- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Novel Protein Purification Approach Using Elastin-Like Polypeptides (ELP) With His-Tag Assistance
Published: Vol 15, Iss 12, Jun 20, 2025 DOI: 10.21769/BioProtoc.5342 Views: 3041
Reviewed by: Alessandro DidonnaNuttavut KosemRupam GhoshAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Expression and Purification of the Human Voltage-Gated Proton Channel (hHv1)
Emerson M. Carmona [...] Luis G. Cuello
Jun 20, 2025 1952 Views
Thermus thermophilus CRISPR Cas6 Heterologous Expression and Purification
Junwei Wei [...] Yingjun Li
Jul 20, 2025 2137 Views
Prokaryotic Expression and Purification of the hSox2-HMG Domain
Lijie Yang [...] Jingjun Hong
Aug 20, 2025 2322 Views
Abstract
Protein purification is essential for drug development, antibody production, and structural biology. We propose a cost-effective chromatography method using elastin-like polypeptide (ELP) as an aggregating core. In this approach, a chilled (target protein)-ELP fusion is loaded onto an immobilized metal affinity chromatography (IMAC) column equilibrated with low-salt buffer. Impurities are removed with warm high-salt buffer washes. Warming the column above the ELP’s transition temperature (Tm) triggers ELP aggregation, physically trapping the target protein between beads. Subsequent stringent washing (high salt/imidazole) eliminates residual contaminants. Finally, cooling with cold low-salt buffer reverses aggregation, eluting the purified target protein. This method eliminates the need for advanced chromatography systems while achieving high purity through dual mechanisms: (1) IMAC affinity and (2) temperature-dependent physical capture. The ELP’s reversible phase transition offers a simplified yet efficient purification platform, particularly valuable for lab-scale production of challenging proteins.
Key features
• This protocol requires an elastin-like polypeptide tag at the C-terminus of the target protein.
• This protocol requires a His-Tag at the N-terminus of the target protein.
• This protocol requires the use of colored/chromogenic proteins to enable real-time visual monitoring of chromatographic progression.
• This protocol yields a highly pure protein by manually operating a Ni-bound resin at two different temperatures.
Keywords: AggregationGraphical overview
Background
Protein purification is pivotal for various uses, especially in structure-based drug discovery, where high purity levels are crucial [1,2]. Recent advancements in molecular biology techniques, such as PCR, have spurred the production of recombinant proteins, allowing for their large-scale production and purification [3,4]. These proteins have diverse applications, including vaccines, therapeutics, and diagnostics [4]. Escherichia coli is often the organism of choice for producing recombinant proteins due to its rapid growth, cost-effectiveness, and high yield [5–8]. By introducing genes into E. coli via plasmids, controlled protein expression can be achieved upon induction.
Protein purification methods, such as precipitation or chromatography, are employed based on the protein's characteristics. Affinity chromatography is particularly favored for its efficiency in purifying recombinant proteins [9]. Various strategies have been developed to enhance this process. Fusion proteins with tags like glutathione S-transferase (GST), maltose binding protein (MBP), or polyhistidine are common as they aid in production and purification [10–12]. Signal peptides can facilitate periplasmic secretion, reducing contamination [13,14]. Aggregation tags induce protein precipitation for easier purification. GST or MBP fusion proteins are typically purified using glutathione or maltose resins, while polyhistidine tags use resins with immobilized metal ions. Periplasmic secretion is advantageous due to fewer contaminating proteins compared to cytoplasmic secretion. Aggregation tags manipulate factors like temperature or salt concentration for purification [15]. The elastin-like polypeptide (ELP) uses inverse temperature cycling (ITC), which requires high-temperature centrifugation, presenting challenges for low-temperature centrifuges [16].
This study introduces a novel method called aggregation-assisted chromatography for purifying fusion proteins with both precipitation and polyhistidine tags. The technique employs an immobilized metal affinity chromatography (IMAC) column, with adjustments in temperature and salt concentration to achieve high purity. By controlling the buffer temperature, this method allows for high-purity manual purification without the need for sophisticated chromatography equipment. Although switching the order of the two tags may not significantly affect overall protein expression, it is possible that the ELP, due to its intrinsically disordered nature, could influence the folding of the downstream protein.
Materials and reagents
Biological materials
1. E. coli strain specialized for protein production, Rosetta2(DE3)pLysS (Merck, catalog number: 71403)
2. DH5α (Enzyomics, catalog number: CP011)
3. pVP65KR-SacB-I48-based plasmid harboring a desired gene [17–20]. SnapGene files of all plasmids will be provided upon request
Reagents
1. Imidazole (Duksan, catalog number: 7097)
2. Sodium phosphate, dibasic (Na2HPO4) (Duksan, catalog number: 1490)
3. Sodium phosphate, monobasic (NaH2PO4) (Duksan, catalog number: 3989)
4. Sodium chloride (NaCl) (Duchefa Biochemie, catalog number: T0520.1000)
5. Trypton (Duchefa Biochemie, catalog number: T1332.0500)
6. Yeast extract (Duchefa Biochemie, catalog number: T1333.0500)
7. IPTG (Gold Biotechnology, catalog number: I2481C)
8. Triton X-100 (MP Biomedicals, catalog number: 02194854-CF)
9. SfaAI (ThermoFisher, catalog number: ER2091)
10. MssI (ThermoFisher, catalog number: ER1342)
11. Rapid DNA Ligation kit (ThermoFisher, catalog number: K1422)
12. Gel Extraction kit (Cosmogenetech, catalog number: CMG0112)
Solutions
1. LB medium (see Recipes)
2. 1 M IPTG (see Recipes)
3. 0.2 M sodium phosphate, dibasic (see Recipes)
4. 0.2 M sodium phosphate, monobasic (see Recipes)
5. PBS (see Recipes)
6. Binding buffer (see Recipes)
7. Washing buffer (see Recipes)
8. Elution buffer (see Recipes)
9. 20% Triton X-100 (see Recipes)
Recipes
1. LB medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Trypton | 1% | 10 g |
| Yeast extract | 0.5% | 5 g |
| NaCl | 1% | 10 g |
| Total | n/a | 1,000 mL |
Sterilize the medium by autoclaving at 121 °C for 20 min.
2. 1 M IPTG
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| IPTG | 1 M | 2.38 g |
| Total | n/a | 10 mL |
Aliquot the solution into 1mL portions and store at -20 °C.
3. 0.2 M sodium phosphate, dibasic
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Na2HPO4 | 0.2 M | 28.4 g |
| Total | n/a | 1,000 mL |
4. 0.2 M sodium phosphate, monobasic
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaH2PO4 | 0.2 M | 24.0 g |
| Total | n/a | 1,000 mL |
5. PBS
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 0.2 M sodium phosphate, dibasic | 10 mM | 25 mL |
| 0.2 M sodium phosphate, monobasic | 10 mM | 25 mL |
| NaCl | 150 mM | 58.44 g |
| Total | n/a | 1,000 mL |
Sterilize the buffer by autoclaving at 121 °C for 20 min.
6. Binding buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 0.2 M sodium phosphate, dibasic | 10 mM | 25 mL |
| 0.2 M sodium phosphate, monobasic | 10 mM | 25 mL |
| NaCl | 150 mM | 58.44 g |
| Imidazole | 10 mM | 0.68 g |
| Total | n/a | 1,000 mL |
7. Washing buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 0.2 M sodium phosphate, dibasic | 10 mM | 25 mL |
| 0.2 M sodium phosphate, monobasic | 10 mM | 25 mL |
| NaCl | 150 mM | 58.44 g |
| Imidazole | 250 mM | 17.02 g |
| Total | n/a | 1,000 mL |
8. Elution buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 0.2 M sodium phosphate, dibasic | 10 mM | 25 mL |
| 0.2 M sodium phosphate, monobasic | 10 mM | 25 mL |
| NaCl | 150 mM | 58.44 g |
| Imidazole | 500 mM | 34.04 g |
| Total | n/a | 1,000 mL |
9. 20% Triton X-100
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Triton X-100 | 20% (v/v) | 20 mL |
| Total | n/a | 100 mL |
Laboratory supplies
1. 3 L baffled polycarbonate Erlenmeyer flasks (Corning, catalog number: 431253)
2. 50 mL centrifuge tubes (Corning, catalog number: CLS430829)
3. 10 μL pipette tips (Axygen, catalog number: T-300-L)
4. 200 μL pipette tips (Axygen, catalog number: T-200-C)
5. 1,250 μL pipette tips (Axygen, catalog number: MTX-1250-C)
6. 20 mL syringe with a Luer lock tip (Beckton Dickinson, catalog number: 302830)
7. 1/16” male/Luer female adaptor (Cytiva, catalog number: 18111251)
Equipment
1. High-speed centrifuge (Hanil, model: Supra R17)
2. Pipetman (Gilson, catalog number: F167350)
3. Racks (Korea Ace Scientific, catalog number: KA.UB33-27)
4. Water bath (Branson, catalog number: CPX1800H-E)
5. Shaking incubator (Vision Scientific, catalog number: VS-37SI)
6. Deep freezer (-80 °C) (Nihon Freezer, model: MY BIO)
7. Ultrasonic processor (Sonics, model: VCX 750)
8. HisTrap HP 5 mL column (Cytiva, catalog number: 17524801)
9. UV-Vis spectrophotometer (Scinco, model number: S-3100)
Procedure
A. Plasmid construction
1. Prepare gene fragments either by gene synthesis or PCR. The gene fragments should include AsiSI and PmeI sites at the 5' and 3' ends, respectively.
2. Digest 5 μg of the vector pVP65KR-SacB-I48 with SfaAI and MssI following the manufacturer’s protocol. Purify the linearized vector by gel extraction following the manufacturer’s protocol.
3. Digest 1 μg of the gene fragments with SfaAI and MssI following the manufacturer’s protocol. Purify the linearized vector by gel extraction following the manufacturer’s protocol.
4. Ligate 100 ng each of the enzyme-treated vector and the gene fragment by following the manufacturer’s protocol.
5. Bring 5 μL of the ligation mixture into 100 μL of the chemically competent DH5α by following the manufacturer’s protocol.
6. Select the colony harboring the correctly ligated plasmid. The target protein is expressed as a fusion with MBP, mCherry, and ELP. Endogenously produced TVMV protease cleaves off the MBP-mCherry segment, resulting in the target protein flanked by an N-terminal 8×His tag and a C-terminal ELP tag (Figure 1).
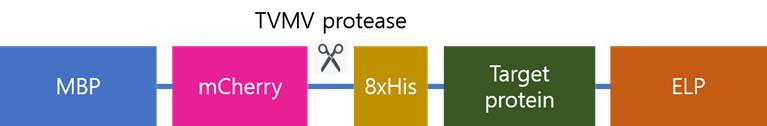
Figure 1. Schematic representation of the recombinant protein expressed from the constructed plasmid. The full-length fusion protein is initially produced, and TVMV protease cleaves specifically between mCherry and the 8xHis tag. As a result, the processed target protein retains the 8xHis tag, allowing it to bind to the affinity column, while the MBP-mCherry fragment does not bind and is removed during purification.
B. E. coli culturing for protein production
1. Using a disposable toothpick, gently scrape the surface of the frozen Rosetta2(DE3)pLysS cell stock harboring the desired plasmid and inoculate it into 500mL of LB medium.
2. Grow the culture in the shaking incubator at 37 °C and 270 rpm.
3. Add IPTG to a final concentration of 0.1 mM when the OD600 reaches 0.8.
4. Grow the culture at 14 °C and 270 rpm for 18 h.
5. Harvest the cells the next day. The cell pellet should appear very dark purple due to the co-expression of mCherry protein in the construct (Figure 2).

Figure 2. Cell pellet upon harvesting. The grown cells exhibit a dark pinkish color due to the co-expressed mCherry protein. If the cells do not show this color, the desired protein has not been produced well.
6. Resuspend the collected cells in 20 mL of PBS.
7. Transfer the resuspended cells to a 50 mL conical tube and store it at -80 °C.
C. Preparation of soluble fraction of cell lysate
1. Thaw the frozen cells in the water bath at ambient temperature (around 22 °C).
2. Lyse the cells using an ultrasonicator. Place the tube containing the cells in the ice. The ON and OFF times are 5 and 50 s, respectively. The total duration of ON time is 8 min. Set applied power to 60% of the full power (750 W).
3. Add 20% Triton X-100 to a final concentration of 1%.
4. Centrifuge the cell lysate at 24,192× g for 30 min at 4 °C.
5. Transfer the supernatant to a new 50 mL conical tube. If proceeding to the next step, place the tube in the ice. Otherwise, store it at -80 °C.
D. Preparation of manual chromatography
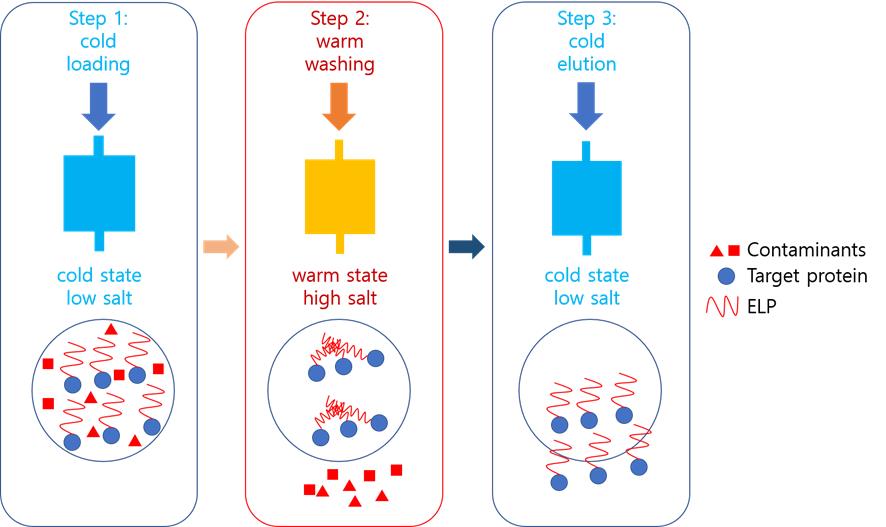
1. Prepare a bucket of ice and set the temperature of the water bath to 37 °C.
2. Place the column and syringe in the ice and wait 10 min before use. Dispense 20 mL of binding buffer into a 50 mL tube. Do the same for the washing buffer and elution buffer. Place the three tubes in the water bath. Then, add 20 mL of PBS and 20 mL of elution buffer to each tube and place the two tubes on ice.
3. Prepare a syringe with a Luer lock and connect it to the adaptor (Figure 3).
Caution: It is strongly recommended to use a syringe with a Luer lock. A syringe with a Luer slip cannot hold the pressure from the plunger, and the connection to the column may break.
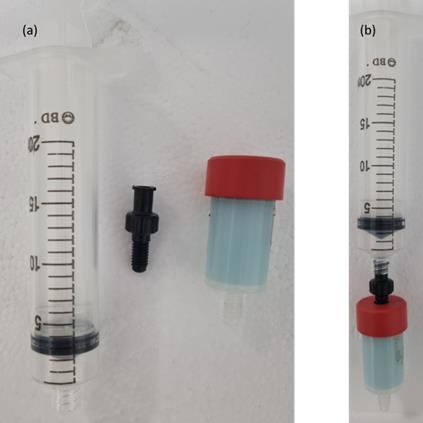
Figure 3. Syringe, adaptor, and column. Luer lock syringe, adaptor, and HisTrap column: (a) separate, and (b) assembled.
E. Purification of the desired protein
E1. Equilibration
1. Aspirate 20 mL of chilled PBS with a syringe.
Caution: Try not to draw any air into the syringe. Air drawn in can dry the column.
2. Connect the syringe to the column with the adaptor.
3. Slowly press the plunger to equilibrate the chilled column with PBS. A drop-by-drop flow rate at the column outlet is sufficient.
Caution: Too much pressure will damage the resin of the column. As the rubber gasket of the syringe plunger wears down, the force required to push it increases.
4. Detach the syringe from the column. Place the column on ice.
E2. Loading
1. Aspirate the chilled supernatant with a syringe.
2. Connect the syringe to the column and slowly press the plunger to load the chilled column with the supernatant. Collect the flowthrough fraction in the 50 mL conical tube, and label it properly. The column will turn dark pink due to the mCherry protein (Figure 4A).
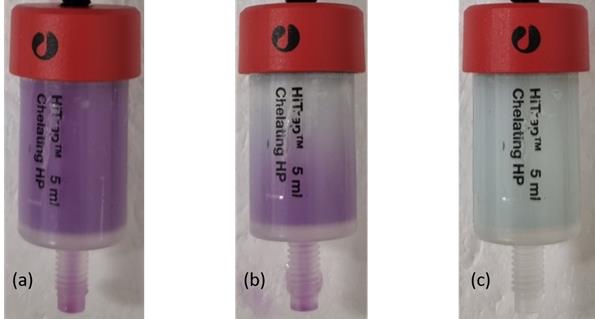
Figure 4. Manual column operation. (A) Upon loading, the color of the column turns dark pink due to the mCherry protein. (B) Upon washing, the pink color diminishes as the mCherry protein is washed away. (C) After washing, the column resumes a pale green color.
3. Detach the syringe from the column and reload it if necessary by repeating steps 1 and 2.
Caution: Use the squeeze bottle to rinse the syringe tip with at least 10 mL of water every time you attach it to the column. Keep the column on ice and plugged with a stopper while not loading it.
E3. Washing
1. Aspirate the 20 mL warmed (to 37 °C) binding buffer with a syringe. This starts the washing step.
2. Connect the syringe to the column and slowly press the plunger to wash and warm the column. Collect the wash fraction in the 50 mL conical tube and label it properly. The column will turn pale green as the mCherry protein is washed away (Figure 4B, C).
3. Detach the syringe from the column. Place the column on the bench (not on ice).
4. Aspirate the 20 mL warmed (to 37 °C) washing buffer with a syringe.
5. Connect the syringe to the column and slowly press the plunger to wash the warmed column. Collect the wash fraction in the 50 mL conical tube and label it properly.
6. Detach the syringe from the column. Place the column on the bench (not on ice).
7. Aspirate the 20 mL warmed (to 37 °C) elution buffer with a syringe.
8. Connect the syringe to the column and slowly press the plunger to wash the warmed column. Collect the wash fraction in the 50 mL conical tube and label it properly.
9. Detach the syringe from the column. Place the column and syringe on ice and wait 10 min.
E4. Elution
1. Aspirate the 20 mL chilled elution buffer with a syringe. This starts the elution step.
2. Connect the syringe to the column and slowly press the plunger to elute the protein from the chilled column. Collect the wash fraction in the 50 mL conical tube and label it properly.
3. Detach the syringe from the column. Place the column on ice.
4. Repeat steps 1 through 3.
E5. Re-equilibration
1. Aspirate the chilled 20 mL PBS with a syringe. This starts the re-equilibration step.
2. Connect the syringe to the column with the adaptor.
3. Slowly press the plunger to equilibrate the column with PBS.
4. Store the column at 4 °C for future use. For longer storage, wash the column with 20 mL of 20% ethanol.
Data analysis
We analyzed the purification results using SDS-PAGE [20]. A warm elution buffer was used to wash the column. As the buffer contained 150mM NaCl and 500mM imidazole, we believe that most nonspecifically bound proteins were effectively removed, as evidenced by the SDS-PAGE results (Figure 5). Deliberately overloading the gel can be useful for detecting trace contaminants. We tested nine proteins (mostly under 13kDa), and among them, MBP was the only one that eluted with the warm elution buffer. Notably, even MBP was only partially eluted under these conditions.
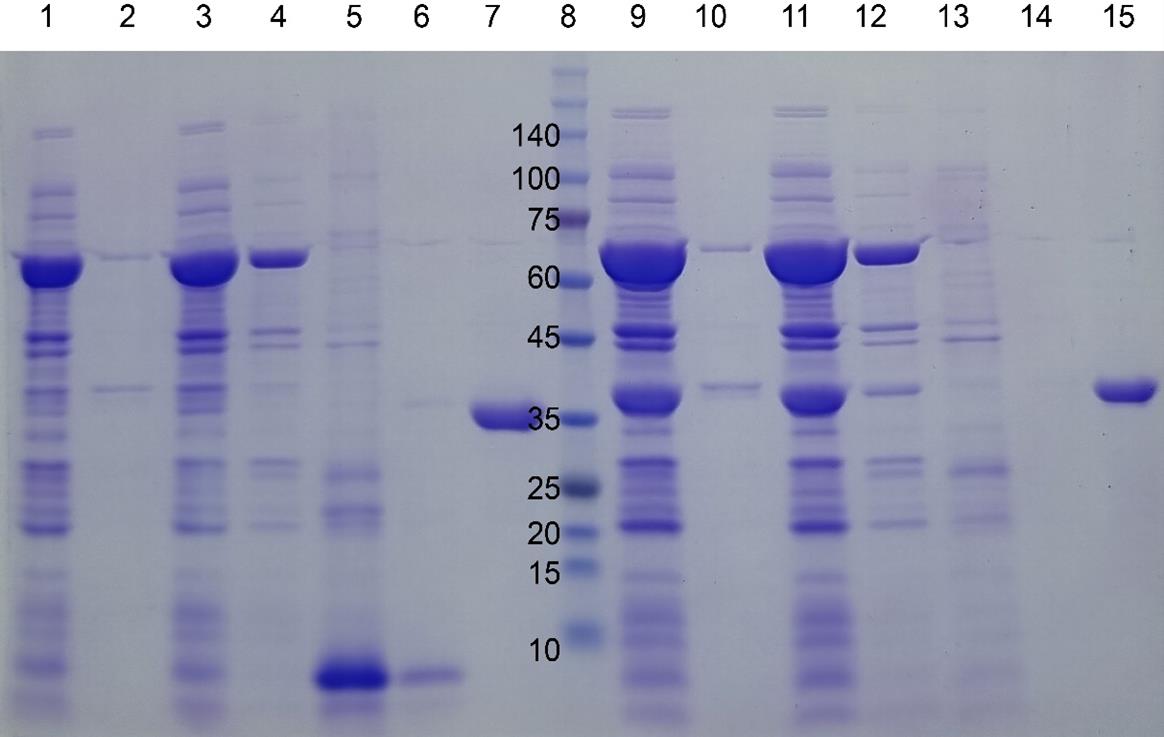
Figure 5. SDS-PAGE gel images of four target proteins fused to I48 during the purification steps. mazE-I48 (32.2k), lanes 1–7, and ccdB-I48 (34.5k), lanes 8–15. Lanes 1 and 2: soluble and insoluble fractions of cell lysate; lane 3: flowthrough fraction upon loading onto HisTrap column; lanes 4, 5, and 6: elution fractions with 10, 250, and 500 mM imidazole at room temperature; lane 7: elution fraction with 500 mM imidazole at ice cold temperature; lane 8: size marker; lanes 9 and 10: soluble and insoluble fractions of cell lysate; lane 11: flowthrough fraction upon loading onto HisTrap column; lanes 12, 13, and 14: elution fractions with 10, 250, and 500 mM imidazole at room temperature; lane 15: elution fraction with 500 mM imidazole at ice-cold temperature. Reprinted/adapted from Shin and Chae [20] under an open-access Creative Commons CC BY license.
Validation of protocol
This protocol or parts of it has been used and validated in the following research article:
• Shin and Chae [20]. Aggregation-Dispersion Chromatography: Application of Elastin-like Polypeptides. Separations (Figures 2 and 3).
General notes and troubleshooting
General notes
1. This method works most successfully with smaller proteins. The largest protein that was successfully purified was 12.9 kDa. The maltose binding protein was partially successful. Based on our analysis, while it is challenging to define a precise optimal size range (in kDa) for the target protein (guest) without experimental validation, we hypothesize that it should generally be smaller than the ELP (host scaffold) to ensure proper aggregation of ELPs. For larger target proteins, increasing the ELP size (e.g., longer chain length) may improve tight aggregation. Furthermore, to maintain the transition temperature within the ambient range, selecting an ELP variant with reduced aggregation propensity is recommended. Empirical testing will be critical to determine system-specific thresholds.
2. This method recommends the use of an indicator protein (color) to visually monitor the progress of chromatography in real-time. For practical manual operation, we implemented a color indicator to eliminate dependence on UV monitoring. The absence of this visual marker would necessitate either UV-based measurements or fraction analysis by SDS-PAGE.
3. This colored protein also serves as an indicator of successful expression of the target protein. Our plasmid construct contains a single large open reading frame comprising MBP, mCherry, a His-tag, the target protein, and finally ELP. A TVMV protease cleavage site is located between mCherry and the His-tag. The TVMV protease is co-expressed at low levels from the same plasmid, enabling the cleavage of the MBP-mCherry portion from the target protein, which remains fused to the His-tag and ELP. Therefore, the more intense the pink color, the higher the level of target protein production.
4. This method may work with batch-style chromatography.
Troubleshooting
Problem 1: The desired protein elutes early.
Possible cause: The protein is larger than what this method can handle with HisTrap HP resin (34 μm diameter).
Solution: A column with larger resins, such as FF (90 μm), may help.
Problem 2: The desired protein does not elute at all.
Possible cause: The protein molecules remained aggregated at the elution step.
Solution: Omit NaCl in the loading and elution steps.
Acknowledgments
The specific contributions of each author were as follows: Conceptualization, H.B.S. and Y.K.C.; Investigation, H.B.S. and Y.K.C. Writing—Original Draft, Y.K.C.; Writing—Review & Editing, Y.K.C.; Funding acquisition, Y.K.C.; Supervision, Y.K.C. This research was funded by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2021R1F1A1046500). The original research paper in which the protocol was described and validated is Shin and Chae [20].
Competing interests
The authors declare no conflicts of interest.
Ethical considerations
This protocol has not used any human or animal subjects.
References
- Batool, M., Ahmad, B. and Choi, S. (2019). A Structure-Based Drug Discovery Paradigm. Int J Mol Sci. 20(11): 2783. https://doi.org/10.3390/ijms20112783
- Labrou, N. E. (2014). Protein Purification: An Overview. In: Labrou, N. E. (Ed.). Protein Downstream Processing: Design, Development and Application of High and Low-Resolution Methods. 3–10. Humana Press, https://doi.org/10.1007/978-1-62703-977-2_1
- Wingfield, P. T. (2015). Overview of the Purification of Recombinant Proteins. Curr Protoc Protein Sci. 80(1): eps0601s80. https://doi.org/10.1002/0471140864.ps0601s80
- Tripathi, N. K. and Shrivastava, A. (2019). Recent Developments in Bioprocessing of Recombinant Proteins: Expression Hosts and Process Development. Front Bioeng Biotechnol. 7: e00420. https://doi.org/10.3389/fbioe.2019.00420
- Rosano, G. L. and Ceccarelli, E. A. (2014). Recombinant protein expression in Escherichia coli: advances and challenges. Front Microbiol. 5: e00172. https://doi.org/10.3389/fmicb.2014.00172
- Baneyx, F. (1999). Recombinant protein expression in Escherichia coli. Curr Opin Biotechnol. 10(5): 411–421. https://doi.org/10.1016/s0958-1669(99)00003-8
- Rudge, S. R. and Ladisch, M. R. (2019). Industrial Challenges of Recombinant Proteins. In: Silva, A. C., Moreira, J. N., Lobo, J. M. S. and Almeida, H. (Eds.) Current Applications of Pharmaceutical Biotechnology. Springer International Publishing, 1–22. https://doi.org/10.1007/10_2019_120
- Jayakrishnan, A., Wan Rosli, W. R., Tahir, A. R. M., Razak, F. S. A., Kee, P. E., Ng, H. S., Chew, Y. L., Lee, S. K., Ramasamy, M., Tan, C. S., et al. (2024). Evolving Paradigms of Recombinant Protein Production in Pharmaceutical Industry: A Rigorous Review. Sci. 6(1): 9. https://doi.org/10.3390/sci6010009
- Zhang, Z. X., Nong, F. T., Wang, Y. Z., Yan, C. X., Gu, Y., Song, P. and Sun, X. M. (2022). Strategies for efficient production of recombinant proteins in Escherichia coli: alleviating the host burden and enhancing protein activity. Microb Cell Fact. 21(1): 191. https://doi.org/10.1186/s12934-022-01917-y
- Pina, A. S., Lowe, C. R. and Roque, A. C. A. (2014). Challenges and opportunities in the purification of recombinant tagged proteins. Biotechnol Adv. 32(2): 366–381. https://doi.org/10.1016/j.biotechadv.2013.12.001
- Lebendiker, M. and Danieli, T. (2011). Purification of Proteins Fused to Maltose-Binding Protein. Methods Mol Biol. 681: 281–293. https://doi.org/10.1007/978-1-60761-913-0_15
- Harper, S. and Speicher, D. W. (2011). Purification of Proteins Fused to Glutathione S-Transferase. Methods Mol Biol. 681: 259–280. https://doi.org/10.1007/978-1-60761-913-0_14
- Loughran, S. T. and Walls, D. (2011). Purification of Poly-Histidine-Tagged Proteins. Methods Mol Biol. 681: 311–335. https://doi.org/10.1007/978-1-60761-913-0_17
- Karyolaimos, A. and de Gier, J. W. (2021). Strategies to Enhance Periplasmic Recombinant Protein Production Yields in Escherichia coli. Front Bioeng Biotechnol. 9: e797334. https://doi.org/10.3389/fbioe.2021.797334
- Lin, Z., Zhao, Q., Xing, L., Zhou, B. and Wang, X. (2015). Aggregating tags for column-free protein purification. Biotechnol J. 10(12): 1877–1886. https://doi.org/10.1002/biot.201500299
- Hassouneh, W., Christensen, T. and Chilkoti, A. (2010). Elastin‐Like Polypeptides as a Purification Tag for Recombinant Proteins. Curr Protoc Protein Sci. 61(1): eps0611s61. https://doi.org/10.1002/0471140864.ps0611s61
- Chae, Y. K. and Kim, H. (2021). Development of an Autoinducible Plasmid for Recombinant Protein Production. Protein Pept Lett. 28(12): 1398–1407. https://doi.org/10.2174/0929866528666211105113750
- Chae, Y. K., Shin, H. B. and Woo, T. R. (2022). Identification of interaction partners using protein aggregation and NMR spectroscopy. PLoS One. 17(9): e0270058. https://doi.org/10.1371/journal.pone.0270058
- Chae, Y. K., Um, Y. and Kim, H. (2021). A simple and sensitive detection of the binding ligands by using the receptor aggregation and NMR spectroscopy: a test case of the maltose binding protein. J Biomol NMR. 75: 371–381. https://doi.org/10.1007/s10858-021-00381-x
- Shin, H. B. and Chae, Y. K. (2024). Aggregation-Dispersion Chromatography: Application of Elastin-like Polypeptides. Separations. 11(12): 335. https://doi.org/10.3390/separations11120335
Article Information
Publication history
Received: Mar 11, 2025
Accepted: May 14, 2025
Available online: May 30, 2025
Published: Jun 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Chae, Y. K. and Shin, H. B. (2025). A Novel Protein Purification Approach Using Elastin-Like Polypeptides (ELP) With His-Tag Assistance. Bio-protocol 15(12): e5342. DOI: 10.21769/BioProtoc.5342.
Category
Biochemistry > Protein > Isolation and purification
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









