- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Transplantation of Cultured Myoblasts Into Intact Skeletal Muscle and Analysis of Muscle Contraction Force in Mice Model
Published: Vol 15, Iss 16, Aug 20, 2025 DOI: 10.21769/BioProtoc.5413 Views: 1784
Reviewed by: Xiaokang WuIshita ChandelAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Assessing the Presence of Hematopoietic Stem and Progenitor Cells in Mouse Spleen
Isabelle J. Marié [...] David E. Levy
Jun 5, 2022 3758 Views

An Optimized Ex Vivo Protocol for Quantitative Electrophysiological Assessment of Neuromuscular Junctions and Skeletal Muscle Function Using the Aurora System
Can Cui [...] Wing Hoi Cheung
Jun 20, 2025 1936 Views
Electroporation of Whole-Mount Postnatal Rodent Retinas for Advanced Functional Assays
Chien-Ting Huang [...] Chih-Tien Wang
Jan 20, 2026 124 Views
Abstract
Cell transplantation is a promising strategy for treating age-related muscle atrophy, but its critical application remains limited. Cultured myoblasts, unlike freshly isolated muscle stem cells, show poor engraftment efficiency and fail to contribute effectively to muscle regeneration. Moreover, successful engraftment generally requires prior muscle injury, as skeletal muscle regeneration is typically triggered by a damaged microenvironment. These limitations present major obstacles for applying cell therapy to sarcopenia, where muscle degeneration occurs without injury. In this protocol, we describe a novel approach that enables the transplantation of cultured myoblasts into intact skeletal muscle without the need for preexisting injuries or genetic modification. By combining myoblasts with extracellular matrices (ECM), such as Matrigel, which mimic the native muscle niche and support cell survival, adhesion, proliferation, and differentiation, we achieve efficient engraftment and increased muscle mass without the need for preexisting injury. The ECM also provides a scaffold and retains bioactive factors that enhance the regenerative capacity of transplanted cells. This is the first protocol that enables robust myoblast engraftment in non-injury muscle conditions, offering a practical tool for studying and potentially treating sarcopenia.
Key features
• Cultured myoblasts mixed with extracellular matrix components are transplanted into intact skeletal muscle.
• Contraction force measurement of the tibialis anterior muscle in vivo.
• Cell transplantation without muscle injury would be applied for the treatment of sarcopenia.
Keywords: Skeletal muscleGraphical overview
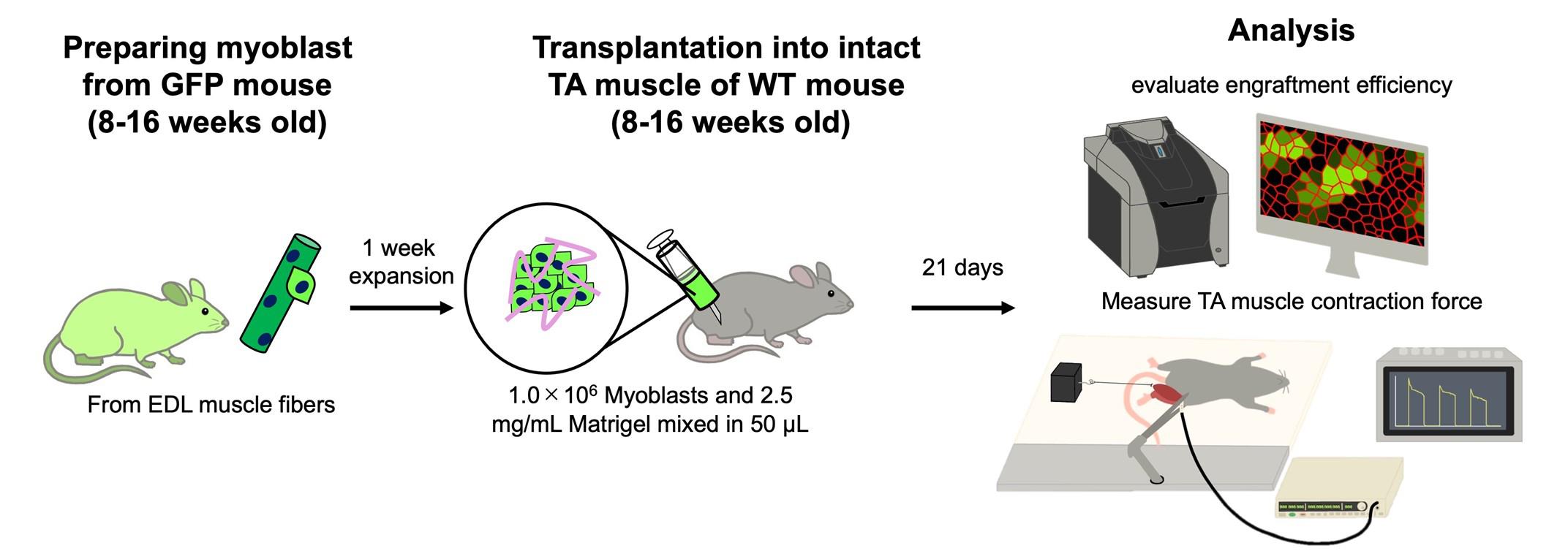
Background
Age-related muscle atrophy is a major global concern, contributing to reduced quality of life and life expectancy. One promising approach to treat skeletal muscle atrophy is cell transplantation using muscle progenitor cells. Skeletal muscle has a high potential to regenerate itself, and muscle stem cells called satellite cells contribute to myogenesis by responding to damaged muscle cells (myofibers). Upon myofibers being damaged, satellite cells activate into myoblasts, and they begin proliferation and differentiation into myofibers. This regenerative potential of myoblasts forms the basis for cell-based therapies targeting skeletal muscle disorders. However, since the first attempt to repair muscle tissue through myoblast transplantation [1], the low efficiency of cell engraftment has been a challenge. Satellite cells are limited in number and need to be expanded in vitro as myoblasts to achieve enough numbers for cell transplantation [2,3], while myoblasts being cultured ex vivo show low transplantation efficiency, and most of the transplanted cells cannot contribute to myogenesis and are eliminated [4–6].
Another issue in achieving cell therapy for age-related skeletal muscle atrophy is that the transplanted cells rarely engraft into intact tissue [4,6,7]. Inducing injury to the muscle tissue is considered an essential step for successful cell engraftment [8,9] because the microenvironment (niche) in damaged muscle promotes myogenesis. Many types of cells and secreted factors accelerate muscle regeneration, including the activation of satellite cells or the proliferation and differentiation of myoblasts [3,10,11]. In the intact tissue, satellite cells stay in a quiescent state, and myogenesis is not conducted. Transplanted cells cannot create myofibers in this niche. From these findings, many researchers consider that cell therapy is hardly available for the treatment of sarcopenia, which does not show a significant collapse of myofibers.
We have recently established a method to successfully engraft cultured myoblasts into intact skeletal muscle tissue [12]. Our approach focused on creating a supportive niche that allows myoblasts to proliferate and differentiate. In general, extracellular matrix (ECM) components must be coated for the adhesion of myoblasts to the culture dishes, and ECM also plays a critical role in in vivo muscle regeneration, which promotes the activation of satellite cells and myoblast proliferation and differentiation [3,10,11]. We mix myoblasts and Matrigel, which are widely used as ECM components, and transplant them into intact muscle. We found that the myoblasts injected with Matrigel exhibited enhanced engraftment efficiency, and muscle weight was increased by transplantation [12]. This article provides a detailed protocol for myoblast transplantation and functional analysis of the transplanted tibialis anterior muscle. A limitation of our method is that transplanted myoblasts generate both mature and immature myofibers without enhancing muscle contraction force. Methodological improvements are needed to promote myofiber maturation and increase muscle strength.
Materials and reagents
Biological materials
1. C57BL/6 wild-type mice (8–16 weeks old, from Japan SLC, Inc.)
2. CAG-EGFP mice (8–16 weeks old, from Japan SLC, Inc.)
Reagents
1. Type 1 collagenase (Worthington Biochemical Corporation, catalog number: LS004196)
2. DMEM GlutaMAX (Gibco, catalog number: 10569044)
3. Penicillin-Streptomycin-Amphotericin B suspension (Fujifilm Wako, catalog number: 161-23181)
4. Bovine serum albumin (BSA) (Sigma, catalog number: A9418)
5. Phosphate-buffer saline (PBS) (Gibco, catalog number: 18912014)
6. Accutase (Innovative Cell Technologies, catalog number: AT104)
7. Matrigel (Corning, catalog number: 354230)
8. DMEM, no glucose, no glutamine, no phenol red (Gibco, catalog number: A1443001)
9. Fetal bovine serum (FBS) (Gibco, catalog number: 10437-028)
10. GlutaMAX (Thermo Fisher, catalog number: 35050061)
11. Chicken embryo extract (USBiological Life Sciences, catalog number: C3999)
12. FGF-basic, murine, recombinant (Pepro Tech Inc., catalog number: 450-33)
13. Trypsin-EDTA (Gibco, catalog number: 25200072)
14. NaCl (Wako, catalog number: 196-01671)
15. Domitor (ZENOAQ, catalog number: AHE1)
16. Mitazolam (Maruishi Pharmaceutical Corporation, catalog number: 1124401A1052)
17. Butorphanol (Meiji Animal Health Corporation, catalog number: VETLI5-B)
18. Anti-anesthetic (ZENOAQ, catalog number: AHC1)
19. Tissue-Tek O.C.T (Sakura Finetek, catalog number: 45833)
20. 4% paraformaldehyde (Nacalai Tesque, catalog number: 09154-85)
21. Goat serum (Vector Laboratories, catalog number: S-1000-20)
22. Laminin-alpha2 antibody (Enzo Life Sciences, catalog number: ALX-804-190, from rat)
23. Alexa Fluor 594 anti-rat (Invitrogen, catalog number: A-11007)
24. VectaShield mounting medium with DAPI (Vector Laboratories, catalog number: H-1200)
25. KCl (Wako, catalog number: 163-03545)
26. CaCl2·2H2O (Wako, catalog number: 035-00455)
27. KH2PO4 (Wako, catalog number: 169-04245)
28. MgSO4·7H2O (Wako, catalog number: 138-00415)
29. NaHCO3 (Wako, catalog number: 191-01305)
30. Disinfectants (Orimatsu, catalog number: WK-Ⅱ-75)
Solutions
1. Myofiber isolation medium (see Recipes)
2. BSA solution (see Recipes)
3. Collagenase solution (see Recipes)
4. Saline (see Recipes)
5. Myoblast growth medium (see Recipes)
6. Myoblast transplantation solution (see Recipes)
7. Anesthetic (see Recipes)
8. Anti-anesthetic (see Recipes)
9. Immunohistochemistry blocking solution (see Recipes)
10. KRB buffer (see Recipes)
Recipes
1. Myofiber isolation medium
Prepare under a clean bench. Add the Penicillin-Streptomycin-Amphotericin B suspension directly to the DMEM GlutaMAX bottle and store at 4 °C.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Penicillin-Streptomycin-Amphotericin B suspension | 1.0% | 5.0 mL |
| DMEM GlutaMAX (500 mL bottle) | 500 mL |
2. BSA solution
Add 5 g of BSA powder to 80 mL of PBS. The final volume will be approximately 100 mL after dissolution. After dissolving BSA, inactivate at 60 °C for 30 min. After the solution cools down at room temperature (RT), separate the solution into approximately 10 mL in a 25 mL tube using a 0.22-μm filter and store at 4 °C.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| BSA | 5.0% | 5.0 g |
| PBS | 80 mL |
3. Collagenase solution
Dissolve collagenase in a 5 mL tube and sterilize with a 0.22-μm filter. Transfer the solution to a 15 mL tube for the myofiber incubation. Use 2.5 mL per two extensor digitorum longus (EDL) muscles from one mouse. Warm to 37 °C before use.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Type 1 collagenase | 0.25% | 6.25 mg |
| Myofiber isolation medium | 2.5 mL |
4. Saline
Make in a glass bottle and autoclave at 121 °C for 20 min. Store at 4 °C.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaCl | 0.9% | 0.9 g |
| H2O | 99.1% | 80 mL |
| Total | Adjust the final volume with H2O | 100 mL |
5. Myoblast growth medium
Store the medium at 4 °C. We recommend using it within 1 week [13]. Warm the medium at 37 °C before use.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| FBS | 30.0% | 30.0 mL |
| Chicken embryo extract | 1.0% | 1.0 mL |
| Penicillin-Streptomycin-Amphotericin B suspension | 1.0% | 1.0 mL |
| Basic-FGF (stock: 50 µg/mL) | 10 ng/mL | 20.0 μL |
| GlutaMAX | 1.0% | 1.0 mL |
| No glucose DMEM | 67.0% | 67.0 mL |
| Total | 100 mL |
6. Myoblast transplantation solution
Prepare on ice as the original concentration of Matrigel easily gellifies at RT.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Matrigel (original: 8.2 mg/mL) | 2.5 mg/mL | 30.49 μL |
| DMEM GlutaMAX | 19.51 μL | |
| Saline | 50.00 μL | |
| Total | 100 μL |
7. Anesthetic
Use sterilized saline, store at 4 °C, and cover with aluminum foil to block the light.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Domitor | 1.0 mg/mL | 0.75 mL |
| Mitazolam | 5.0 mg/mL | 2.00 mL |
| Butorphanol | 5.0 mg/mL | 2.50 mL |
| Saline | 7.25 mL | |
| Total | 12.50 mL |
8. Anti-anesthetic
Use sterilized saline, store at 4 °C, and cover with aluminum foil to block the light.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Anti-anesthetic | 5.0 mg/mL | 1.20 mL |
| Saline | 8.80 mL | |
| Total | 10.0 mL |
9. Immunohistochemistry blocking solution
Store at 4 °C.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Goat serum | 20.0% | 400 μL |
| PBS | 1,600 μL | |
| Total | 2.0 mL |
10. KRB buffer
Prepare each stock solution in advance and store at 4 °C. Mix the reagents in co-washed containers. Make fresh and warm at 37 °C before use.
Caution: If it contains impurities, the liquid turns white.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 5.0 M NaCl | 117 mM | 0.171 g |
| 1.0 M KCl | 4.7 mM | 118 μL |
| 1.0 M CaCl2·2H2O | 2.5 mM | 63 μL |
| 0.5 M KH2PO4 | 1.2 mM | 60 μL |
| 0.5 M MgSO4·7H2O | 1.2 mM | 60 μL |
| 1.0 M NaHCO3 | 24.6 mM | 0.052 g |
| Total | Adjust the final volume with H2O | 25 mL |
Laboratory supplies
1. 0.22 μm filter (MERCK, catalog number: SLGVR33RB)
2. Glass Pasteur pipette (Iwaki, catalog number: IK-PAS-5P)
3. 100 mm cell culture dishes (Iwaki, catalog number: 3020-100)
4. 50 mm deep dishes (Thermo Fisher, catalog number: 124-17)
5. Serological pipettes, 5 mL (FastGene, catalog number: FG3007)
6. Serological pipettes, 10 mL (FastGene, catalog number: FG3008)
7. Serological pipettes, 25 mL (FastGene, catalog number: FG3009)
8. Serological pipettes, 50 mL (FastGene, catalog number: FG3010)
9. Pipettes (SARTORIUS, catalog number: LH-729010/20/30/50/60/70)
10. 10 μL filter pipette tips (BMBio, catalog number: WEF-10RS)
11. 100 μL filter pipette tips (BMBio, catalog number: WEF-100RS)
12. 200 μL filter pipette tips (BMBio, catalog number: WEF-200RS)
13. 1,250 μL filter pipette tips (NICHIRYO, catalog number: 00-LRT-F1250RB)
14. 1.5 mL tubes (Watson, catalog number: 131-715C)
15. 5.0 mL tubes (SANSYO Co., Ltd, catalog number: ST-500)
16. 15 mL centrifuge tubes (Iwaki, catalog number: 2325-015N)
17. 25 mL centrifuge tubes (Iwaki, catalog number: 2362-025N)
18. 50 mL centrifuge tubes (Iwaki, catalog number: 2345-050N)
19. 100 mL centrifuge tubes (Iwaki, catalog number: 2355-100N)
20. Cell counter (OneCell, catalog number: 2-7124-02)
21. Surgical tape (NICHIBAN, catalog number: No. 25)
22. Twenty-six-gauge, 1 mL capacity plastic syringe (JMS Co., Ltd., catalog number: JS-VM2613R)
23. CO2 gas (Nissan TANAKA, catalog number: 87799)
24. APS coated slide glass (Matsunami, catalog number: 83-1631)
25. Cork stand (Uchiyama Manufacturing Corp., catalog number: 56735254)
26. Cover glass (Matsunami, catalog number: 83-0223/83-0225)
Equipment
1. Ultrapure water production equipment (SARTORIUS, catalog number: H2O mm-T)
2. High-pressure steam sterilizer (TOMY, catalog number: LPS-300)
3. Stereomicroscope (Leica, catalog number: M165 C)
4. Bio clean bench (PHCbi, catalog number: MCV-B131F)
5. Bio-shaker (TAITEC, catalog number: BR-40LF)
6. CO2 incubator (PHCbi, catalog number: MCO-170AICUVD)
7. Inverted microscope (EVIDENT, catalog number: CKX53)
8. Centrifuge for 15 mL tubes (HERMLE, catalog number: Z 207 A)
9. Centrifuge for 1.5 mL tubes (KUBOTA, catalog number: 3520)
10. Cryostat (Leica, catalog number: CM 1950)
11. Fluorescence microscope (KEYENCE, catalog number: BZ-810)
12. Mouse muscle tension measurement system (Uchida Denshi)
13. Electronic stimulator (Nihon Kohden, catalog number: SEN-3401)
Software and datasets
1. KEYENCE BZ-X series Image Analyzer (KEYENCE, version: BZ-X800_Analyzer.exe, 1.1.2.4)
2. ImageJ-Fiji (Version: 2.16.0/1.54p)
Procedure
A. Myofiber isolation and myoblast culture
1. The myofibers are isolated using protocols developed previously [14–16].
2. Prepare a myofiber isolation medium on a clean bench. Store the prepared medium at 4 °C in a refrigerator.
3. Prepare 0.25% collagenase solution in a tissue culture hood, filter sterilize with a 0.22 μm filter, and warm at 37 °C in a bio-shaker.
4. Euthanize GFP mice by cervical dislocation and remove the EDL muscle. Immerse EDL muscles in collagenase solution at 45 rpm for 2 h at 37 °C.
5. Prepare a glass Pasteur pipette. Coat a 50 mm deep dish and the glass Pasteur with 5% BSA to avoid myofiber adhesion.
6. Separate muscle tissue into single myofibers using a glass Pasteur pipette. Transfer only single muscle fibers to a new dish.
7. Coat Matrigel to a 100 mm dish and incubate at RT for at least 30 min. Start warming the cell culture medium at 37 °C.
8. (Continuation from step A6) Collect all single myofibers from one GFP mouse in a 25 mL tube and let it stand for 5 min to precipitate myofibers and remove the myofiber isolation medium (less than 500 μL).
9. Add 1.0 mL of accutase to the 25 mL tube containing single myofibers and let it react at RT for 10 min on a clean bench.
10. Add 5.0 mL of culture medium to a 100 mm dish from step A7 in advance to keep it wet. Add 5.0 mL of the medium to the 25 mL tube containing the myofibers, mix, and slowly transfer to the 100 mm dish.
11. After 2 min, transfer the dish into an incubator (37 °C, 5% CO2).
12. Change the culture medium every 24 h, starting on the third day after seeding the myofibers.
B. Myoblast transplantation into intact tibialis anterior (TA) muscle
1. Wash the culture dish twice with PBS.
2. Add 1.0 mL of trypsin-EDTA and incubate at 37 °C for 2 min.
3. After the detachment of myoblasts from the culture dish, add 6–8 mL of the culture medium, pipette gently, and collect the cells in a 15 mL tube. Centrifuge at 800× g for 5 min.
4. Remove the supernatant. Add 1 mL of new culture medium, resuspend, and collect the cells in a 1.5 mL tube.
5. Dilute the cell suspension solution to 1/10 or 1/20 and count the number of cells.
6. Transfer 1.4 million myoblasts into a 1.5 mL tube.
7. Centrifuge the 1.5 mL tube containing the cells at 500× g for 5 min and discard the supernatant. Then, gently suspend the cells in 70 μL of saline or Matrigel solution (1.0 million cells/50 μL).
Note: Prepare a larger volume of cell suspension, as some may be lost during collection with a syringe.
8. Anesthetize mice on a hot plate.
9. Measure the weight of WT mouse and intraperitoneally inject 5 μL of anesthetizing solution per gram of body weight into the mouse.
10. Wipe the mouse limb with 70% ethanol and shave the hair near the tibialis anterior (TA) muscle with a shaver.
11. Fix the 5 mL tube with surgical tape and place the foot of the mouse on it (Figure 1A). At this point, the TA muscle should bulge toward the experimenter. Apply surgical tape to bind the back of the foot and the 5 mL tube to fix the mouse’s foot. Set the foot, TA muscle, and knee horizontally (Figure 1B).
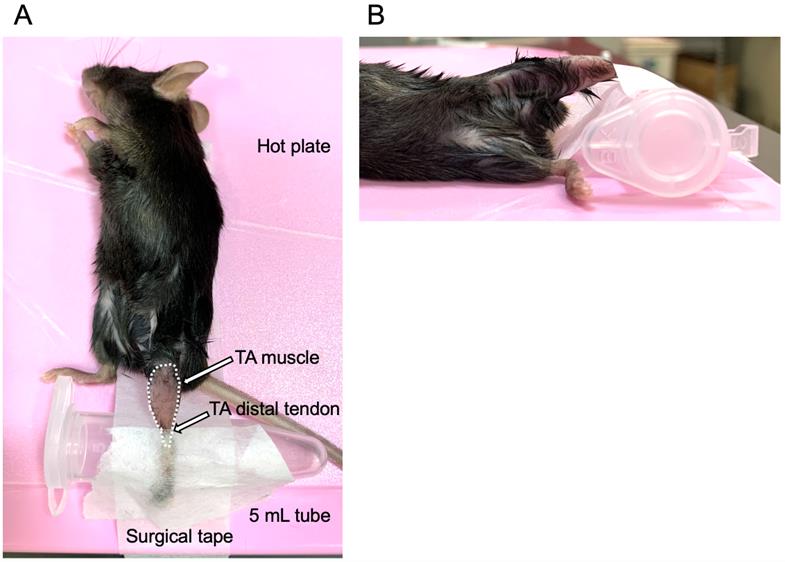
Figure 1. Fix the mouse for myoblast injection into the tibialis anterior (TA) muscle. (A) Image from above: lay the mouse on the hot plate and place the hind leg on a 5.0 mL tube. Fix the leg using surgical tape. The broken white line indicates the area of the TA muscle; white arrows indicate the TA distal tendon. (B) Image from the side: place the TA muscle horizontally to the hot plate.
12. Rinse the syringe with saline 10 times by aspirating and releasing saline.
Note: Do not neglect this step because the new syringe may have some residuals or coatings on the surface, which may affect the myoblast engraftment.
13. Inject myoblasts into the intact TA muscle. Load 50 μL of myoblast transplantation solution from step B7 and insert the syringe needle 2 mm above the distal tendon of the TA muscle, 1–2 mm next to the tibia (Figure 2A). At this point, with the needle inserted, bevel up toward the muscle surface.
14. Push the needle straight to the knee side, parallel to the tibia (Figure 2B), and hold for 10 s.
Note: If the needle is barely visible through the TA muscle tissue, the depth of the needle is approximately 500 μm from the muscle surface (Figure 2C).
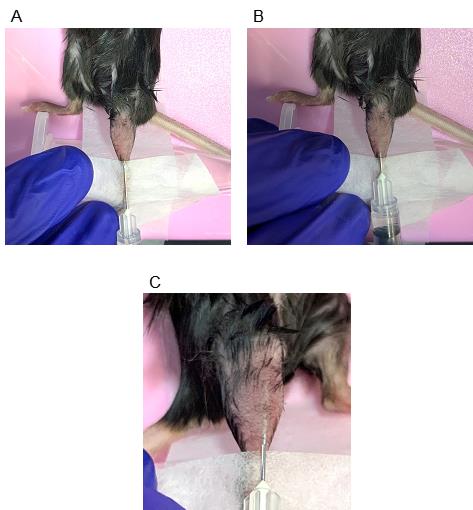
Figure 2. Starting position and direction of syringe injection. (A) Starting position of the injection from above: start the injection 1–2 mm above the distal tibialis anterior (TA) muscle tendon. (B) The end of a syringe insertion. Push the needle parallel to the tibia and horizontally to the muscle. Be sure not to penetrate the TA muscle. (C) Ideal depth of the needle. When the needle is barely visible from outside the body, it is approximately 500 μm below the surface of the TA muscle.
15. Once the needle is fully inserted, withdraw it slightly and inject the solution gradually, using an approximate rate of 10 μL per 1 mm. Then, expel all the solution before removing the needle from the muscle tissue. Complete this process within 1–2 min.
16. Check if any solution has leaked after injection.
17. Repeat steps B9–15 for transplantation to the opposite leg.
Note: We usually compare conditions within the same mouse; for example, the left TA muscle is injected with myoblasts mixed with saline, and the right TA muscle is injected with myoblasts mixed with Matrigel.
18. After complete transplantation, wake the mouse by an intraperitoneal injection of an anti-anesthetic agent in the same dose as the anesthetics.
19. Euthanize mice by cervical dislocation 21 days after transplantation.
20. Remove the TA muscle and measure muscle weight. Detailed protocols for cryosectioning and immunostaining are described in previous studies [12,17]
21. Cut both edges of the TA muscle with a razor blade and let the TA muscle stand on a 1 × 1 cm cork stand, cut using scissors. The knee side of the muscle should be in contact with the cork stand.
22. Submerge the muscle with O.C.T. and freeze it quickly with isopentane cooled in liquid nitrogen. Then, dry the tissue in a cryostat at -25 °C and store it at -80 °C until use.
Note: Cool isopentane using liquid nitrogen; when it starts changing into a solid state, the temperature is suitable for freezing. Put the TA muscle tissue into cold isopentane and shake it rapidly to freeze completely.
23. Cutting the sections and attaching them to the glass slide should be done in a cryostat. Attach the sample stand to the cryostat, and place the glass slide on the section cut by the cryostat (Figure 3).
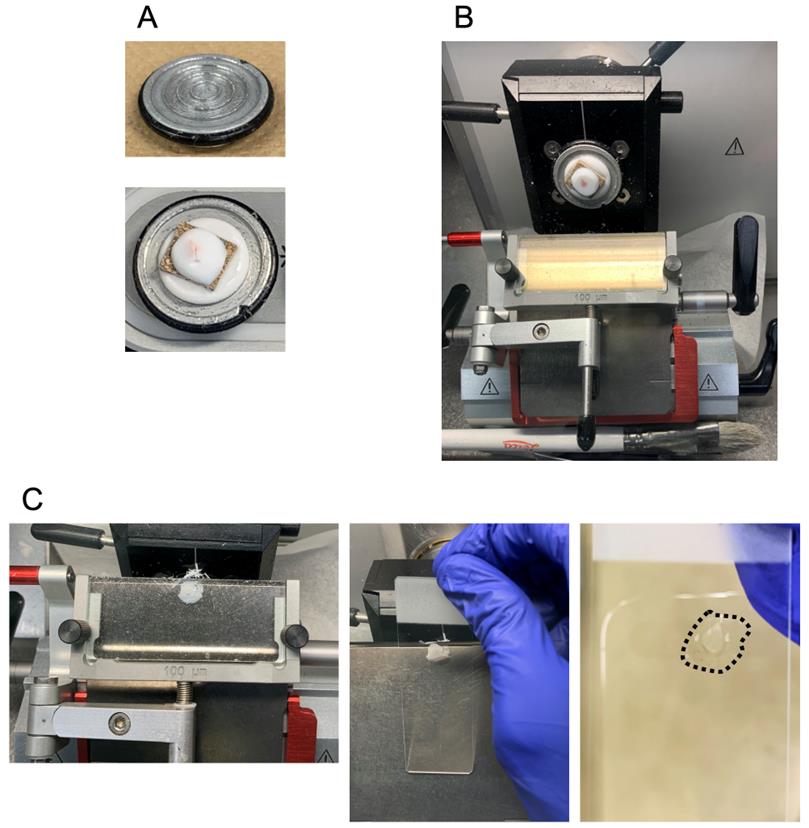
Figure 3. Preparation of a tibialis anterior (TA) muscle cryosection with a cryostat. (A) Mount a cork stand on the sample holder with O.C.T. The upper and lower images show the sample holder and mounting process, respectively. (B) TA muscle tissue sectioning. (C) Attaching the TA muscle section to the glass slide. The left image shows 10-µm TA muscle cryosections. The middle image indicates the process of attaching a cryosection to the glass slide, and the attached section is shown as a black broken line in the right image.
24. After attaching the sections to the glass slide, place the slide at RT with light-shielding using aluminum foil while cutting other samples.
Note: If the sections are not shielded from light, autofluorescence will increase and make it difficult to detect the GFP signal. It is also better to shorten the time between cutting the muscle sections and obtaining the GFP signal.
25. Capture the GFP image with a fluorescence microscope. The sections are then stored at -80 °C until staining.
26. Laminin-α2 and DAPI are stained using the method from previous studies [12,17].
27. Laminin-α2, DAPI, and GFP images are then merged using KEYENCE BZ-810 microscopy analysis software (Figure 4).
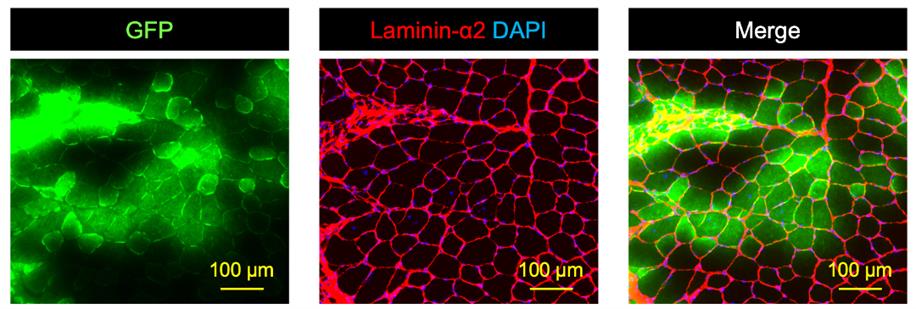
Figure 4. Original GFP signal, immunostaining of Laminin-α2 mounting with DAPI, and merged image of the two. The left image shows the original GFP (green) signal taken using a fluorescent microscope immediately after cryosectioning. The middle image, which is the same section as the left image, is an immunostaining of laminin-α2 (red) mounting with DAPI (blue). The right image indicates the merged image of GFP, laminin-α2, and DAPI. Scale bars are 100 µm.
28. Quantify the number of GFP+ muscle fibers surrounded by laminin-α2 with ImageJ.
C. TA muscle contraction
1. Measure muscle strength 21 days after transplantation [12].
2. Prepare the KRB buffer and heat it to 37 °C in the heat block.
3. Turn on the hot heater of the contraction system and software (Figure 5). The hot heater maintains the system at 37 °C during an experiment (Figure 5A).
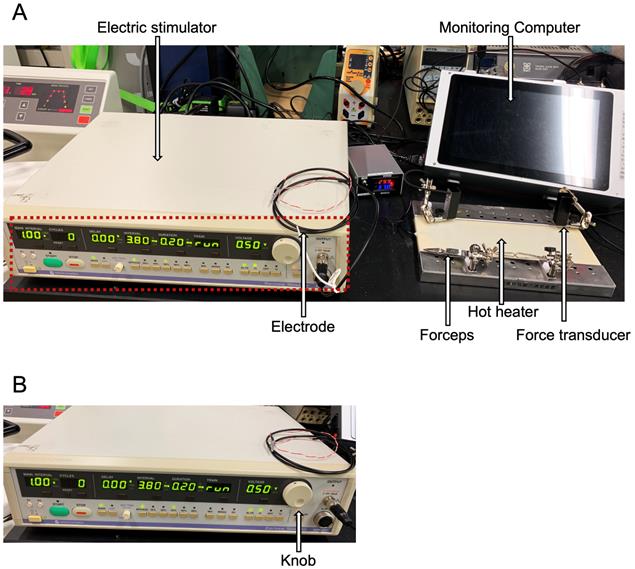
Figure 5. Tibialis anterior (TA) muscle contraction system. (A) Overview of the TA muscle contraction system. An electric stimulator, electrode, monitoring computer, forceps, hot heater, and force transducer are indicated by white arrows. (B) Expanded image of an electric stimulator made by Nihon Kohden shown in the red broken line in panel A; the value of a stimulation voltage or train can be changed with a knob shown by a white arrow.
4. Calibrate 200× g to 10.0 V with a calibration weight (Figure 6).
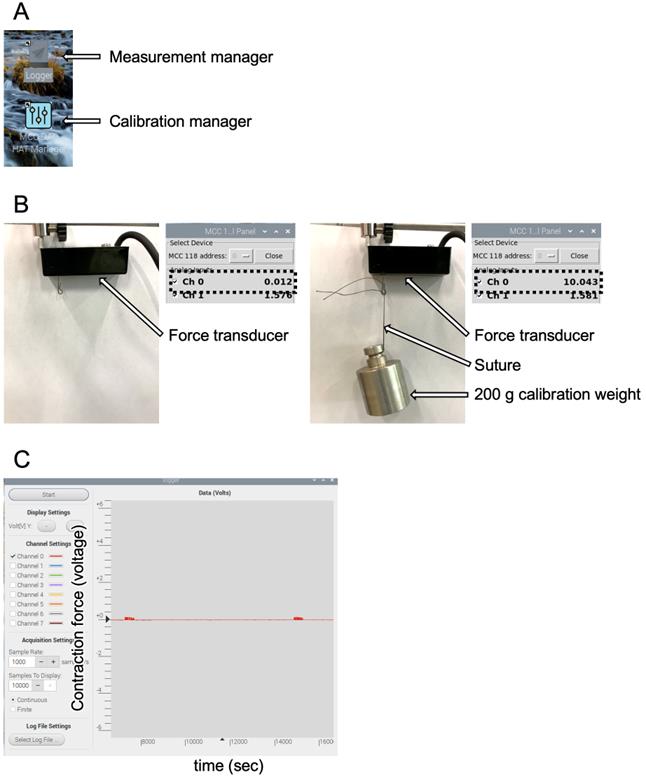
Figure 6. Monitoring computer software. (A) Applications made by Uchida Denshi. (B) Calibration process with a calibration manager. The left image shows the basal value adjusted to 0.0 V (shown by a broken black line), and the right image shows the force transducer calibrated for 10.0 V to 200 g with a calibration weight pointed by white arrows. The adjusted voltages are shown as broken black lines. (C) Measurement manager; the vertical axis is the contraction force (voltage) and the horizontal axis is time (seconds).
5. Anesthetize the mouse as described in Section B.
6. Incise the skin of the mouse's hind leg. At this time, there will be bleeding; wipe it off with paper to ensure visibility. Be careful not to damage the muscle, tendon, or nerve.
7. Prepared a suture to connect the distal TA tendon and the force transducer. Make a loop with a diameter of 3 mm (Figure 7A). Pass the suture between the TA and EDL tendons (Figure 7B, C).
8. Tie an overhand knot around the TA tendon, repeating this twice. Make sure to connect the string strongly so that it does not come off from the TA tendon during the contraction force measurement (Figure 7B, C).
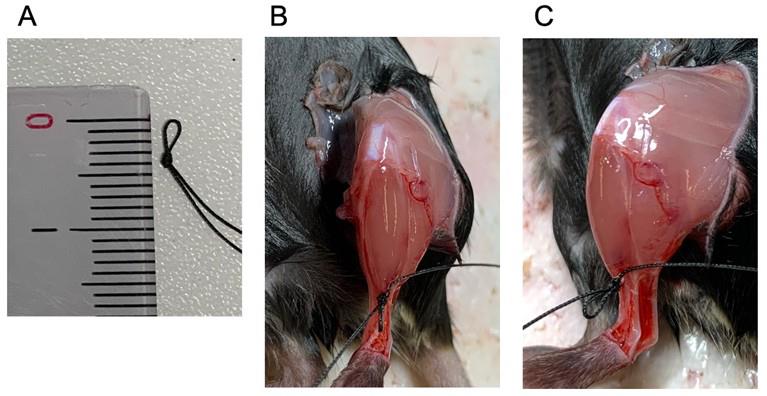
Figure 7. The suture used for connecting the tibialis anterior (TA) distal tendon and the force transducer. (A) Loop with a diameter of 3 mm with the suture. (B, C) The suture is tied to the distal tendon of the TA muscle, shown from above (B) and from the side (C). The loop sits in front of the TA muscle.
9. Cut the distal TA tendon with a scalpel. Using tweezers, pull out only the TA tendon from the ankle side.
10. Cover the TA muscle with paper moistened with KRB buffer. Do not wet the suture and avoid drying out the TA muscle during measurement.
Caution: Wetting the suture may affect the measurement of muscle contraction force.
Carefully expose the common peroneal nerve. Remove the flesh from below the knee toward the outside of the hind. Any debris that might be attached to the nerve should be removed without damaging the nerve (Figure 8).
Note: If some debris remains around the nerve, the response to electrical stimulation will be poor.
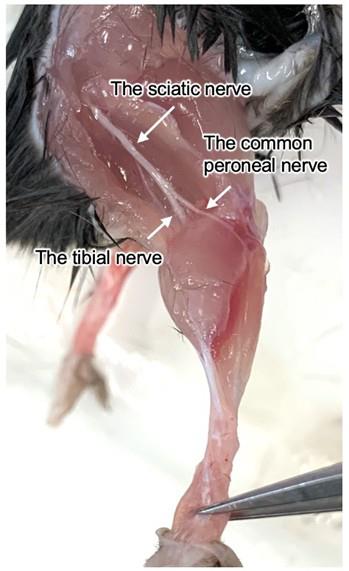
Figure 8. The common peroneal nerve that will be attached to an electrode. The common peroneal nerve can be exposed from next to the edge of the tibialis anterior (TA) muscle and the knee.
11. Once the nerve is exposed, secure the knee with forceps and connect the suture to the force transducer. Set the suture wire so that the transducer, tendon, TA muscle, and knee are in a straight line. Apply electrical stimulation to contract the muscle and verify that the direction of muscle contraction is aligned with the setup (Figure 9 and Video 1).
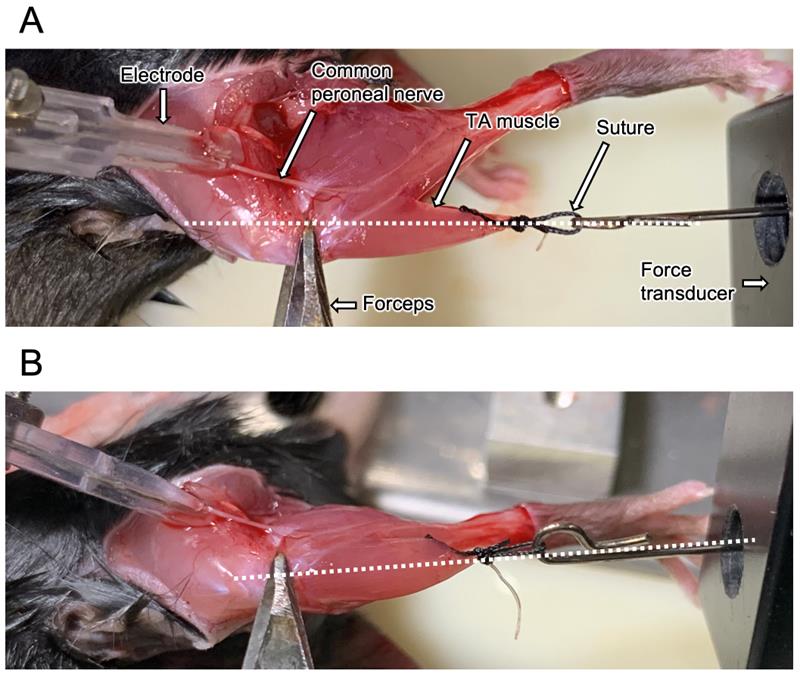
Figure 9. Position to measure the tibialis anterior (TA) muscle contraction force. Place the knee, TA muscle, and force transducer on the same straight line shown as a broken line. (A) Top and (B) side views. Some components are demonstrated by white arrows.
12. Set the value of TRAIN = 60 (values for Nihon Kohden stimulation device, Figure 5B). This condition will avoid exhausting the TA muscle because it contracts and immediately relaxes. In this stimulation, only the contraction peak appears. Gradually increase the stimulation voltage to find the voltage at which the contraction force reaches its peak (Figure 10).
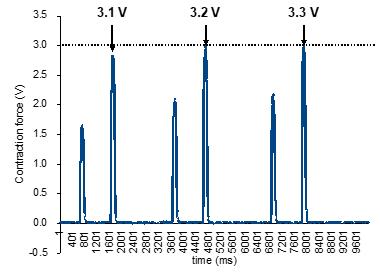
Figure 10. The contraction waves reach a peak following strength stimulation. Set the stimulation voltage to a level that elicits the peak of contraction waves without causing muscle fatigue. Increase the stimulation voltage from 3.1 to 3.3 V, as pointed out by the arrows; at 3.2 V, maximum contraction is reached, being equal to the value of contraction force at 3.3 V, marked with the broken line. In this case, 3.2 V should be used as the stimulation voltage for measuring the maximum contraction force.
13. Allow the muscle to recover without electric stimulation for 1–2 min.
14. Set the value of TRAIN = run (values for Nihon Kohden stimulation device, Figure 5B) to contract muscle completely, as shown in Supplementary Figure 3B of [12]. Stimulate with the voltage defined in step C12 for 10 s and check the form of the contraction wave. Define the peak value of the first contraction wave as the maximum contraction force.
Validation of protocol
This protocol has been used and validated in the following research article:
Dohi et al. [12] Achieving myoblast engraftment into intact skeletal muscle via extracellular matrix. Front Cell Dev Biol (Figures 4A and 5B and Supplementary Figures 1 and 3).
General notes and troubleshooting
General notes
1. When 1.0 × 106 myoblasts per 50 μL are transplanted into intact TA muscle tissue, GFP+ fibers can be detected at approximately 1,200 counts, and the GFP+ area occupies almost 10% of the total TA area 21 days after cell injection. We recommend using as many cells as possible for transplantation (at least 5.0 × 105 cells) as the high number of transplanted cells shows a clear result of their engraftment efficiency.
2. Because cell viability decreases when it passes the syringe needle, we suggest that the injection should be done as slowly as possible.
3. The contraction force of the TA muscle affects how the electrode contacts the nerve; if the muscle does not contract, changing the direction of the electrode should be effective.
Troubleshooting
Problem 1: GFP+ fibers cannot be detected 21 days after transplantation (Figure 11).
Possible cause: The cells were injected into the EDL muscle but not the TA.
Solution: The needle should be injected closer to the tibia and TA muscle surface.
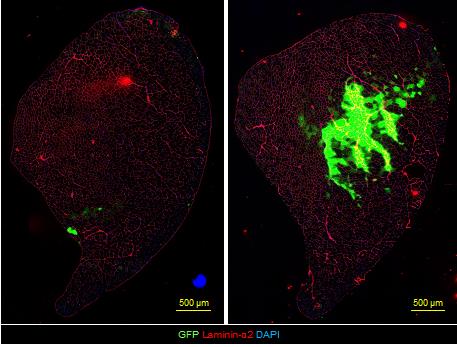
Figure 11. Tissue sections of failed and successful injections. The section in which the needle did not pass the center of the TA muscle (left panel) vs. the section injected in the right position (at approximately 500 μm below the surface of the TA muscle) (right panel). Color indicates GFP (green), laminin-α2 (red), and DAPI (blue). Scale bars, 500 μm.
Problem 2: The TA muscle does not contract even if the direction of the electrode changes.
Possible cause: The fixation of the knee prevents muscle contraction.
Solution: Fix the knee again and provide some electric stimulation.
Acknowledgments
This study was supported by a Japan Society for the Promotion of Science (JSPS) KAKENHI Grant-in-Aid for JSPS Fellows (grant number 24KJ1872) to KD, the Japan Science and Technology Agency via the Fusion Oriented Research for Disruptive Science and Technology program (JST FOREST Program number JPMJFR205K) to YF, a Tokyo Metropolitan Government Advanced Research Grant (R2-2) to Y.F., and TMU Young Selection Research Support for Promising Research awarded to Y.F. This protocol is same to the our previous work [12].
Competing interests
The authors declare no conflicts of interest.
Ethical considerations
The animal study was conducted with the acceptance of the Experimental Animal Committee of Tokyo Metropolitan University (A6-9 and A7-11). The study was conducted in accordance with the local legislation and institutional requirements.
References
- Partridge, T. A., Morgan, J. E., Coulton, G. R., Hoffman, E. P. and Kunkel, L. M. (1989). Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 337(6203): 176–179. https://doi.org/10.1038/337176a0
- Ono, Y., Boldrin, L., Knopp, P., Morgan, J. E. and Zammit, P. S. (2010). Muscle satellite cells are a functionally heterogeneous population in both somite-derived and branchiomeric muscles. Dev Biol. 337(1): 29–41. https://doi.org/10.1016/j.ydbio.2009.10.005
- Yin, H., Price, F. and Rudnicki, M. A. (2013). Satellite Cells and the Muscle Stem Cell Niche. Physiol Rev. 93(1): 23–67. https://doi.org/10.1152/physrev.00043.2011
- Ikemoto, M., Fukada, S. i., Uezumi, A., Masuda, S., Miyoshi, H., Yamamoto, H., Wada, M. R., Masubuchi, N., Miyagoe-Suzuki, Y., Takeda, S., et al. (2007). Autologous Transplantation of SM/C-2.6+ Satellite Cells Transduced with Micro-dystrophin CS1 cDNA by Lentiviral Vector into mdx Mice. Mol Ther. 15(12): 2178–2185. https://doi.org/10.1038/sj.mt.6300295
- Montarras, D., Morgan, J., Collins, C., Relaix, F., Zaffran, S., Cumano, A., Partridge, T. and Buckingham, M. (2005). Direct Isolation of Satellite Cells for Skeletal Muscle Regeneration. Science. 309(5743): 2064–2067. https://doi.org/10.1126/science.1114758
- Sacco, A., Doyonnas, R., Kraft, P., Vitorovic, S. and Blau, H. M. (2008). Self-renewal and expansion of single transplanted muscle stem cells. Nature. 456(7221): 502–506. https://doi.org/10.1038/nature07384
- Azzag, K., Bosnakovski, D., Tungtur, S., Salama, P., Kyba, M. and Perlingeiro, R. C. R. (2022). Transplantation of PSC-derived myogenic progenitors counteracts disease phenotypes in FSHD mice. npj Regener Med. 7(1): 43. https://doi.org/10.1038/s41536-022-00249-0
- Hekmatnejad, B. and Rudnicki, M. A. (2022). Transplantation to study satellite cell heterogeneity in skeletal muscle. Front Cell Dev Biol. 10: e902225. https://doi.org/10.3389/fcell.2022.902225
- Mueller, A. L. and Bloch, R. J. (2019). Skeletal muscle cell transplantation: models and methods. J Muscle Res Cell Motil. 41(4): 297–311. https://doi.org/10.1007/s10974-019-09550-w
- Fuchs, E. and Blau, H. M. (2020). Tissue Stem Cells: Architects of Their Niches. Cell Stem Cell. 27(4): 532–556. https://doi.org/10.1016/j.stem.2020.09.011
- Schüler, S. C., Liu, Y., Dumontier, S., Grandbois, M., Le Moal, E., Cornelison, D. and Bentzinger, C. F. (2022). Extracellular matrix: Brick and mortar in the skeletal muscle stem cell niche. Front Cell Dev Biol. 10: e1056523. https://doi.org/10.3389/fcell.2022.1056523
- Dohi, K., Manabe, Y., Fujii, N. L. and Furuichi, Y. (2025). Achieving myoblast engraftment into intact skeletal muscle via extracellular matrix. Front Cell Dev Biol. 12: e1502332. https://doi.org/10.3389/fcell.2024.1502332
- Furuichi, Y., Kawabata, Y., Aoki, M., Mita, Y., Fujii, N. L. and Manabe, Y. (2021). Excess Glucose Impedes the Proliferation of Skeletal Muscle Satellite Cells Under Adherent Culture Conditions. Front Cell Dev Biol. 9: e640399. https://doi.org/10.3389/fcell.2021.640399
- Pasut, A., Jones, A. E. and Rudnicki, M. A. (2013). Isolation and Culture of Individual Myofibers and their Satellite Cells from Adult Skeletal Muscle. J Visualized Exp. 73: e3791/50074. https://doi.org/10.3791/50074
- Lim, C. L., Ling, K. H. and Cheah, P. S. (2018). Isolation, cultivation and immunostaining of single myofibers: An improved approach to study the behavior of satellite cells. J Biol Methods. 5(1): 1. https://doi.org/10.14440/jbm.2018.219
- Hüttner, S. S., Ahrens, H. E., Schmidt, M., Henze, H., Jung, M. J., Schüler, S. C. and von Maltzahn, J. (2019). Isolation and Culture of Individual Myofibers and Their Adjacent Muscle Stem Cells from Aged and Adult Skeletal Muscle. Methods Mol Biol. 2045: 25–36. https://doi.org/10.1007/7651_2019_209
- Motohashi, N., Minegishi, K., Imamura, M. and Aoki, Y. (2023). Techniques for Injury, Cell Transplantation, and Histological Analysis in Skeletal Muscle. Methods Mol Biol. 2640: 193–205. https://doi.org/10.1007/978-1-0716-3036-5_14
Article Information
Publication history
Received: Apr 27, 2025
Accepted: Jul 10, 2025
Available online: Jul 29, 2025
Published: Aug 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Dohi, K. and Furuichi, Y. (2025). Transplantation of Cultured Myoblasts Into Intact Skeletal Muscle and Analysis of Muscle Contraction Force in Mice Model. Bio-protocol 15(16): e5413. DOI: 10.21769/BioProtoc.5413.
Category
Cell Biology > Cell Transplantation > Isograft transplantation
Cell Biology > Tissue analysis > Electrophysiology
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









