- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Murine Osteoblast and Osteoclast Co-culture on Demineralized Bone Paper for Bone Remodeling
(*contributed equally to this work) Published: Vol 15, Iss 11, Jun 5, 2025 DOI: 10.21769/BioProtoc.5339 Views: 2177
Reviewed by: Valérian DORMOYAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

In Vitro Bone Marrow–Derived Dendritic Cells (BMDC) Generation for Antigen Presentation Assay
Sudhakar Singh [...] Sharvan Sehrawat
Apr 20, 2025 4113 Views

Assessing Human Treg Suppression at Single-Cell Resolution Using Mass Cytometry
Jonas Nørskov Søndergaard [...] James B. Wing
Aug 20, 2025 2843 Views

Isolation and Co-culture of Paneth Cells and Intestinal Stem Cells
Ryosuke Isotani [...] Toshimasa Yamauchi
Sep 20, 2025 3379 Views
Abstract
Continuous and balanced bone remodeling is essential for maintaining mechanical integrity, mineral homeostasis, and hematopoiesis. Dysregulated bone metabolism develops pathological conditions, such as osteoporosis and bone metastasis. Functional and analytical recapitulation of bone remodeling in vitro is critical for advancing our understanding of bone mineral metabolism, disease mechanisms, and drug development. However, conventional models fail to replicate the essential complexity of the bone extracellular matrix (ECM) and the dynamic interplay between bone-forming osteoblasts and bone-resorbing osteoclasts. Recently, we developed an osteoid-mimicking demineralized bone paper (DBP) by thin-sectioning demineralized bovine compact bone matrix. DBP supports osteoblastic mineral deposition and the subsequent transition to bone-lining cells. When co-cultured with bone marrow mononuclear cells under biochemical stimulation, osteoblasts shift their regulatory secretion profiles and effectively induce osteoclastogenesis. The semi-transparent nature of DBP, combined with primary osteogenic cells retrieved from DsRed and eGFP reporter mice, enables longitudinal fluorescent monitoring of these multicellular processes and quantitative analysis. In this protocol, we describe the methods for DBP generation, reconstituting mineralized bone tissue complexity with osteoblasts, and recapitulating the bone remodeling cycle through bone marrow monocytes co-culture under biochemical stimulation, offering a useful platform for the related and broader research community.
Key features
• DBP supports in vivo–relevant osteoblastic mineral deposition and transition to lining cells.
• DBP supports in vivo–relevant recapitulation of osteoblast–osteoclast-driven bone remodeling.
• DBP supports longitudinal fluorescent monitoring of multicellular processes and quantification.
Keywords: Demineralized bone paperGraphical overview
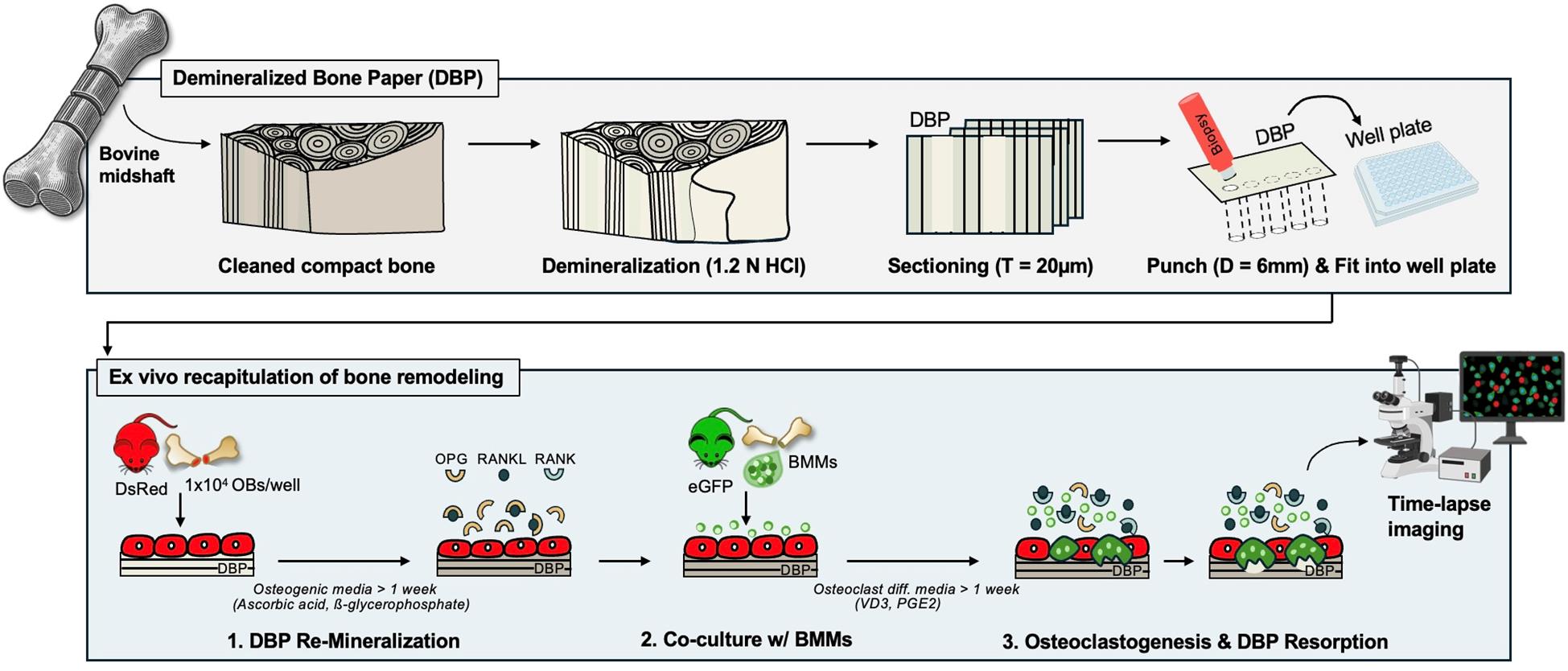
Background
Continuous bone remodeling is essential for maintaining mechanical integrity, mineral homeostasis, and blood formation [1,2]. This process is driven by the coupled actions of bone-resorbing osteoclasts and bone-forming osteoblasts under the spatiotemporal regulation of stimulative and suppressive molecules. Receptor activator of nuclear factor κB ligand (RANKL) is a key stimulatory molecule that induces the differentiation of bone marrow monocytes into multinucleated osteoclasts. Osteoprotegerin (OPG), a decoy receptor for RANKL, serves as a key suppressive molecule that prevents bone remodeling by blocking RANKL-RANK receptor interactions [3,4]. While these multicellular and molecular processes are well established in animal models [5,6] and clinical studies [7,8], their in vitro recapitulation remains a significant challenge.
One important consideration in developing bone tissue models outside the body is the unique hierarchical organization of the bone extracellular matrix (ECM), which consists of collagen fibers and hydroxyapatite crystals at multiple length scales [9]. This mature lamellar bone structure is continuously broken down and rebuilt throughout life. During this process, osteoblasts first deposit a structural collagen matrix, known as osteoid, which then gradually mineralizes to form a lamellar mineralized collagen matrix [10,11]. We hypothesized that mimicking the osteoid-state bone ECM would be a promising strategy to reproduce bone formation and remodeling in vitro. To test this hypothesis, we created demineralized bone paper (DBP) by thin sectioning demineralized bovine compact bone in 2021 [12]. DBP preserves the collagen structure of mature lamellar bone, and based on the sectioning direction, the collagen organization varies significantly [13]. DBP provides semi-transparency for microscopic imaging and mechanical durability for easy experimental handling. The electrostatic charges of the collagen matrix facilitate stable adhesion to tissue culture plastic surfaces [12–15].
So far, we have demonstrated the enabling features of DBP for in vitro recapitulation of bone tissue complexity and remodeling processes with unprecedented analytical power by using primary cells derived from eGFP and DsRed reporter mice. DBP supports rapid and structurally organized mineral deposition by osteoblasts. These osteoblasts then acquire resting-state bone-lining cell phenotypes, including high OPG and low RANKL secretion. Biochemical stimulation of bone-lining cells shifts their OPG and RANKL secretion profiles, activating osteoclastogenesis in co-cultured bone marrow monocytes (BMCs) and inducing subsequent mineral resorption. Upon withdrawal of chemical stimulants, secretion profiles gradually revert to a resting state, and osteoclasts undergo fission [12,14]. The semi-transparent DBP, combined with genetically labeled osteogenic and hematopoietic cells, enables longitudinal fluorescent monitoring of these multicellular processes [13,15]. In this protocol paper, we compile the related methodological sections for (i) DBP preparation, (ii) DBP remineralization with primary murine osteoblasts, and (iii) the recapitulation of osteoclastogenesis via co-culture of primary murine BMCs under biochemical stimulation.
Materials and reagents
Biological materials
Animals
1. DsRed mice (Jackson Laboratory, catalog number: 006051)
2. eGFP mice (Jackson Laboratory, catalog number: 003291)
Reagents
1. Chloroform (Fisher Chemical, catalog number: C607-4)
2. Methanol (Fisher Chemical, catalog number: A454-4)
3. Hydrochloric acid (HCl) (Fisher Chemical, catalog number: A144-212)
4. Optimal cutting temperature (OCT) compound (Fisher Healthcare, catalog number: 23-730-571)
5. NucBlue Live ReadyProbes (Invitrogen, catalog number: R37605) and Hoechst33342 (Invitrogen, catalog number: H1399)
6. Fluorescent 5-FAM conjugated collagen hybridized peptide (CHP) (3Helix, catalog number: FLU300)
7. Collagenase (Life Technologies, catalog number: 10.17101015)
8. Fetal bovine serum (FBS) (Sigma-Aldrich, catalog number: F0926-500 mL)
9. Penicillin and streptomycin (PS) (Gibco, catalog number: 15070-063)
10. α-MEM (Gibco, catalog number: 12000-022)
11. 10× Phosphate-buffered saline (PBS) (Fisher Bioreagents, catalog number: BP399500)
12. Sodium bicarbonate (NaHCO3) (Fisher Chemical, catalog number: S233-3)
13. β-glycerophosphate (Sigma, catalog number: G5422-25G)
14. L-ascorbic acid 2-phosphate trisodium salt, 25 G (Wako Chemicals, catalog number: 323-44822)
15. Alkaline phosphatase (ALP) detection kit (Sigma, catalog number: 86C)
16. Vitamin D (VD3) (CAYMAN Chemical Company, catalog number: 71820)
17. Prostaglandin E2 (PGE2) (Cayman Chemical Company, catalog number: 14010)
18. 4% Paraformaldehyde (Thermo Fisher Scientific, catalog number: J19943.K2)
19. Alexa Fluor 488 phalloidin (Invitrogen, catalog number: A12379)
20. Calcein (MP, catalog number: 195087)
21. Alizarin Red S (Acros Organics, catalog number: 400481000)
22. Tartrate-Resistant Acid Phosphatase (TRAP) detection kit (Sigma-Aldrich, catalog number: 387A)
23. Mouse OPG ELISA (R&D Systems, catalog number: DY459)
24. Mouse RANKL ELISA (R&D Systems, catalog number: DY462)
25. Ethanol (Decon Laboratories Inc., catalog number: 2705)
26. Acetic acid (Fisher Scientific, catalog number: A38SI-212)
Solutions
1. 1.2 N HCl (see Recipes)
2. Defatting medium (see Recipes)
3. Collagenase solution (see Recipes)
4. Expansion medium (see Recipes)
5. Osteogenic differentiation medium (see Recipes)
6. Biochemical stimulation medium (see Recipes)
7. Alizarin Red S solution (see Recipes)
8. Calcein staining solution (see Recipes)
Recipes
1. 1.2 N hydrochloric acid (HCl)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 12.1 N HCl | 1.2 N | 40 mL |
| Deionized (DI) water | n/a | 360 mL |
| Total | 400 mL |
Storage: Room temperature in an acid chemical storage cabinet.
2. Defatting medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Chloroform | 50% | 200 mL |
| Methanol | 50% | 200 mL |
| Total | 400 mL |
Storage: Room temperature in a flammable chemical storage cabinet.
3. Collagenase solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| α-MEM | 1× | 4 mL |
| Collagenase, Type II | 800 U | 40 μL |
| Total | 4.04 mL |
Storage: Collagenase at -20 °C. Once reconstituted at 4 °C, use immediately.
4. Expansion medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| α-MEM powder | 10.1 g/L | 10.1 g |
| NaHCO3 | 2.2 g/L | 2.2 g |
| FBS | 10% | 100 mL |
| Pen/Strep | 1% | 10 mL |
| DI water | n/a | 890 mL |
| Total | 1 L |
Storage: Refrigerator at 4 °C; use within 6 weeks.
5. Osteogenic differentiation medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| α-MEM | 1× | 49.42 mL |
| β-glycerophosphate | 10 mM | 500 μL |
| L-ascorbic acid | 125 μM | 80 μL |
| Total | 50 mL |
Storage: Refrigerator at 4 °C, use within 6 weeks.
6. Biochemical stimulation medium
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| α-MEM | 1× | 19.96 mL |
| Vitamin D (VD3) | 10 nM | 20 μL |
| Prostaglandin E2 (PGE2) | 1 μM | 20 μL |
| Total | 20 mL |
Storage: Refrigerator at 4 °C, use within 1 week.
7. Alizarin Red S solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Alizarin Red S | 20 mM | 136.9 mg |
| DI water | n/a | 20 mL |
| Total | 20 mL |
Storage: Room temperature or 4 °C, use within 6 months.
8. Calcein staining solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Calcein (from 1 M stock) | 1 μM | 20 μL |
| DI water | n/a | 20 mL |
| Total | 20 mL |
Storage: Room temperature or 4 °C, use within 6 months.
Laboratory supplies
1. Deionized (DI) water (Milli-Q)
2. Scalpel (Graham Field, catalog number: 2975#10)
3. Razor blade (AccuTec Blades, catalog number: AGBL-7033-0000)
4. Biopsy punch 6 mm (Fisher Scientific, catalog number: 12-460-412)
5. Biopsy punch 10 mm (Fisher Scientific, catalog number: NC9226137)
6. 48-well plate (Falcon, catalog number: 353078)
7. 96-well plate (Fisher Scientific, catalog number: FB012931)
8. T-25 flask (Thermo Fisher Scientific, catalog number: 156340)
9. T-75 flask (Fisher Scientific, catalog number: FB012937)
10. T-175 flask (Fisher Scientific, catalog number: NC1932996)
11. 10 cm untreated Petri dish (Fisher Scientific, catalog number: FB0875712)
12. 1.5 mL microtube (Fisher Scientific, catalog number: 05-408-129)
13. 0.6 mL microtube (Fisher Scientific, catalog number: 05-408-128)
14. 50 mL Falcon conical tube (Fisher Scientific, catalog number: 14-432-22)
Supplies for bone demineralization
15. Bovine femurs (local slaughterhouse or grocery store, age 30–42 months according to USDA)
16. 20-inch hand bone saw (KATA SK5)
17. Cyclic hydrostatic pressure chamber (in-house production)
18. Stainless-steel chamber (in-house production)
19. Arduino controller (in-house production)
Equipment
1. Cryostat (Thermo Fisher Scientific, EprediaTM, catalog number: 95-711-0L, model: CryoStar NX70)
2. Centrifuge (Thermo Fisher Scientific, catalog number: 75004221, model: SorvallTM LegendTM X1 Centrifuge)
3. Tissue culture incubator (Thermo Fisher Scientific, catalog number: 51033557, model: HeracellTM VIOS 160i CO2 Incubator)
4. EVOS XL Core Imaging System (Thermo Fisher Scientific, catalog number: AMEX1000)
5. EVOS M7000 imaging system (Invitrogen, catalog number: AMF7000)
6. -80 °C freezer (Thermo Fisher Scientific, catalog number: TSU400D)
7. -20 °C freezer (Thermo Fisher Scientific, catalog number: FBG25FSSA)
8. 4 °C fridge (Thermo Fisher Scientific, catalog number: GTFBG49RPGA)
9. Auto-Fill LN2 Cryo (Thermo Fisher Scientific, catalog number: 7400, model: CryoPlus 1)
10. Radiographic imaging (Faxitron X-Ray LLC, model: MX-20 Cabinet X-ray System)
11. X-ray scanning (Caliper LifeSciences, model: IVIS Spectrum-CT)
12. Second harmonic generation (SHG) multiphoton microscopy (Nikon, model: A1RMP)
13. Microplate reader (Biotek, model: Synergy 2)
14. Inverted fluorescent microscope (Etaluma, model: Lumascope 720)
15. Resonant scanning multiphoton microscope (Nikon, model: A1MP+)
16. Confocal microscope (Carl Zeiss Microscopy, model: Zeiss Cell Observer SD)
17. Box furnace (Thermo Fisher Scientific, catalog number: 10-549-166)
Software and datasets
1. FIJI (ImageJ 1.54)
2. EVOS Fluorescence Microscope Software
3. NIS-Elements Imaging Software
4. Microsoft Excel
5. Adobe Illustrator
6. BioRender (https://www.biorender.com/)
Procedure
A. Preparation of DBP
Caution: All steps must be performed while wearing appropriate personal protective equipment (PPE).
A1. Preparation of bone samples [Figure 1A(i–ii)]
1. Obtain frozen bovine femurs from a local slaughterhouse or grocery store.
2. Using a bone saw, cut the femurs into ~5 cm sections.
3. Remove the bone marrow from the inner cavity and trim away any muscle and connective tissues using scalpels and forceps.
A2. Defatting the bones (in fume hood) [Figure 1A(iii)]
1. Place the cleaned bone in a glass beaker and submerge in a chloroform/methanol (1:1) solution.
2. Incubate in a chemical fume hood for 24 h.
Caution: Chloroform/methanol mixtures are volatile and hazardous. Keep containers tightly sealed and store away from ignition sources in a flammable chemical storage cabinet.
Note: Remove any remaining connective tissue before starting demineralization.
A3. Demineralization with hydrochloric acid (in fume hood) [Figure 1A(iv–v)]
1. Immerse the defatted bones in 1.2 N hydrochloric acid.
2. Place the container in a cyclic hydrostatic pressure chamber and apply compressed air at 4 bars in 10 s on/off cycles (0.1 Hz).
Caution: The pressure chamber can be placed outside a fume hood, but the ventilation tube must remain inside to prevent inhalation of acid vapors.
3. After 24 h, carefully remove the outer bone layer with a razor blade to ensure acid exposure.
Note: This improves acid penetration and accelerates demineralization.
4. Replace the hydrochloric acid daily for 7–10 days or until bones are fully demineralized.
Note: Monitor demineralization progress by weighing bones daily. Demineralization is complete when weight stabilizes, and bones become manually compressible. Confirm full demineralization using X-ray.
A4. Neutralization and storage [Figure 1A(vi)]
1. Rinse demineralized bones overnight in deionized water to remove residual acid.
2. For short-term storage (>1 week), submerge in ethanol and store at 4 °C.
3. For long-term storage (<1 week), vacuum-seal and store at -20 °C.
Note: Insufficient rinsing can cause rust formation in a cryostat chamber due to residual acid.
A5. Trimming and embedding [Figure 1B(i)]
1. Trim demineralized bone pieces to fit the cryostat mounting disc diameter and blade length.
2. Embed trimmed bones in OCT compound, positioning them parallel to the mounting disc.
3. Freeze at -80 °C for 5 min or until the OCT is fully solidified.
A6. Cryosectioning [Figure 1B(i–ii)]
1. Section OCT-embedded bones into slices with 20 μm thickness using a cryostat to generate DBP.
Note: Demineralized bone can be sliced vertically or transversely depending on the desired collagen alignment. Check collagen alignment under transmitted light microscopy after initial sections.
2. Transfer DBP into 50 mL centrifuge tubes and wash 5× with deionized water to remove residual OCT.
A7. Decellularization [Figure 1B(ii)]
1. Fill the 50 mL centrifuge tubes containing DBP with 1.2 N HCl.
2. Incubate at room temperature with gentle shaking for 30–60 min.
Note: Confirm decellularization by DAPI staining for nuclei, followed by PBS washes, and fluorescent imaging.
A8. Neutralization and storage [Figure 1B(ii)]
1. Wash decellularized DBP ~20× with deionized water to remove residual HCl.
2. Store in 70% ethanol at 4 °C.
Note: DBP is typically used within 1 month; however, it can be stored for longer periods, although gradual collagen degradation may occur over time.
A9. Preparation for DBP-attached 96-/48-well plates [Figure 1B(iii)]
1. Cut DBP into 6 mm (for 96-well plate) or 10 mm (for 48-well plate) circles using biopsy punches.
Note: DBP can also be prepared in custom sizes to fit 24-/12-well formats.
2. In a biosafety cabinet, wash the punched DBP five times with 70% ethanol, followed by PBS three times.
3. Using sterile forceps, transfer DBP into the well plates and air-dry overnight to promote stable adhesion.
Note: Pre-wetting wells with PBS improves DBP adhesion and reduces wrinkling, which can otherwise impair imaging and cell attachment.
A10. Characterization of DBP [Figure 1C(i-iii)]
1. Use brightfield and second harmonic generation (SHG) imaging to evaluate collagen alignment (e.g., vertical vs. transverse orientation).
2. Confirm decellularization using DAPI staining after A7 to verify the absence of nuclei.
3. Apply collagen hybridizing peptide (CHP) staining to assess the integrity of the triple-helical structure of collagen fibers.
Note: A positive control is prepared by heating DBP with 80 °C water for 1 min to denature collagen. Denatured collagen exposes unfolded strands, resulting in increased CHP binding, while intact DBP shows minimal signal.
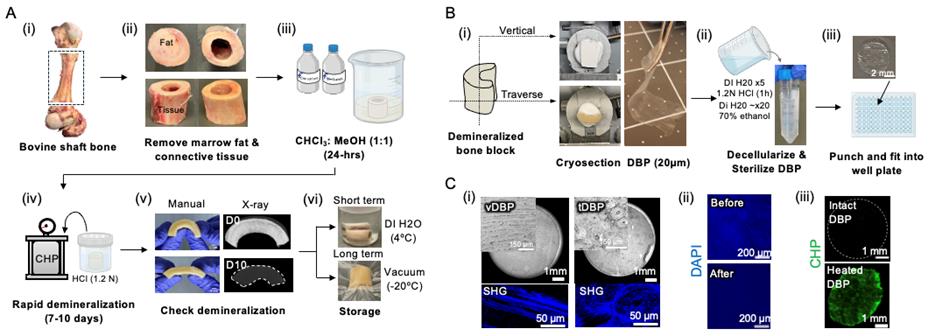
Figure 1. Fabrication and characterization of demineralized bone paper (DBP). (A) Bone sourcing and demineralization: (i) Whole bovine femur sectioned to obtain shaft bone blocks. (ii) Removal of marrow and soft tissue to obtain clean shafts. (iii) Defatting in 1:1 chloroform:methanol for 24 h. (iv) Demineralization in 1.2 N HCl under cyclic hydrostatic pressure. (v) Confirmation of demineralization by manual inspection and X-ray imaging. (vi) Storage: short-term (>1 week) in 70% ethanol at 4 °C; long-term (<1 week) vacuum-sealed at -20 °C. (B) DBP generation: (i) Orientation for vertical or transverse sectioning into 20 μm thick slices. (ii) Decellularization in 1.2 N HCl for 1 h; sterilized and stored in 70% ethanol. (iii) Biopsy-punched DBP tailored to 96-/48-well plate sizes. (C) Structural and compositional analysis: (i) Microscopic images of vertically aligned DBP (vDBP) and transversely aligned DBP (tDBP) showing distinct collagen alignments. (ii) DAPI staining confirms decellularization via the absence of nuclei post–HCl wash. (iii) Collagen hybridizing peptide (CHP) staining indicates preserved triple helical structure of collagen fibers in DBP.
B. Preparation of primary murine osteoblasts [Figure 2(i–vi)]
1. Collect mouse femur and tibia in cold PBS.
2. Remove surrounding soft tissue using forceps and gauze.
3. Trim the ends of the bones using a scalpel or surgical scissors.
4. Prepare a 0.5 mL microtube by punching a hole in the bottom with a needle or sharp tweezers.
5. Insert the bone into the microtube with the cut end facing downward. Place this tube inside a 1.5 mL microtube to extract bone marrow.
6. Centrifuge at 10,000× g for 30 s. The red bone marrow will collect in the lower tube, and the remaining bone will appear white.
7. Transfer the bones to a Petri dish containing 10 mL of sterile PBS.
8. Using a scalpel, cut the bones into 1–2 mm pieces.
Note: Bone fragments larger than 2 mm may slow the release of osteoprogenitors. Remove any remaining soft tissue at this step.
9. Transfer the chopped bone pieces into a T-25 cell culture flask. Add 3 mL of expansion medium and 30 μL of collagenase II (800 units). Incubate at 37 °C overnight.
10. The next day, terminate collagenase digestion and wash the bone chips 3× with sterile PBS to remove non-adherent cells and tissue debris.
11. Add 4 mL of fresh expansion medium and incubate at 37 °C to allow osteoprogenitors to migrate from the bone chips.
12. Replace 4 mL of medium every 3 days. Continue the culture until osteoprogenitors reach 70%–80% confluency.
13. Harvest and reseed the osteoprogenitors into a T-75 flask. Keep the bone chips in the original T-25 flask to allow further migration and expansion of osteoprogenitors.
Note: Osteoprogenitors are typically harvested 4–5 times from the same bone chips.
14. Once osteoprogenitors reach 70%–80% confluency in the T-75 flask, transfer them to a T-175 flask for further expansion.
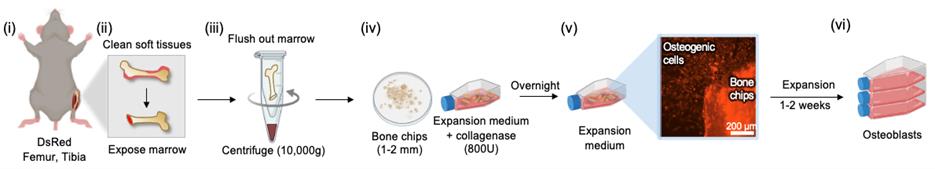
Figure 2. Isolation and expansion of osteoblasts from DsRed murine bone. (i) Isolation of femurs and tibias. (ii) Removal of soft tissue and cutting of bone ends to expose marrow. (iii) The open-ended bone is placed in a perforated 0.5 mL tube nested inside a 1.5 mL tube, then centrifuged to flush out marrow. (iv) Marrow-depleted bones are cut into 1–2 mm chips and digested overnight with collagenase. (v) Osteogenic cells migrate from bone chips and begin proliferation. (vi) Osteoblasts are expanded by subculture over 1–2 weeks.
C1. Cell culture (Figure 3A)
1. Prepare the osteogenic differentiation medium as described above.
2. Prepare DBP-attached 96-/48-well plates, following section A9.
3. Seed DsRed-expressing osteogenic cells onto DBP-containing wells.
Note: The recommended osteoblast seeding densities to reach full confluency in 3–4 days are as follows:
96-well plate: 10,000 cells/well in 100 μL of osteogenic medium.
48-well plate: 50,000 cells/well in 200 μL of osteogenic medium.
Lower seeding densities will require more time to reach confluency.
4. Culture the cells in osteogenic differentiation medium at 37 °C in a humidified incubator with 5% CO2 for 1–2 weeks, replacing the medium every 3 days. Monitor cell morphology and mineralization by observing darkening of the DBPs under a brightfield microscope.
C2. Assessment of osteoblast differentiation [Figure 3B(i)]
5. At designated time points (e.g., Day 7, 14), assess cytoskeletal organization and osteoblast differentiation.
a. Fix the cells with 4% paraformaldehyde for 10 min at room temperature.
b. Stain with phalloidin following the manufacturer’s instructions and visualize actin filaments and cytoskeletal organization via fluorescence microscopy.
c. Stain with an alkaline phosphatase (ALP) detection kit following the manufacturer’s instructions and assess ALP activity, a key marker of osteoblasts, using brightfield microscopy.
Assessment of osteoblast mineralization [Figure 3B(ii)]
6. At designated time points (e.g., Day 7 or 14), assess mineralization using one of the following approaches:
Approach 1: Imaging and brightness analysis
a. Capture brightfield images of the wells every 2 days.
b. Analyze the images using ImageJ to quantify brightness (inverse of darkness) on the DBPs, indicating mineral deposition.
c. Plot brightness changes over time to evaluate mineralization progression.
Approach 2: Calcein staining
a. Prepare a 1–2 μM Calcein solution in calcium- and magnesium-free PBS.
b. Aspirate the culture medium and rinse the wells with calcium- and magnesium-free PBS.
c. Add the Calcein solution to each well and incubate for 30 min at 37 °C, protected from light.
d. Visualize deposited minerals under a fluorescence microscope and conduct quantitative analysis.
Note: Calcein staining can be done during live osteoblast culture.
Approach 3: Alizarin Red S staining
a. Aspirate the culture medium and rinse wells with PBS.
b. Wash the wells 2× with deionized water to remove excess fixative.
c. Add 20 mM Alizarin Red S solution and incubate for 20 min at room temperature, protected from light.
d. Rinse the wells thoroughly with deionized water until no Alizarin Red S dye is released.
e. Image DBPs stained with Alizarin Red S.
f. Solubilize Alizarin Red S by adding 50% acetic acid and incubate for 30 min at room temperature.
g. Transfer the solution to new wells and measure absorbance at 405 nm using a microplate reader.
Note: The values from replicate wells were averaged to quantify mineral deposition. Analyze the mineralization data from the selected approach, comparing results across different time points or experimental conditions.
Assessment of ECM organization [Figure 3B(iii)]
7. At designated time points (e.g., Day 7 or 14), assess ECM organization via imaging and brightness analysis.
a. Fix the cells cultured on DBP with 4% paraformaldehyde for 10 min at room temperature.
b. Carefully detach the DBP from the wells.
c. Transfer the DBP onto a glass slide and visualize secreted collagen fibers using second harmonic generation (SHG) multiphoton microscopy.
d. Subject the DBP to heat treatment at 500 °C for 5 h in a furnace to decompose organic collagen and preserve mineralized structures.
e. Mount the remaining inorganic matrix on an aluminum stub using carbon tape and analyze mineral composition and structure using scanning electron microscopy coupled with energy-dispersive X-ray spectroscopy (SEM-EDX).
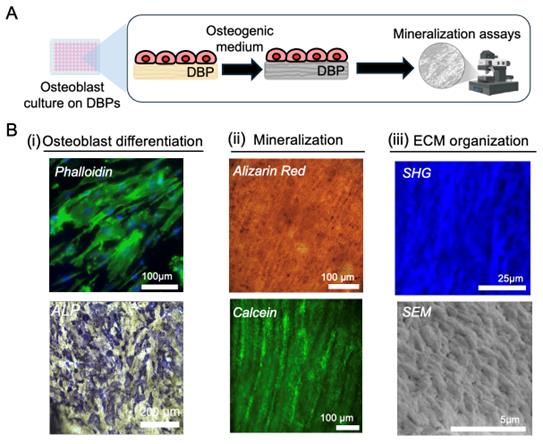
Figure 3. Osteoblastic mineralization on demineralized bone paper (DBP). (A) Schematic of the mineralization assay. Osteoblasts are seeded onto DBP, cultured in osteogenic differentiation medium for a designated period, and mineral deposition is quantitatively assessed. (B) Characterization of mineralization. (i) Osteoblast differentiation: Cells adhere and proliferate along DBP collagen, visualized by phalloidin staining (green: actin; blue: nuclei). Alkaline phosphatase (ALP) staining indicates osteogenic enzymatic activity. (ii) Mineralization: Alizarin Red staining and Calcein labeling visualizes mineral deposition. (iii) Extracellular matrix (ECM) organization: Second harmonic generation (SHG) imaging captures collagen fiber organization; scanning electron microscopy (SEM) shows mineral granule deposition aligned with collagen orientation.
D. Preparation of BMCs from mouse femurs and tibia [Figure 4(i–v)]
1. Harvest BMCs from murine long bones following Section C, steps 1–4.
Note: Use transgenic reporter mice expressing different fluorescence (e.g., DsRed for osteoblasts, eGFP for BMCs) to enable clear visualization and tracking of multicellular processes in fluorescence imaging.
2. Plate the isolated bone marrow cells in a T-75 flask with expansion medium and incubate overnight at 37 °C in a humidified incubator with 5% CO2 to allow adherent cells to attach.
3. Gently collect the non-adherent (floating) cells without disturbing the adherent layer. Use this BMC-enriched fraction as osteoclast precursors for osteoblast co-culture experiments.
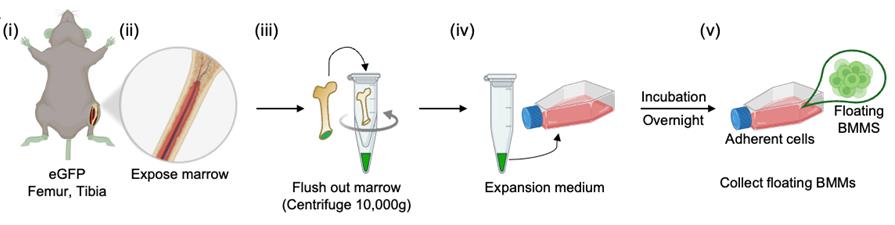
Figure 4. Isolation of bone marrow monocytes from eGFP murine bones. (i) Harvesting of femurs and tibias. (ii–iii) Bone marrow is flushed from an open-ended bone into a collection tube. (iv) Flushed marrow is plated in expansion medium and incubated overnight to allow attachment of stromal cells. (v) Floating bone marrow monocytes are collected the next day for co-culture experiments.
E. Recapitulation of osteoclastogenesis via co-culture of osteoblasts and BMCs on DBP under biochemical stimulation
Cell culture (Figure 5A)
1. Seed osteoblasts onto DBP and control tissue culture plastic (TCP), and culture in osteogenic medium for 1 week for remineralization, replacing the medium every 3 days.
Note: Recommended osteoblast seeding densities are as follows:
96-well plate: 10,000 cells/well in 100 μL of osteogenic medium.
48-well plate: 50,000 cells/well in 200 μL of osteogenic medium.
2. After 1 week of osteoblast culture, add BMCs to each well with the biochemical stimulation medium, including vitamin D3 (VD3) and prostaglandin E2 (PGE2). Maintain the co-culture at 37 °C in a humidified incubator with 5% CO2, replacing the medium every 3 days.
Note: Recommended BMCs seeding densities are as follows:
96-well plate: 100,000 cells/well in 150 μL of biochemical stimulation medium.
48-well plate: 500,000 cells/well in 200 μL of biochemical stimulation medium.
Assessment of osteoclast differentiation and activity [Figure 5B(i–iii)]
3. At designated time points, assess osteoclast differentiation and activity using one of the following methods:
Approach 1: Time-lapse fluorescence imaging and osteoclastic resorption tracking.
a. Perform time-lapse fluorescence imaging to monitor osteoclast differentiation and resorptive activity. VD3/PGE2-stimulated osteoblasts increase RANKL secretion, inducing BMC differentiation into multinucleated osteoclasts.
b. Capture images at defined intervals during VD3/PGE2 stimulation to observe osteoclast dynamics, including fusion/fission activity and resorption behavior.
c. Perform additional imaging following VD3/PGE2 withdrawal to assess osteoclast survival and resorptive activity post-stimulation.
Note: TCP serves as a control substrate, exhibiting artifact osteoclast behaviors such as oversized multinucleated cells due to excessive precursor fusion and apoptosis. In contrast, DBP supports physiologically relevant osteoclast differentiation and resorptive activity due to the preserved bone extracellular matrix complexity.
Approach 2: Actin and nuclear staining to confirm mature osteoclasts.
a. Aspirate the culture medium without disturbing the cells.
b. Wash the cells three times with PBS to remove residual media.
c. Fix cells with 4% paraformaldehyde for 15 min at room temperature.
d. Wash with PBS to remove excess fixative.
e. Stain actin filaments with phalloidin following the manufacturer’s instructions to visualize actin ring structures characteristic of mature osteoclasts.
f. Wash with PBS and counterstain with DAPI for 10 min to visualize nuclei.
g. Image under fluorescence microscopy to identify multinucleated osteoclasts.
Approach 3: Osteoclast tartrate-resistant acid phosphatase (TRAP) activity assay.
a. Follow steps a–d from Approach 2 to prepare the cells.
b. Perform TRAP staining according to the manufacturer’s instructions to identify and quantify active osteoclasts based on TRAP enzymatic activity.
c. Image TRAP-positive multinucleated osteoclasts using fluorescence microscopy.
Note: Ensure consistent imaging settings for comparison across experimental groups.
Approach 4: ELISA-based quantification of osteoclast regulatory factors.
a. Collect conditioned media at defined time points before, during, and after VD3/PGE2 stimulation to assess RANKL and OPG secretion dynamics.
b. Perform ELISA following the manufacturer’s instructions, ensuring proper standard curve generation and sample dilution.
Note: Dilute OPG samples to ensure measurements fall within the linear range of the standard curve and prevent signal saturation. A dilution ratio should be optimized in each experiment.
c. Compare RANKL/OPG ratios across time points.
Note: An increase in RANKL and a decrease in OPG indicate the molecular milieu that promotes osteoclast differentiation.
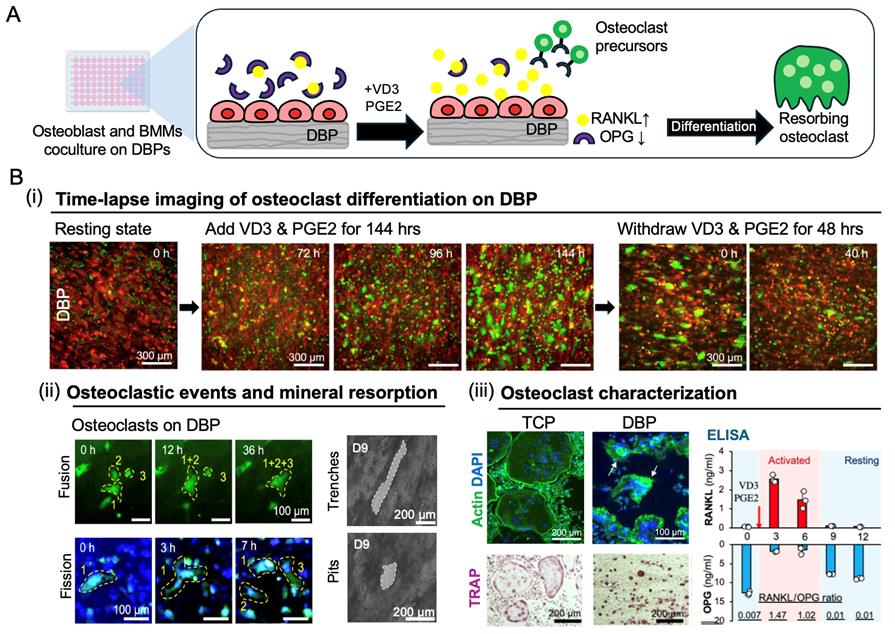
Figure 5. Recapitulation of a bone remodeling cycle via co-culture of DsRed osteoblasts and eGFP bone marrow monocytes on demineralized bone paper (DBP) under biochemical stimulation. (A) Schematic of the co-culture assay. DsRed-expressing osteoblasts are cultured on DBP to reconstitute a mineralized collagen matrix and adopt a resting bone-lining phenotype characterized by high osteoprotegerin (OPG) and low receptor activator of nuclear factor κB ligand (RANKL) secretion. eGFP-labeled bone marrow monocytes are then seeded and co-cultured in osteoclast differentiation medium containing vitamin D3 (VD3) and prostaglandin E2 (PGE2), which stimulate osteoblasts to increase RANKL and decrease OPG secretion. This shift in the RANKL/OPG ratio induces monocyte differentiation into multinucleated osteoclasts that resorb the mineralized matrix. (B) (i) Time-lapse fluorescence imaging of before stimulation (0 h), after VD3/PGE2 treatment (72, 96, 144 h), and following withdrawal (0, 40 h) captures dynamic and reversible osteoclast phenotype transitions. (ii) Time-course fluorescence imaging reveals osteoclast fusion (0–36 h) and fission (0–7 h) events. Brightfield imaging shows trench- and pit-type resorption patterns. (iii) Phalloidin (actin), DAPI (nuclei), and TRAP staining confirm multinucleated osteoclast formation on both tissue culture plastic (TCP) and DBP. Osteoclasts on DBP are significantly smaller than those on TCP and closely resemble in vivo osteoclast size. ELISA quantifies time-dependent changes in the RANKL/OPG secretion ratio during the remodeling cycle. Together, the in vivo–relevant osteoclast size, observed fusion–fission dynamics, and osteoblast secretion profile shifts underscore the biological significance of DBP in recapitulating the intrinsic regulatory roles of native bone extracellular matrix (ECM).
Data analysis
1. Quantitative analysis of bone remodeling was carried out using time-lapse imaging and fluorescence microscopy to track cellular activity over time.
2. Image processing and quantification of osteoblast and osteoclast activity were performed using ImageJ (FIJI). Spatial distribution patterns of osteogenic and osteoclastic activity were analyzed by dividing the DBP surface into concentric zones and mapping fluorescence intensity along with morphological changes.
3. Bone surface coverage, mineral deposition, and osteoclast activity were evaluated through fluorescence imaging and quantitative spatial mapping, with heatmaps generated to visualize activity patterns across different regions of the DBP surface.
4. To assess molecular changes in osteoblast activity, the RANKL/OPG ratio was determined using enzyme-linked immunosorbent assays (ELISA) under resting and stimulated conditions.
5. Osteoblast and osteoclast migration was tracked using the TrackMate in ImageJ.
6. High-content imaging was used to analyze osteoblast–osteoclast interactions, incorporating multiplex fluorescence imaging to capture direct cell–cell contacts.
7. Statistical analyses were conducted using SPSS (IBM). Data were expressed as mean ± standard deviation, and group comparisons were performed using t-tests and one-way ANOVA, followed by post hoc Bonferroni tests to assess significant differences between conditions.
8. Data were tested for normality, and significance was set at p < 0.05. Results were visualized through heatmaps, scatter plots, and trendline regression to assess spatial correlations in bone remodeling activity.
Validation of protocol
This protocol or parts of it has been used and validated in the following research articles:
• Park et al. [12]. Trabecular bone organoid model for studying the regulation of localized bone remodeling. Science Advances (Figures 1–4).
• Park et al. [14]. Functional and analytical recapitulation of osteoclast biology on demineralized bone paper. Nature Communication (Figures 2–4 and 6).
• Yoon et al. [13]. Collagen structures of demineralized bone paper direct mineral metabolism. Journal of Bone & Mineral Research-Plus. (Figures 1, 3, 5).
• Ryan et al. [15]. AI-assisted label-free monitoring bone mineral metabolism on demineralized bone paper. ACS Biomaterials Science & Engineering. (Figures 1–3).
The protocol is robust and reproducible, as confirmed by multiple publications.
General notes and troubleshooting
1. If the DBP tears or folds during sectioning, try adjusting the blade sharpness or sectioning speed to improve consistency. Always sterilize with 70% ethanol and rinse thoroughly with PBS to remove any ethanol residue that might interfere with cell adhesion.
2. When transferring the DBP biopsy into a 96-well plate, use forceps to carefully place it in PBS. Gently press it flat with forceps to ensure it sits evenly at the bottom. Let the DBP dry overnight to allow proper adhesion, which helps prevent movement during cell culture and imaging.
3. If osteoblasts fail to deposit minerals within 4 days, check that the osteogenic differentiation medium contains the necessary supplements (ascorbic acid, β-glycerophosphate). Mineralization can be confirmed using brightfield imaging.
4. Multinucleated osteoclasts should appear within 3–6 days following stimulation with VD3/PGE2. If differentiation is inconsistent, check that BMC seeding density is appropriate and that differentiation factors are fresh and at the correct concentrations.
5. Fluorescent time-lapse imaging is recommended for tracking osteoblast and osteoclast behavior. Using two distinct fluorophores can help differentiate between the two cell types. If cell movement appears inconsistent or difficult to analyze, adjusting imaging intervals and fluorescence exposure can help reduce photobleaching and improve image clarity.
Acknowledgments
We thank all contributors of the initial publications that laid the foundation for the development of DBP and its applications in co-culture and bone remodeling assays.
Funding: This work was supported by NIH grants (R01CA237171, R33CA291301) and an NSF grant (1944188). S.A. was partly supported by a NIH Biotechnology Traineeship (T32 GM13509), and H.Y. was partly supported by the Ottogi Ham Taiho Foundation grant.
Author contributions: H.Y. and S.A. drafted the original manuscript. D.C. and Y.H. contributed to reproducibility validation and protocol refinement. J.L. provided overall supervision and edited the manuscript.
Competing interests
J.L. is a co-founder of MetaBone Inc. and holds equity. The other authors have no competing interests to declare.
Ethical considerations
All animal procedures in this study were approved by the Institutional Animal Care and Use Committee at the University of Massachusetts Amherst (Institutional Protocol No. 4652). Experiments involving mice were conducted following all applicable federal, state, and institutional guidelines to ensure ethical treatment and compliance with regulatory standards.
References
- Wang, L., You, X., Zhang, L., Zhang, C. and Zou, W. (2022). Mechanical regulation of bone remodeling. Bone Res. 10(1): 16. https://doi.org/10.1038/s41413-022-00190-4
- Bolamperti, S., Villa, I. and Rubinacci, A. (2022). Bone remodeling: an operational process ensuring survival and bone mechanical competence. Bone Res. 10(1): 48. https://doi.org/10.1038/s41413-022-00219-8
- Boyce, B. F. and Xing, L. (2008). Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 473(2): 139–146. https://doi.org/10.1016/j.abb.2008.03.018
- Udagawa, N., Koide, M., Nakamura, M., Nakamichi, Y., Yamashita, T., Uehara, S., Kobayashi, Y., Furuya, Y., Yasuda, H., Fukuda, C., et al. (2021). Osteoclast differentiation by RANKL and OPG signaling pathways. J Bone Miner Metab. 39(1): 19–26. https://doi.org/10.1007/s00774-020-01162-6
- Kong, Y. Y., Yoshida, H., Sarosi, I., Tan, H. L., Timms, E., Capparelli, C., Morony, S., Oliveira-dos-Santos, A. J., Van, G., Itie, A., et al. (1999). OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 397(6717): 315–323. https://doi.org/10.1038/16852
- Min, H., Morony, S., Sarosi, I., Dunstan, C. R., Capparelli, C., Scully, S., Van, G., Kaufman, S., Kostenuik, P. J., Lacey, D. L., et al. (2000). Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J Exp Med. 192(4): 463–474. https://doi.org/10.1084/jem.192.4.463
- Kearns, A. E., Khosla, S. and Kostenuik, P. J. (2008). Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev. 29(2): 155–192. https://doi.org/10.1210/er.2007-0014
- Riches, P. L., McRorie, E., Fraser, W. D., Determann, C., van't Hof, R. and Ralston, S. H. (2009). Osteoporosis associated with neutralizing autoantibodies against osteoprotegerin. N Engl J Med. 361(15): 1459–1465. https://doi.org/10.1056/NEJMoa0810925
- Ma, C., Du, T., Niu, X. and Fan, Y. (2022). Biomechanics and mechanobiology of the bone matrix. Bone Res. 10(1): 59. https://doi.org/10.1038/s41413-022-00223-y
- Ravazzano, L., Colaianni, G., Tarakanova, A., Xiao, Y. B., Grano, M. and Libonati, F. (2024). Multiscale and multidisciplinary analysis of aging processes in bone. NPJ Aging. 10(1): 28. https://doi.org/10.1038/s41514-024-00156-2
- Salhotra, A., Shah, H. N., Levi, B. and Longaker, M. T. (2020). Mechanisms of bone development and repair. Nat Rev Mol Cell Biol. 21(11): 696–711. https://doi.org/10.1038/s41580-020-00279-w
- Park, Y., Cheong, E., Kwak, J. G., Carpenter, R., Shim, J. H. and Lee, J. (2021). Trabecular bone organoid model for studying the regulation of localized bone remodeling. Sci Adv. 7(4). https://doi.org/10.1126/sciadv.abd6495
- Yoon, H., Park, Y., Kwak, J. G. and Lee, J. (2024). Collagen structures of demineralized bone paper direct mineral metabolism. JBMR Plus. 8(8): ziae080. https://doi.org/10.1093/jbmrpl/ziae080
- Park, Y., Sato, T. and Lee, J. (2023). Functional and analytical recapitulation of osteoclast biology on demineralized bone paper. Nat Commun. 14(1): 8092. https://doi.org/10.1038/s41467-023-44000-9
- Ryan, P., Yoon, H., Amin, S., Chambers, J. J. and Lee, J. (2025). AI-Assisted Label-Free Monitoring Bone Mineral Metabolism on Demineralized Bone Paper. ACS Biomater Sci Eng. 11(4): 2096–2105. https://doi.org/10.1021/acsbiomaterials.4c02349
Article Information
Publication history
Received: Mar 21, 2025
Accepted: May 11, 2025
Available online: May 26, 2025
Published: Jun 5, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Amin, S., Yoon, H., Choi, D., Hsu, Y. and Lee, J. (2025). Murine Osteoblast and Osteoclast Co-culture on Demineralized Bone Paper for Bone Remodeling. Bio-protocol 15(11): e5339. DOI: 10.21769/BioProtoc.5339.
Category
Biological Engineering > Biomedical engineering
Cell Biology > Cell isolation and culture > Co-culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









