Clean oospore suspension (40× magnification).
Acknowledgments
Conceptualization, M.T.M. & MA.V.N.; Investigation, M.T.M.; Writing—Original Draft, M.T.M.; Writing—Review & Editing, M.T.M. & MA.V.N.; Funding acquisition, A.F.M.; Supervision, MA.V.N. The work presented here was supported by Biomar Microbial Technologies. This protocol was adapted from the original article [12]. We also thank Neyla Álvarez García for her technical assistance throughout the process.
Competing interests
The authors declare no conflicts of interest.
References
- Fernández-Ortuño, D., Escobar-Niño, A., Fernández-Acero, F. J. and Niño-Sánchez, J. (2023). Nuevas herramientas biotecnológicas para el control de enfermedades: Botrytis cinerea como modelo. Fitopatología. 16–23.
- Thines, M. (2014). Phylogeny and evolution of plant pathogenic oomycetes—a global overview. Eur J Plant Pathol. 138(3): 431–447. https://doi.org/10.1007/s10658-013-0366-5
- Sullam, K. E. and Musa, T. (2021). Ecological Dynamics and Microbial Treatments against Oomycete Plant Pathogens. Plants. 10(12): 2697. https://doi.org/10.3390/plants10122697
- Kamoun, S., Furzer, O., Jones, J. D. G., Judelson, H. S., Ali, G. S., Dalio, R. J. D., Roy, S. G., Schena, L., Zambounis, A., Panabières, F., et al. (2014). The Top 10 oomycete pathogens in molecular plant pathology. Mol Plant Pathol. 16(4): 413–434. https://doi.org/10.1111/mpp.12190
- Sanogo, S., Lamour, K., Kousik, C. S., Lozada, D. N., Parada-Rojas, C. H., Quesada-Ocampo, L. M., Wyenandt, C. A., Babadoost, M., Hausbeck, M. K., Hansen, Z., et al. (2023). Phytophthora capsici, 100 Years Later: Research Mile Markers from 1922 to 2022. Phytopathology. 113(6): 921–930. https://doi.org/10.1094/phyto-08-22-0297-rvw
- Barchenger, D. W., Lamour, K. H. and Bosland, P. W. (2018). Challenges and Strategies for Breeding Resistance in Capsicum annuum to the Multifarious Pathogen, Phytophthora capsici. Front Plant Sci. 9: e00628. https://doi.org/10.3389/fpls.2018.00628
- Wang, T., Gao, C., Cheng, Y., Li, Z., Chen, J., Guo, L. and Xu, J. (2020). Molecular Diagnostics and Detection of Oomycetes on Fiber Crops. Plants. 9(6): 769. https://doi.org/10.3390/plants9060769
- Zhang, S., Wang, Y., Cai, J., Liu, D., Yan, Y., Zhang, H., Li, L., Wang, X., Xiang, W., Zhang, J., et al. (2022). Discovery of febrifugine with specific anti-Phytophthora oomycete activity isolated from Dichroa febrifuga Lour. Ind Crops Prod. 178: 114651. https://doi.org/10.1016/j.indcrop.2022.114651
- Wu, J., Xue, Z., Miao, J., Zhang, F., Gao, X. and Liu, X. (2020). Sensitivity of Different Developmental Stages and Resistance Risk Assessment of Phytophthora capsici to Fluopicolide in China. Front Microbiol. 11: e00185. https://doi.org/10.3389/fmicb.2020.00185
- Loreto, Ã. S., Tondolo, J. S. M., Pilotto, M. B., Alves, S. H. and Santurio, J. M. (2014). New Insights into the In Vitro Susceptibility of Pythium insidiosum. Antimicrob Agents Chemother. 58(12): 7534–7537. https://doi.org/10.1128/aac.02680-13
- Clinical and Laboratory Standards Institute (2008). Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; Approved Standard—Second Edition. M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA.
- Vinuesa, M. A. and Fernández, A. (2021). A Simple Antifungal assay for Testing Actinomycetes and Other Microbial Extracts. Methods Mol Biol. 2296: 217–225. https://doi.org/10.1007/978-1-0716-1358-0_13
- Marcianò, D. and Toffolatti, S. L. (2023). Methods for Fungicide Efficacy Screenings: Multiwell Testing Procedures for the Oomycetes Phytophthora infestans and Pythium ultimum. Microorganisms. 11(2): 350. https://doi.org/10.3390/microorganisms11020350
- Rogozhin, E. A., Vasilchenko, A. S., Barashkova, A. S., Smirnov, A. N., Zavriev, S. K. and Demushkin, V. P. (2020). Peptide Extracts from Seven Medicinal Plants Discovered to Inhibit Oomycete Phytophthora infestans, a Causative Agent of Potato Late Blight Disease. Plants. 9(10): 1294. https://doi.org/10.3390/plants9101294
- Romero, F. and Fernández, A. (2021). Screening Fermentation and Extract Generation. Methods Mol Biol. 2296: 209–216. https://doi.org/10.1007/978-1-0716-1358-0_12
Article Information
Publication history
Received: Jun 12, 2025
Accepted: Aug 18, 2025
Available online: Sep 2, 2025
Published: Sep 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Trigal Martínez, M., Fernández Medarde, A. and Vinuesa Navarro, M. Á. (2025). In Vitro Screening of Microbial Extracts Against the Oomycetes Phytophthora capsici and Pythium ultimum. Bio-protocol 15(18): e5451. DOI: 10.21769/BioProtoc.5451.
Category
Microbiology > Antimicrobial assay
Microbiology > Microbe-host interactions
Plant Science > Plant immunity > Host-microbe interactions
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link







