- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Cryopreservation of Bulk-Produced Primary Rat Oligodendrocyte Progenitor Cells
(§Technical contact: hanki313@ajou.ac.kr) Published: Vol 15, Iss 12, Jun 20, 2025 DOI: 10.21769/BioProtoc.5345 Views: 1426
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Cryopreservation Method for Preventing Freeze-Fracture of Small Muscle Samples
Namrata Ghag [...] Nashwa Cheema
Jan 5, 2025 1943 Views

Microfluidic Cultures of Basal Forebrain Cholinergic Neurons for Assessing Retrograde Cell Death by Live Imaging
Srestha Dasgupta [...] Wilma J. Friedman
Jan 5, 2025 1860 Views

Time-Lapse Super-Resolution Imaging and Optical Manipulation of Growth Cones in Elongating Axons and Migrating Neurons
Masato Sawada [...] Kazunobu Sawamoto
Mar 20, 2025 2139 Views
Abstract
Primary oligodendrocyte cultures are a crucial driving force for in vitro research on oligodendrocytes (OLs) and myelin. Various methods are available to obtain oligodendrocyte lineage cells, primarily from neonatal rodent brains or human induced pluripotent stem cells (iPSCs). In this protocol, we describe a step-by-step procedure for detaching and cryopreserving primary rat oligodendrocyte progenitor cells (OPCs), followed by the thawing, proliferation, and differentiation of the cryopreserved OPCs. After freezing in a serum-free cryopreservation medium, the OPCs can be preserved at -80 °C for up to two months without notable changes in viability, proliferation, or differentiation into mature OLs. Cryopreserved OPCs can be differentiated into mature OLs with robust myelin processes and the capacity to wrap around neuron-mimicking structures. Combined with the author’s method for primary OL culture, which allows for bulk production of OPCs, OPC cryopreservation may substantially improve the efficiency of in vitro OL research.
Key features
• This protocol recommends the use of a specific culture method that enables the simple, bulk production of primary rat OPCs.
• Through this protocol, researchers may obtain large numbers of cryopreserved OPCs, which can be reserved for up to two months.
• This protocol facilitates the planning of in vitro experiments and reduces the effort required to maintain adequate numbers of primary OPCs for large-scale experiments.
Keywords: Oligodendrocyte progenitor cell (OPC)Graphical overview
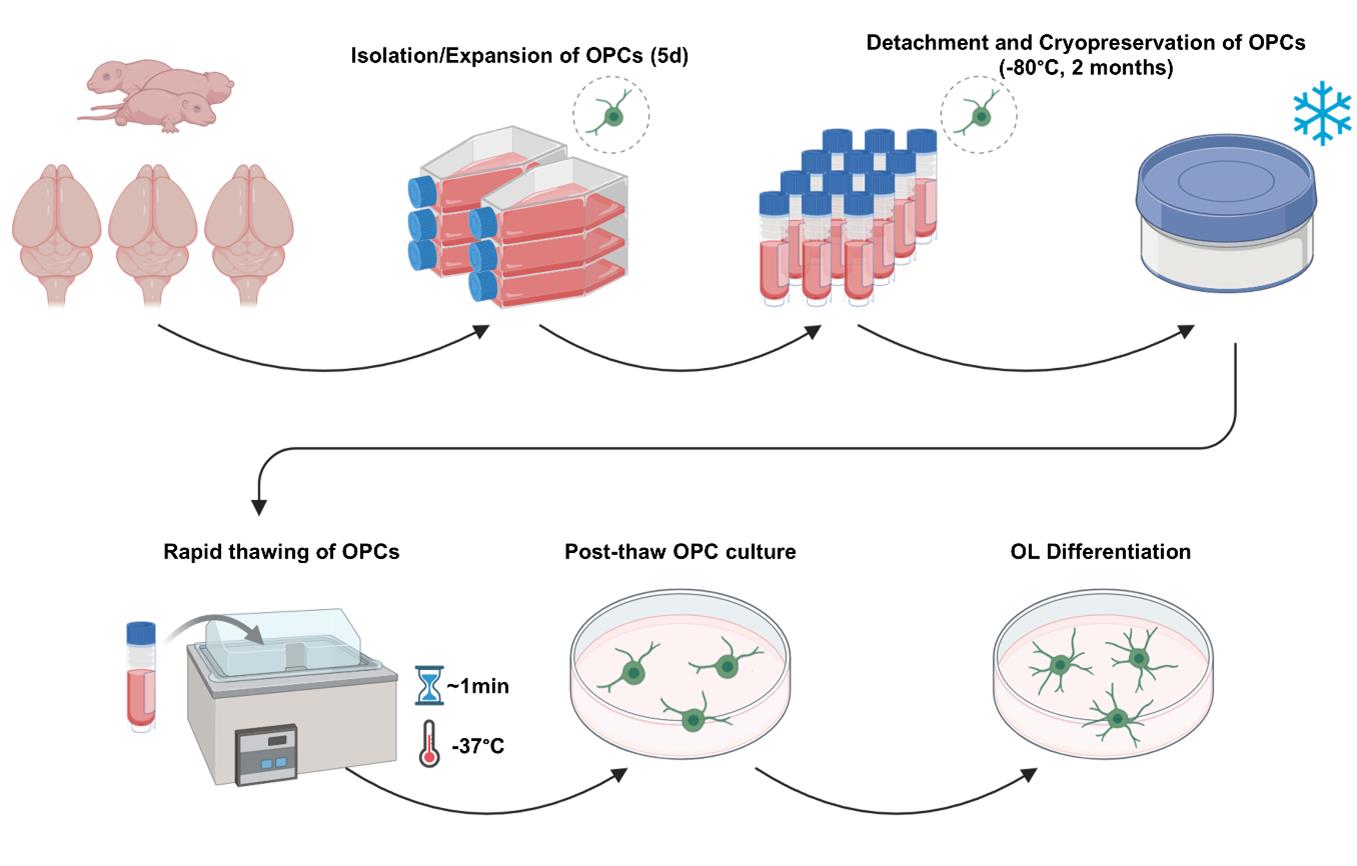
Procedure for the bulk generation, cryopreservation, thawing, and post-thaw culture of primary rat oligodendrocyte progenitor cells (OPCs)
Background
Oligodendrocytes (OLs) are the myelinating glial cells in the central nervous system (CNS). The myelination, saltatory conduction, and metabolic support that OLs provide are vital to normal CNS function [1–3]. The deterioration of OLs and myelin ultimately leads to neurodegeneration, which is implicated in numerous CNS disorders such as multiple sclerosis, ischemic brain disease, neurodegenerative diseases, or traumatic brain injury [4–8].
In vitro, primary OL cultures are effective for observing various biological processes of the OL lineage, including oligodendrocyte progenitor cell (OPC) proliferation, migration, differentiation into myelinating OLs, and myelination. Primary OL cultures derived from either neonatal rodent brains or human induced pluripotent stem cells (hIPSCs) are primarily used for in vitro OL research [9–13]. However, despite the advantage of accurately reproducing OL function, primary OL cultures are generally not an efficient research model due to their lengthy culture periods, low cell yield, and high material and technical requirements.
In this protocol, we outline a step-by-step process for cryopreserving OPCs derived in bulk from neonatal rat brains. Cryopreserved OPCs can be maintained for up to two months without compromising viability, proliferative capacity, and differentiation into myelinating OLs [14]. Combined with an efficient, high-yield primary OL culture method we have recently developed, researchers may obtain large numbers of OPC reserves, which may reduce the effort needed to constantly generate and maintain primary OL cultures and facilitate in vitro OL research.
Materials and reagents
Biological materials
1. Sprague-Dawley rats (postnatal Day 1, wild type, of either sex) (Orient Bio)
Reagents
1. Distilled water (DW) (autoclaved, sterile)
2. 70% ethanol (in distilled water)
3. Isopropanol
4. Poly-D-lysine (PDL) (Sigma-Aldrich, catalog number: P6407)
5. OptiprepTM density gradient medium (Sigma-Aldrich, catalog number: D1556)
6. Phosphate-buffered saline (PBS), no calcium, no magnesium (Cytiva, catalog number: SH30256.01)
7. AccumaxTM (Merck Millipore, catalog number: SCR006)
8. Papain suspension (Worthington, catalog number: LS003126)
9. CELLBANKER® 2 (Zenogen Pharma)
10. Hank’s balanced salt solution (HBSS), no calcium, no magnesium (Gibco, catalog number: 14175-095)
11. Hibernate-A medium (Thermo Fisher Scientific, catalog number: A1247501)
12. Dulbecco’s modified Eagle’s medium/Ham’s F12 (DMEM/F12) (Thermo Fisher Scientific, catalog number: 11320033)
13. Neurobasal medium (NBM) (Thermo Fisher Scientific, catalog number: 21103049)
14. Penicillin/streptomycin (Cytiva, catalog number: SV30010)
15. GlutaMAXTM (Thermo Fisher Scientific, catalog number: 35050-061)
16. B27 supplement (Thermo Fisher Scientific, catalog number: 17504-044)
17. N2 supplement (Thermo Fisher Scientific, catalog number: 17502048)
18. Platelet-derived growth factor-AA (PDGF-AA) (Peprotech, catalog number: 100-13A)
19. Basic fibroblast growth factor (bFGF) (Peprotech, catalog number: 100-18B)
20. Epidermal growth factor (EGF) (Peprotech, catalog number: AF-100-15)
21. Triiodothyronine (thyroid hormone T3) (Sigma, catalog number: T6397)
Solutions
1. PDL coating solution (see Recipes)
2. Cell detachment solution (see Recipes)
3. Dissection medium (see Recipes)
4. Papain dissociation medium (see Recipes)
5. 12% OptiprepTM medium (see Recipes)
6. OPC medium (see Recipes)
7. Post-thaw OPC medium (see Recipes)
8. OL differentiation medium (see Recipes)
Recipes
1. PDL coating solution
Make fresh on the day of use.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Poly-D-Lysine | 0.01 mg/mL | 10 mL |
| DW (autoclaved, sterile) | n/a | 90 mL |
| Total | n/a | 100 mL |
2. Cell detachment solution
Make fresh on the day of use.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Accumax | n/a | 10 mL |
| PBS | n/a | 20 mL |
| Total | n/a | 30 mL |
3. Dissection medium
Make fresh on the day of use. Keep in a 37 °C water bath until the moment of use.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Penicillin-streptomycin (100×) | 1× | 1 mL |
| GlutaMAXTM (100×) | 1× | 1 mL |
| Hibernate-A medium | n/a | 98 mL |
| Total | n/a | 100 mL |
4. Papain dissociation medium
Make fresh on the day of use. Activate in a 30 °C water bath for 30 min before use. The initially opaque medium should be translucent by the time of use. While the optimal temperature for the activation and usage of papain is 30 °C, 37 °C is a usable alternative for simplicity.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Papain suspension (~20 units/mg protein) | ~1 mg protein/mL | 100 μL |
| Dissection medium | n/a | 4 mL |
| Total | n/a | 4.1 mL |
5. 12% OptiprepTM medium
Make fresh on the day of use. Invert 3–5 times to obtain a homogenous mixture.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| OptiprepTM | 12% | 1.2 mL |
| Dissection medium | n/a | 8.8 mL |
| Total | n/a | 10 mL |
6. OPC medium
Make fresh on the day of use. Invert 3–5 times to obtain a homogenous mixture.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Penicillin-streptomycin (100×) | 1× | 1 mL |
| GlutaMAXTM (100×) | 1× | 1 mL |
| B27 supplement (50×) | 1× | 2 mL |
| PDGF-AA (30 μg/mL) | 30 ng/mL | 100 μL |
| bFGF (10 μg/mL) | 10 ng/mL | 100 μL |
| EGF (10 μg/mL) | 10 ng/mL | 100 μL |
| DMEM/F12 | n/a | 95.7 mL |
| Total | n/a | 100 mL |
7. Post-thaw OPC medium
Make fresh on the day of use. Invert 3–5 times to obtain a homogenous mixture.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Penicillin-streptomycin (100×) | 1× | 1 mL |
| GlutaMAXTM (100×) | 1× | 1 mL |
| B27 supplement (50×) | 1× | 2 mL |
| PDGF-AA (30 μg/mL) | 30 ng/mL | 100 μL |
| bFGF (10 μg/mL) | 10 ng/mL | 100 μL |
| DMEM/F12 | n/a | 95.8 mL |
| Total | n/a | 100 mL |
8. OL differentiation medium
Make fresh on the day of use. Invert 3–5 times to obtain a homogenous mixture.
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Penicillin-streptomycin (100×) | 1× | 1 mL |
| GlutaMAXTM (100×) | 1× | 1 mL |
| B27 supplement (50×) | 1× | 2 mL |
| N2 supplement (100×) | 1× | 1 mL |
| Triiodothyronine (40 μg/mL) | 40 ng/mL | 100 μL |
| Neurobasal medium | n/a | 94.9 mL |
| Total | n/a | 100 mL |
Laboratory supplies
1. T75 culture flasks (vent-seal) (Corning, catalog number: 430641U)
2. Culture surfaces (plates, coverslips) (SPL Life Sciences, catalog numbers: 30012, 30024, 20009)
3. 10 cm Petri dishes (SPL Life Sciences, catalog number: 10093)
4. Conical tubes (15 and 50 mL) (SPL Life Sciences, catalog numbers: 50015, 50050)
5. Cryopreservation vials (Thermo Fisher Scientific, catalog number: 368632)
Equipment
1. Surgical scissors (large, small) (Fine Science Tools, catalog numbers: 14001-14, 14558-11)
2. Forceps (Fine Science Tools, catalog number: 11000-16)
3. Curved forceps (Fine Science Tools, catalog number: 91197-00)
4. Freezing container (Thermo Fisher Scientific, catalog number: 5100-0001)
5. Cell culture incubator (37 °C, 5% CO2) (PHCbi, catalog number: MCO-18AC-PK)
6. Water bath (Coretech, catalog number: HQ-DW22)
7. Tabletop centrifuge (compatible with 15 mL conical tubes) (Labogene, catalog number: 416)
8. LUNA-FLTM dual fluorescence cell counter (Logos Biosystems, catalog number: L20001)
9. Brightfield microscope (Olympus, catalog number: CKX53)
10. Laminar flow hood
11. -80 °C freezer
Procedure
This protocol has been developed using neonatal Sprague-Dawley (SD) rat OPCs. For the isolation and expansion of rat OPCs (Section C), we recommend using the “E3 method,” a two-step differential centrifugation method using the density gradient medium OptiprepTM. The E3 method excels in its simple procedure and high yield, which makes it suitable for the bulk generation of frozen OPC reserves [15,16]. A detailed protocol of the E3 method can be found in [16].
For cryopreservation, we demonstrate freezing of OPCs at -80 °C for up to two months, using the serum-free cryopreservation media CELLBANKER® 2. We advise against the use of animal serum-containing cryoprotectants as they may alter the cell fate of OPCs. An isopropanol cell freezing container is required for cell survival during freezing.
A. Poly-D-lysine (PDL) coating
Note: Primary OPCs/OLs require surface coating for adhesion. Prepare PDL-coated culture surfaces the day before use.
1. Add PDL coating solution and cover the cell culture surface(s).
Note: The recommended volumes of PDL coating solution for one well of 96, 24, 12, and 6-well plates are 0.2, 0.5, 1, and 2 mL, respectively. The recommended volume for a 100 mm dish and T75 flask is 5 mL.
2. Incubate for 1 h at room temperature (RT) (~23 °C).
3. Discard the PDL coating solution and rinse three times with DW.
4. Air-dry the surfaces for 10 min at RT and store at RT, protected from light.
B. Dissection and extraction of postnatal day 1 (P1) rat cerebral cortices
1. Anesthetize the P1 rat pups on ice.
2. Decapitate the pups and transfer the heads into 15 mL of ice-cold HBSS (in a 50 mL conical tube).
3. Transfer the heads into 15 mL of ice-cold 70% ethanol. Then, transfer into a different tube with ice-cold HBSS.
4. Place the heads into a Petri dish with 15 mL of HBSS.
5. Cut along the midline of the scalp with surgical scissors and remove the scalp.
6. Cut along the midline of the skull with surgical scissors and remove the skull.
7. Scoop the brain out of the skull and isolate the two hemispheres using curved forceps.
8. Remove the meninges using curved forceps and transfer the hemispheres into ice-cold Accumax (two hemispheres per 1 mL of Accumax in one 15 mL conical tube).
C. Isolation and expansion of rat OPCs
Note: This section is part of the E3 method, which enables a simple, high-yield primary OL culture [15,16].
1. Mechanically dissociate the hemispheres in Accumax ten times with a 1 mL pipette tip. This should result in a nearly homogenous brain tissue suspension with no evident tissue chunks.
2. Add the papain dissociation solution to the tubes (4 mL per brain).
3. Incubate the tissue suspension with the papain dissociation solution at 30 °C for 30 min.
Note: For convenience, 37 °C may suffice.
4. Centrifuge at 200× g for 5 min at RT, discard the supernatant, and resuspend the pellet in 1 mL of 12% OptiprepTM medium.
5. Triturate the pellet 10–20 times through a 10 μL pipette tip, attached to a 1 mL tip. Add 3 mL of 12% OptiprepTM medium. The total volume in one 15 mL tube (containing two hemispheres) should be 4 mL of 12% OptiprepTM medium.
6. Perform the first differential centrifugation at 200× g for 15 min at RT.
7. Transfer the supernatant to a different tube and add 4 mL of dissection media to dilute. The diluted cell suspension should now be 8 mL, and the concentration of OptiprepTM should be lowered to 6%.
Note: Discard the pellet from the first centrifuging cycle, which contains microglia and red blood cells.
8. Perform the second differential centrifugation at 200× g for 15 min at RT. Discard the supernatant and resuspend the cell pellet in 1 mL of OPC medium.
9. Count the number of cells and seed 1 × 104 cells/cm2 on T75 flasks (10 mL of OPC medium per flask).
Note: An estimate of one brain per two flasks can be used.
10. Maintain the cells in a 37 °C, 5% CO2 cell culture incubator for five days. Supplement with 30 ng/mL PDGF-AA to the flasks on Day 3. Upon observation by brightfield microscopy, confluent colonies of bipolar OPCs should be visible on Day 5.
D. Detachment and cryopreservation of OPCs
1. Prepare 5 mL of cell detachment solution per T75 flask in a 50 mL tube and equilibrate to RT.
2. Discard the media in the flasks with the OPCs and add 5 mL per flask of cell detachment solution.
3. Incubate for 5 min in a 37 °C, 5% CO2 cell culture incubator. Observe the rounding of cells on a brightfield microscope (Figure 1).
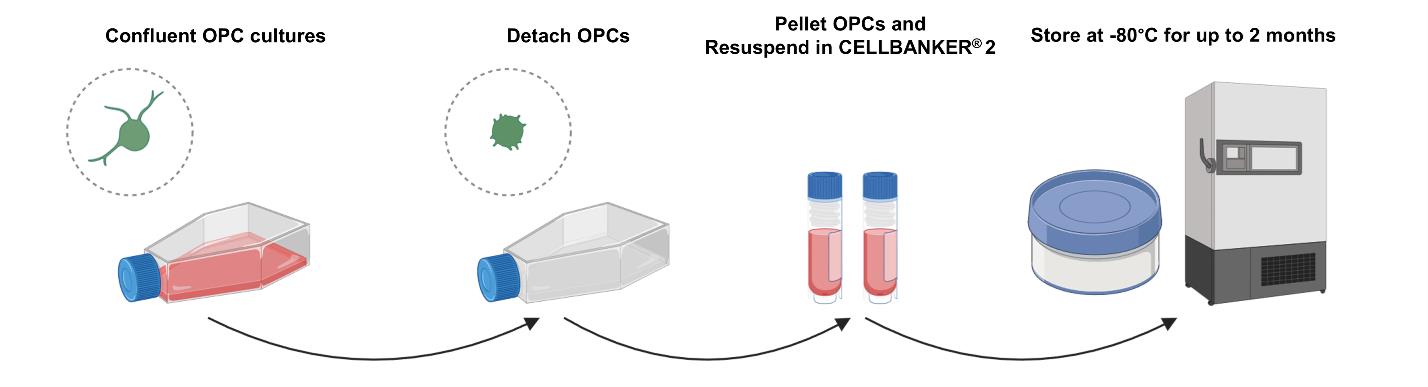
Figure 1. Procedure for the detachment and cryopreservation of neonatal rat oligodendrocyte progenitor cells (OPCs). Confluent OPC cultures, after five days of expansion, are detached from the surfaces with the cell detachment solution. After pelleting by centrifugation, the OPCs can be stored at -80 °C for up to two months in CELLBANKER® 2 with an isopropanol cell freezing container.
4. Hit the flasks on their sides to detach the OPCs. Transfer the cell detachment solution, now containing the detached OPCs, to appropriate conical tubes for centrifugation.
5. Pellet the cells at 200–500× g for 5 min at RT. Discard the supernatant and resuspend the pellet in 1 mL per flask of CELLBANKER® 2. Equilibrate CELLBANKER® 2 to RT before use (Figure 1).
6. Count the number of OPCs with a cell counter and transfer the OPCs to cryovials. Generally, 2–3 × 106 OPCs per vial (1 mL of CELLBANKER® 2 per vial) will suffice.
Note: To simplify, an estimate of two cryovials per one T75 flask of OPCs will suffice.
7. Place the vials in a freezing container with isopropanol and store at -80 °C for up to two months (Figure 1).
E. Thawing, stabilization, and differentiation of OPCs
1. Place the OPC cryovial to be thawed in a 37 °C water bath.
2. Rapidly thaw the vial for approximately 1 min (Figure 2).
3. Pellet the OPCs at 200–500× g for 5 min at RT. Discard the supernatant and resuspend the OPC pellet in 1 mL of post-thaw OPC medium.
Note: The post-thaw OPC medium does not contain EGF, which is used in the expansion stage to facilitate OPC proliferation and increase cell yield. If EGF is included in the post-thaw OPC medium, it causes the clumping of OPCs, which may result in cell death during the following differentiation into mature OLs.
4. Count the number of OPCs and seed at 1 × 104 OPCs/cm2 onto PDL-coated culture surfaces, in post-thaw OPC medium. Stabilize for two days (Figure 2).
5. To induce differentiation into mature OLs, exchange the media to OL differentiation media and maintain for 3–4 days (Figure 2).
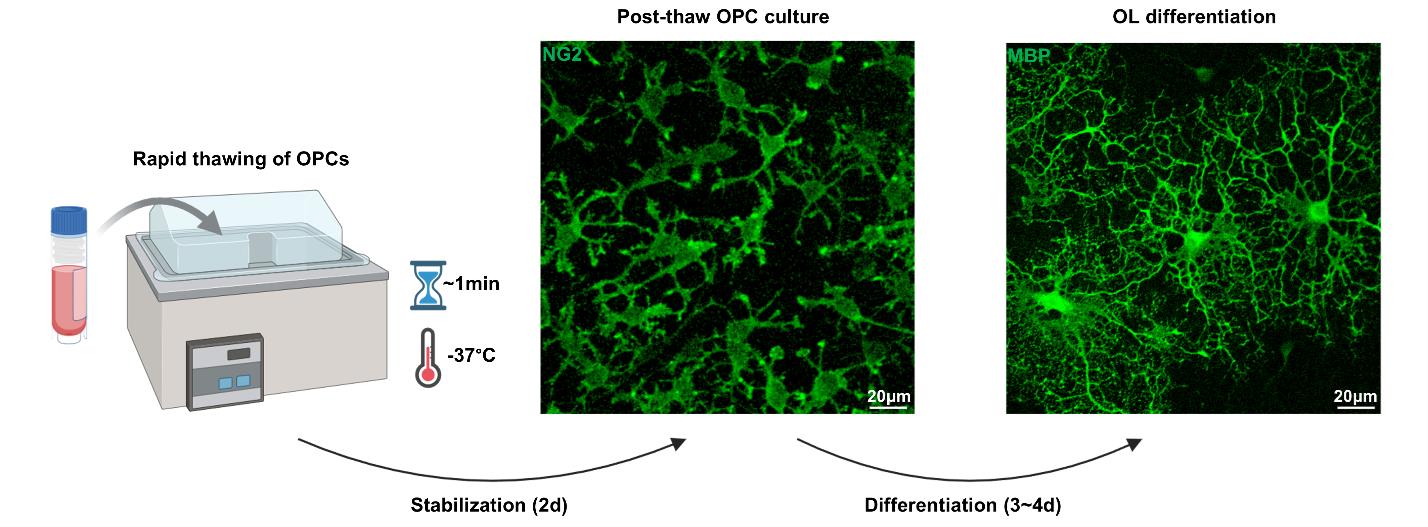
Figure 2. Rapid thawing and cultivation of neonatal rat oligodendrocyte progenitor cells (OPCs). At the time of use, the cryopreserved OPCs are rapidly thawed for ~1 min at 37 °C and promptly stabilized in post-thaw OPC medium and maintained for two days. After two days of stabilization, the OPCs can be subsequently differentiated into mature OLs by exchanging the culture media to OL differentiation medium. Images are OPCs and differentiated OLs, immunostained for NG2 and MBP. MBP = myelin basic protein, NG2 = neuroglia2, OL = oligodendrocyte.
Validation of protocol
This protocol has been used and validated in the following research article [14]:
Kim et al. [14]. Cryopreservation of primary neonatal rat oligodendrocytes and recapitulation of in vitro oligodendrocyte characteristics. Frontiers in Cellular Neuroscience.
The method for the dissection of neonatal rats, isolation and expansion of OPCs, and OL culture media solutions used in this article has been validated in and derived from the following articles [15,16]:
Kim et al. [15]. A primary culture method for the easy, efficient, and effective acquisition of oligodendrocyte lineage cells from neonatal rodent brains. Heliyon.
Kim et al. [16]. Protocol for the acquisition and maturation of oligodendrocytes from neonatal rodent brains. STAR Protocols.
General notes and troubleshooting
General notes
1. All steps throughout the protocol should be performed in a laminar flow hood where applicable.
2. Although we have not optimized this specific protocol for OPC cryopreservation for use with neonatal mice, we have validated that the primary OL culture method (the E3 method) is compatible with mice. For the specific purpose of obtaining large numbers of OPC reserves, rats have a substantial advantage over mice due to the larger brain size and higher number of cells.
3. While we have not validated longer periods of cryopreservation using liquid nitrogen, it may be a viable option if needed. The OPCs may show mildly diminished proliferative activity if the storage period at -80 °C exceeds two months.
Troubleshooting
Problem 1: Decreased viability at thaw (the expected viability post-thaw is ~94%).
Possible cause: Inadequate speed of thawing or freeze-thaw during storage at -80 °C.
Solution: Make sure that the 37 °C water bath has reached 37 °C before adding the OPC cryovials to thaw. Also, place the cryovials where they can be less impacted by increases in temperature due to the opening of the -80 °C freezer.
Problem 2: OPCs not detaching from the cell culture surface.
Possible cause: Spontaneous differentiation.
Solution: Although the protocol specifies five days of OPC expansion, if the OPC colonies become confluent at earlier timepoints, we advise detaching them at that point. Increased cell density can cause the OPCs to initiate differentiation, and differentiated OLs may become difficult or impossible to detach. If needed, the proportion of Accumax in the cell detachment solution (initially set to 1/3 of the total solution, to match the enzyme concentration of Accutase) can be increased to 1/2 of the total solution.
Acknowledgments
Author contributions: H.K.: Conceptualization, Methodology, Validation, Investigation, Writing - Original Draft, Writing - Review & Editing, Visualization., R.A.: Validation, Investigation, Writing - Review & Editing., B.J.K.: Validation, Writing - Review & Editing., H.J.C.: Validation, Writing - Review & Editing., J.Y.C.: Conceptualization, Methodology, Resources, Writing - Original Draft, Writing - Review & Editing, Visualization, Supervision, Project administration, Funding acquisition.
Funding: This work was supported by National Research Foundation of Korea (NRF) grants funded by the Korean government (MSIT; Ministry of Science and ICT) (RS-2024-00335969, RS-2019-NR040055) to J.Y.C.
Figures throughout the article were created with the assistance of Biorender.com. https://BioRender.com/kqaygac.
The original research paper in which the protocol was described and validated is Kim et al [14].
Competing interests
The authors declare no conflicts of interest.
Ethical considerations
The Institutional Animal Care and Use Committee of Ajou University School of Medicine reviewed and approved the use of animals for this protocol. The devised animal experiments complied with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
References
- Bradl, M. and Lassmann, H. (2009). Oligodendrocytes: biology and pathology. Acta Neuropathol. 119(1): 37–53. https://doi.org/10.1007/s00401-009-0601-5
- Krämer-Albers, E. M. and Werner, H. B. (2023). Mechanisms of axonal support by oligodendrocyte-derived extracellular vesicles. Nat Rev Neurosci. 24(8): 474–486. https://doi.org/10.1038/s41583-023-00711-y
- Philips, T. and Rothstein, J. D. (2017). Oligodendroglia: metabolic supporters of neurons. J Clin Invest. 127(9): 3271–3280. https://doi.org/10.1172/jci90610
- Duncan, I. D. and Radcliff, A. B. (2016). Inherited and acquired disorders of myelin: The underlying myelin pathology. Exp Neurol. 283: 452–475. https://doi.org/10.1016/j.expneurol.2016.04.002
- Deshmukh, V. A., Tardif, V., Lyssiotis, C. A., Green, C. C., Kerman, B., Kim, H. J., Padmanabhan, K., Swoboda, J. G., Ahmad, I., Kondo, T., et al. (2013). A regenerative approach to the treatment of multiple sclerosis. Nature. 502(7471): 327-332. https://doi.org/10.1038/nature12647
- Molina-Gonzalez, I., Miron, V. E. and Antel, J. P. (2022). Chronic oligodendrocyte injury in central nervous system pathologies. Commun Biol. 5(1): 1274. https://doi.org/10.1038/s42003-022-04248-1
- Simkins, T. J., Duncan, G. J. and Bourdette, D. (2021). Chronic Demyelination and Axonal Degeneration in Multiple Sclerosis: Pathogenesis and Therapeutic Implications. Curr Neurol Neurosci Rep. 21(6): 26. https://doi.org/10.1007/s11910-021-01110-5
- Kedia, S. and Simons, M. (2025). Oligodendrocytes in Alzheimer’s disease pathophysiology. Nat Neurosci. 28(3): 446–456. https://doi.org/10.1038/s41593-025-01873-x
- McCarthy, K. D. and de Vellis, J. (1980). Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue.. J Cell Biol. 85(3): 890–902. https://doi.org/10.1083/jcb.85.3.890
- Dugas, J. C. and Emery, B. (2013). Purification of Oligodendrocyte Precursor Cells from Rat Cortices by Immunopanning. Cold Spring Harb Protoc. 2013(8): 745–758. https://doi.org/10.1101/pdb.prot070862
- Emery, B. and Dugas, J. C. (2017). Corrigendum: Purification of Oligodendrocyte Lineage Cells from Mouse Cortices by Immunopanning. Cold Spring Harb Protoc. 2017(11): pdb.corr102954. https://doi.org/10.1101/pdb.corr102954
- Brewer, G. J. and Torricelli, J. R. (2007). Isolation and culture of adult neurons and neurospheres. Nat Protoc. 2(6): 1490–1498. https://doi.org/10.1038/nprot.2007.207
- Yamashita, T., Miyamoto, Y., Bando, Y., Ono, T., Kobayashi, S., Doi, A., Araki, T., Kato, Y., Shirakawa, T., Suzuki, Y., et al. (2017). Differentiation of oligodendrocyte progenitor cells from dissociated monolayer and feeder-free cultured pluripotent stem cells. PLoS One. 12(2): e0171947. https://doi.org/10.1371/journal.pone.0171947
- Kim, H., Kim, B. J., Koh, S., Cho, H. J., Kim, B. G. and Choi, J. Y. (2025). Cryopreservation of primary neonatal rat oligodendrocytes and recapitulation of in vitro oligodendrocyte characteristics. Front Cell Neurosci. 18: e1520992. https://doi.org/10.3389/fncel.2024.1520992
- Kim, H., Kim, B. J., Koh, S., Cho, H. J., Jin, X., Kim, B. G. and Choi, J. Y. (2024). A primary culture method for the easy, efficient, and effective acquisition of oligodendrocyte lineage cells from neonatal rodent brains. Heliyon. 10(8): e29359. https://doi.org/10.1016/j.heliyon.2024.e29359
- Kim, H., Kim, B. J., Koh, S., Cho, H. J., Jin, X., Kim, B. G. and Choi, J. Y. (2024). Protocol for the acquisition and maturation of oligodendrocytes from neonatal rodent brains. STAR Protoc. 5(4): 103327. https://doi.org/10.1016/j.xpro.2024.103327
Article Information
Publication history
Received: Apr 4, 2025
Accepted: May 15, 2025
Available online: May 30, 2025
Published: Jun 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Kim, H., Afzal, R., Kim, B. J., Cho, H. J. and Choi, J. Y. (2025). Cryopreservation of Bulk-Produced Primary Rat Oligodendrocyte Progenitor Cells. Bio-protocol 15(12): e5345. DOI: 10.21769/BioProtoc.5345.
Category
Neuroscience > Cellular mechanisms > Cell isolation and culture
Cell Biology > Cell isolation and culture > Cryopreservation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link