- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
The Mouse Social Frailty Index (mSFI): A Standardized Protocol
Published: Vol 15, Iss 8, Apr 20, 2025 DOI: 10.21769/BioProtoc.5272 Views: 1789
Reviewed by: Marco Pagliusi Jr.Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
A Social Stimulation Paradigm to Ameliorate Memory Deficit in Alzheimer's Disease
Qiaoyun Ren [...] An Liu
Aug 5, 2024 1762 Views
Pupillometry: A Simple and Automatic Way to Explore Implicit Cognitive Processing
Tian Yuan [...] Yi Jiang
Apr 5, 2025 1277 Views
Training Mice to Perform Attentional Set-Shifting Under Head Restraint
Katarina Kalajzic [...] Timothy Spellman
Sep 5, 2025 1454 Views
Abstract
The advent of geroscience engendered the development of approaches to quantify the aging process and estimate biological age on an individual level. Recognizing that declines observed in aging are not only physical but also social led us to develop a mouse Social Frailty Index (mSFI) designed to quantify age-related impairments of social functioning in mice. The mSFI consists of seven behavioral assays that measure essential facets of social behavioral functioning in mice: social communication, social interaction, and social functional ability. The assays that comprise the mSFI are all minimally disruptive, relatively simple to execute, and optimized for compatibility with longitudinal studies utilizing experimental interventions relevant to geroscience. The mSFI is conducted over AM and PM sessions spanning a maximum of 3.5 days, using materials common to most animal facilities. The data for all assays is obtained observationally, manually recorded, and entered into predefined template sheets that automate the computation of the mSFI. We have demonstrated the validity and applicability of the mSFI across multiple laboratory sites and experiments. This index has proven to discriminate between differential trajectories of biological aging driven by sex, progeria, or social stress-relevant contexts. The mSFI represents a novel index to quantify trajectories of biological aging in mice, and its application may help elucidate the social dimensions of the aging process.
Key features
• The mSFI is a comprehensive assessment for the evaluation of impairment in social behavioral functioning related to aging in mice.
• Minimally disruptive, relatively simple, commonly used high-throughput assays of spontaneous social behavior that are optimized for compatibility with longitudinal studies of aging.
• The protocol spans AM and PM sessions over 3.5 days maximum; the sequence of individual assays is flexible by design.
• The mSFI requires materials common to most animal research facilities.
• mSFI application is compatible with most experimental treatments, social behavioral paradigms, longitudinal within-subject designs, and genetically modified mouse models.
Keywords: Social frailtyGraphical overview
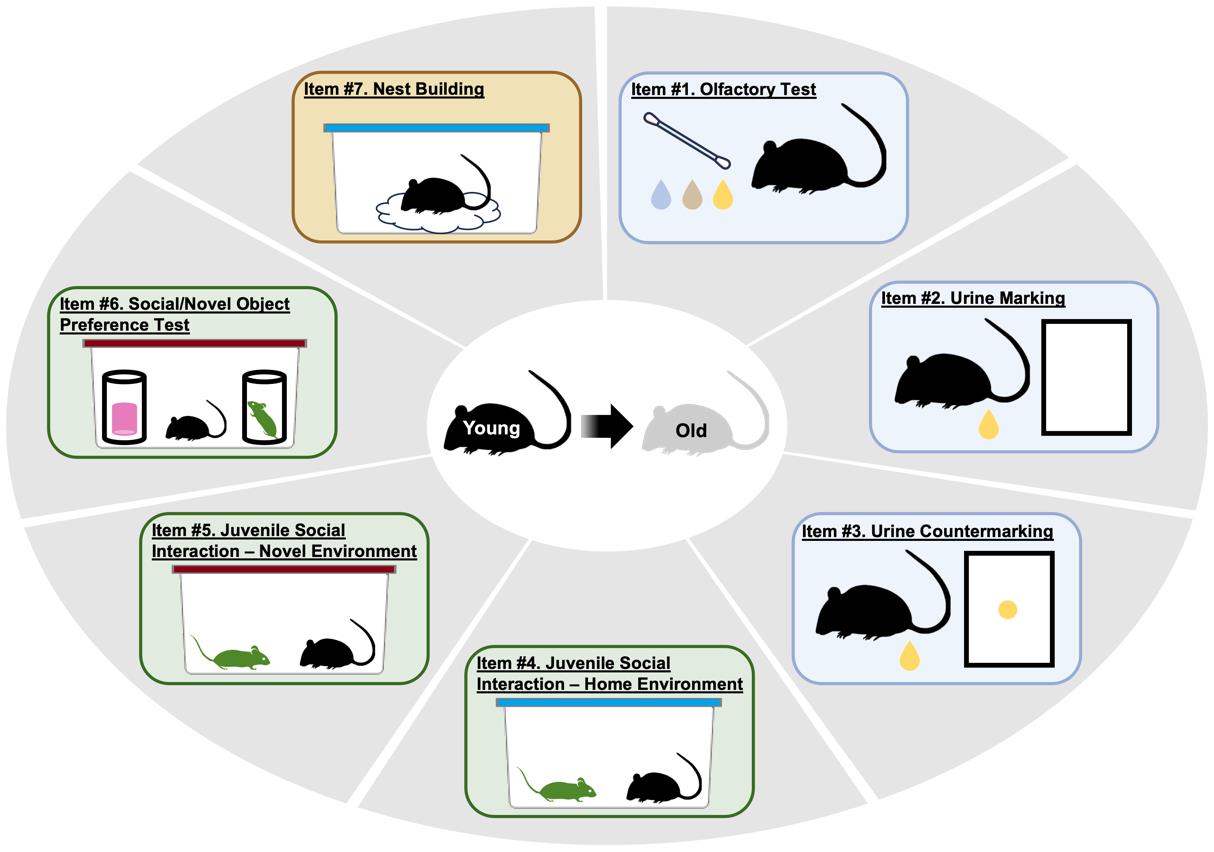
The mouse social frailty index (mSFI)
Background
As a consequence of societal advances allowing modern humans to live longer than ever before, aging now represents the single largest risk factor for chronic diseases and mortality [1–4]. Although the aging process is characterized by gradual and progressive decline, individuals of the same chronological age may manifest a different biological age, with some experiencing a variety of age-related morbidities while others remain relatively healthy [5–10]. Given this marked heterogeneity in the aging process, it is imperative that the growing field of geroscience be equipped with measures that reliably capture the quality of biological aging on an individual level in order to better understand and leverage mechanisms underlying the basic biology of aging to lengthen healthspan [11–17]. Frailty, an age-related state of increased vulnerability to adverse health outcomes, has emerged as a reliable and valid outcome in the quantification of functional impairment and biological aging [18–21]. Indices based on an accumulation of physical health deficits over time have been constructed for both humans and mice, demonstrating high evolutionarily conserved predictive validity for age-related morbidity and mortality while providing high utility as a robust indicator of biological aging for clinical practice and preclinical research [22–30].
However, many of the factors that produce disparities in healthspan and dictate the pace of biological aging across species are socially bound and linked to social determinants [31–37]. In this light, social frailty, an age-related lack of social skills and resources necessary to fulfill basic social needs and achieve well-being, has been proposed as a construct and measured in humans, showing predictive validity for mortality [38,39]. Despite the widespread use of mice as an animal model for geroscience, and studies demonstrating evolutionarily conserved declines in their social capabilities with age, no extant method has been able to quantify the social aspects of frailty in laboratory mice [40–43]. To fill this gap, we recently developed a novel approach to quantify social frailty in mice [44]. The mouse Social Frailty Index (mSFI) is based on the deficit accumulation model of frailty [20,22,45] and consists of seven minimally disruptive, relatively simple, commonly used assays that measure essential facets of social behavioral functioning in mice: social communication, social interaction, and social functional ability. The mSFI is conducted over a maximum of 3.5 days, in AM and PM sessions. During each session, each mouse is tested from a few minutes to one hour in duration (unless otherwise specified). Behavioral data are either directly recorded during testing or quantified later in assays that are based on image quantification via freely available software. The obtained values are then entered into predefined spreadsheet templates that automate the calculation of the mSFI. In practice [44], the mSFI showed a progressive, graded increase with chronological age in group-housed C57BL6 mice as well as sensitivity to i) sex, ii) social isolation and chronic subordination stress, and iii) mouse models of progeria. Our published data [44] provide robust support for the initial validity and applicability of the mSFI as a useful measure for preclinical geroscience research. This protocol describes and operationalizes the procedure for conducting the mSFI.
Materials and reagents
Biological materials
1. Juvenile (4–5 weeks old) stimulus mice matched to the sex and strain of experimental mice (see General Note 1). Testing can be conducted with up to five mice per observer, per round of observation.
a. Juvenile mice can be reused in subsequent rounds of observation, so the amount required is one that is sufficient to provide one round of experimental mice with sex- and strain-matched juveniles. For example, 5 juvenile mice could be used for 20 experimental mice. This would result in four rounds of observation.
b. If juvenile mice are of the same coat color as the experimental mouse, mark the tail of the juveniles with a bright-colored marker for ease of observation and to differentiate between juveniles themselves.
Reagents
1. Tap water
2. 1:10,000 pure vanilla extract (McCormick & Co., Inc., Hunt Valley, MD) diluted in tap water
3. Pooled intact male & female mouse urine purchased from a commercial vendor (Biochemed Pharmacologicals, catalog number: NC1569592), ~2.2 mL/20 mice as a social odor (see also General Notes 1 and 2)
4. 70% EtOH spray
Laboratory supplies
1. Small static cages (186 mm × 298 mm × 128 mm) without bedding, nesting material, food, or water bottle
2. Small static cages (186 mm × 298 mm × 128 mm) with a layer of bedding but without nesting material, food, or water bottle
3. Large static cages (257 mm × 483 mm × 152 mm) with bedding but without nesting material, food, water bottle, or metal wire lids
4. Wire mesh cups (e.g., Amazon, Amazon Basics wire mesh pen cup, reference: B07VVB7DSK)
5. Bottles filled with tap water (e.g., EMD Milipore Corporation, Milipore Express® PLUS 0.22 mm PES 150 mL bottle unit) or any other available object to serve as a weight on top of the wire mesh cups to prevent the mouse from tipping them while exploring
6. Novel objects (e.g., Simport Scientific Inc., catalog number: C576, SecurTainerTM II tamper-evident container, 40 mL, reference: C576-40MA). These may be changed based on available resources but must fit in the wire mesh cups and be similar in size to a juvenile mouse
7. Pressed cotton batting nestlets (e.g., Ancare Corp, reference: NES3600)
8. GeneMate 20–200 μL autoclavable single-channel pipettor (ISC BioExpress Inc. or an equivalent model) and pipette tips
9. 15.24 cm cotton swabs (e.g., Tyco Healthcare Group LP, Kendall CurityTM single tipped applicators, reference: 8884540500)
10. 15 mL polystyrene conical tubes (e.g., Corning, Falcon® 15 mL polystyrene centrifuge tube, catalog number: 352095)
11. Tube rack (recommended) (e.g., Heathrow Scientific LLC, 5.39 cm wd, 9.52 cm ht, 17.46 cm lg, polypropylene, Grainger, catalog number: 55PT41)
12. 31.5 × 33.5 cm Whatman 3 MM CHR chromatography blotting paper (Cytiva, catalog number: 3030-335)
13. One stopwatch per experimenter + one additional
14. One clipboard per experimenter
15. Coding sheets for instantaneous sampling
16. Optional: sticky notes to keep track of mouse IDs
17. If needed: cages to temporarily store cage mates or house overnight for nest building
Equipment
1. UV transilluminator (e.g., Ultra-Violet Products, Inc., model: chromato-vue transilluminator 0–62)
2. High-resolution camera (high-resolution phone camera is OK; you must ensure that either the same camera is used across assessments or that megapixel count is identical between cameras used)
Software and datasets
1. Microsoft Excel or any other spreadsheet-based software
2. ImageJ (Version 1.53m [46])
3. Statistical software of your choosing to produce data in a reproducible manner, adequate for the comparisons you are making, based on your experimental design
Procedure
A. Scheduling
1. The mSFI consists of seven tests that take place during seven individual AM and PM sessions over 3.5 days.
2. Typically, testing can start on a Monday and end on a Thursday, allowing Friday to be used for other testing such as the 31-Item Clinical Frailty Index assessment [28].
3. The mSFI test sequence is meant to be flexible such that any test can be scheduled in any of the AM or PM sessions over the 3.5 days. The only exception is the nest-building test, which needs to occur overnight.
4. Special attention should be paid to when the AM and PM sessions occur each day.
a. The AM sessions need to be scheduled early enough so that they do not go into the PM, but not too close to the start of the light cycle. The PM sessions need to be scheduled to give ample time between AM and PM sessions, but not too late that they come close to the start of the dark cycle.
b. When applied to a 12:12 06:00 AM 06:00 PM light cycle, a start time of 8:30 AM works well for AM sessions and 1:30 PM for the PM session. Besides the nest-building test, see each section for specifics.
B. Fulfilling cage needs
1. The mSFI requires behavioral testing environments constructed using caging materials common to most animal research facilities. Prior to starting the mSFI assessment (See General Note 3), you should prepare:
a. One empty (i.e., no bedding, nesting material, food, or water bottle) small static cage (186 mm × 298 mm × 128 mm) per mouse for urine marking.
b. One empty (i.e., no bedding, nesting material, food, or water bottle) small static cage (186 mm × 298 mm × 128 mm) per mouse for urine countermarking.
Note: To limit caging demands, the empty small static cage used in either the urine marking or countermarking assays (whichever is conducted first) can be thoroughly cleaned with 70% EtOH and reused in the remaining assay.
c. One small static cage (186 mm × 298 mm × 128 mm) with bedding, nesting material, food, and water bottle per mouse for juvenile social interaction in a novel environment.
d. One large static cage (257 mm × 483 mm × 152 mm; typically used to house rats) with bedding but without nesting material, food, water bottle, or metal wire lids per mouse for social/novel object preference test.
Note: If the mice being assessed are not individually housed (e.g., they are group-housed or in another housing paradigm), then caging needs will need to be altered based on adaptations for each assay. See notes throughout the following protocol sections for specifics.
C. Assessment environment
1. Provided the availability of suitable working surfaces (tables, shelves, carts, etc.), the mSFI should be conducted in the same room where the experimental mice are housed. If this is not the case, transport the mice to an area with suitable working surfaces. Allow mice at least 1 h to acclimate to the novel environment prior to beginning testing.
2. Assays in the mSFI occur in both the home cage environment (olfactory test, juvenile social interaction in home environment, nest building) and novel cage environments (urine marking, urine countermarking, juvenile social interaction in a novel environment, social/novel object preference test and nest building; see individual sections for specifics). In the case where mice are group-housed, special consideration should be taken for assays that occur in the home cage environment. Mice housed in groups should be assigned to undergo testing in the home cage environment in random order.
a. During testing, group-housed cage mates to be tested subsequently can be housed in temporary cages.
b. When this occurs, the group-housed experimental mice that are undergoing testing should be allowed to freely roam the home cage for 1 min to habituate before behavioral testing occurs.
D. Item 1: Olfactory test, home environment
1. Prepare solutions of tap water in a 15 mL conical tube, 1:10,000 pure vanilla extract diluted in tap water in a 15 mL conical tube, and 0.1 mL/mouse of pooled male and female urine. Bring these to the assessment environment. It is recommended to use a tube holder to organize these during assessment to allow ease of access.
2. Place the experimental mouse home cage on the working surface, remove the cage filter top (if present), and, while maintaining the metal lid and food in place, set the water bottle aside.
3. Each of the three odors (water, vanilla, urine, in that order) should be presented in sequence to the experimental mouse. For each odor, the process is identical:
a. Wet a new cotton swab in stimulus odor solution, making sure to press the cotton tip firmly against the edge of the tube to get rid of excess liquid.
b. Prepare the stopwatch with your preferred hand. This will be used to measure the time spent sniffing the swab.
c. Prepare an extra timer that will be used for 2-minute interval timing.
d. With the cotton swab in your preferred hand, insert the swab approximately 2.5 cm through the wire top near the water bottle insert, starting the 2-min interval timer simultaneously (Figures 1 and 2).

Figure 1. Item 1: Olfactory test apparatus. Sagittal view. This test should be conducted in the experimental mouse’s home cage, and the cage should be configured as depicted above. The 15.24 cm (6 in.) cotton swab should be inserted 2.5 cm (~1 in.) into the cage and held stable throughout odor presentation. Note: There is no mouse in this figure.

Figure 2. Item 1: Olfactory test configuration. Transverse view. This test should be conducted in the experimental mouse’s home cage, and the cage should be configured as depicted above. The 15.24 cm (6 in.) cotton swab should be inserted 2.5 cm (~1 in.) into the cage and held stable throughout odor presentation. Note: There is no mouse in this figure.
e. Observe the mouse behavior intently for 2 min. Start the stopwatch only when the mouse is engaging with the cotton swab (see below) and stop it as immediately as possible when the mouse disengages. Engaging behavior is defined as follows: the mouse’s nose is within approximately 2 cm of the cotton swab, oriented toward the swab, and making visible efforts to inhale the odor (i.e., the mouse is actively sniffing the swab, not simply walking past it); the mouse is chewing, licking and/or gnawing at the swab; the mouse is holding the swab with its forepaws.
f. After each 2 min interval, remove the cotton swab and record the time spent sniffing the odor.
g. Allow a 1 min recovery period before presenting the next odor.
Note: This is the longest assay in the mSFI. At maximum efficiency, it takes 8 min per mouse. With two experienced observers, it takes approximately 2 h to test 20 mice (See General Note 4).
Olfactory test quantification: The measure of interest in the olfactory test is the percentage of social odor (urine) preference.
Formula: % urine preference = time spent sniffing urine (s) / (time spent sniffing water (s) + time spent sniffing vanilla (s) + time spent sniffing urine (s)) × 100.
E. Item 2: Urine marking, novel environment
1. Cut Whatman paper into sheets according to the standard size of cages used in your animal care facility, so that the paper will lay flat and cover the entire bottom of the cage during experimentation.
2. Label the cut Whatman paper with any pertinent identification information (i.e., date, subject ID).
3. Lay labeled sheets of Whatman paper within empty (i.e., no bedding, nesting material, food, or water bottle) small static cages (186 mm × 298 mm × 128 mm), ensuring that the paper lies flat on the bottom of the cage (Figure 3).

Figure 3. Item 2: Urine marking. Chromatography paper should be cut to the dimensions of the standard size of cages used in your animal care facility; alterations may be needed depending on variability between cages. The paper should lie flat at the bottom of the cage and may be curled upward to properly fit the cage. Bottom: paper in the cage without the filter top. Top: paper in the cage with the filter top.
4. Place experimental mice in individual cages with Whatman paper and close the cage tops. Placement of all experimental mice should occur as simultaneously as possible.
5. Once all experimental mice are all in their respective cages, begin timing. Allow mice to freely roam the novel environment and deposit their urinary marks for 1 h.
6. At the end of the 1 h period, return the mice to their home cages.
7. Remove the marked Whatman paper, clean any feces, and hang to dry overnight to allow for the fixation of urinary marks.
Note: In its entirety, the urine-marking assay takes approximately 1.25 h per 20 mice with two observers. This does not include quantification time.
Urine-marking quantification: details on quantification and analysis can be found in section G.
F. Item 3: Urine countermarking, novel environment
1. Cut Whatman paper into sheets according to the standard size of cages used in your animal care facility so that the paper will lay flat and cover the entire bottom of the cage during experimentation.
a. Label the cut Whatman paper with any pertinent identification information (i.e., date, subject ID).
b. Using a pencil, outline a 1.5 cm diameter circle in the center of the Whatman paper. This will serve as a reference for where to aliquot the stimulus urine immediately before testing.
2. Lay labeled sheets of Whatman paper within empty (i.e., no bedding, nesting material, food, or water bottle) small static cages (186 mm × 298 mm × 128 mm), ensuring that the paper lies flat on the bottom of the cage.
3. Aliquot 10 μL of the pooled urine into the centrally located reference outline. This will serve as the stimulus for countermarking behavior (Figure 4).

Figure 4. Item 2: Urine countermarking. Chromatography paper should be cut to the dimensions of the standard size of cages used in your animal care facility; alterations may be needed depending on variability between cages. The paper should lie flat at the bottom of the cage and may be curled upward at the corners to properly fit the cage. Ten microliters of pooled urine should be aliquoted in the center of the cage. A circle outline has been superimposed here for reference of where the aliquot would be. Bottom: marked paper in the cage without the filter top. Top: marked paper in the cage with the filter top.
4. Immediately place experimental mice in individual cages with Whatman paper and close the cage tops. Placement of all experimental mice should occur as simultaneously as possible.
5. Once all experimental mice are all in their respective cages, begin timing. Allow mice to freely roam the novel environment and deposit their urinary marks for 1 h.
6. At the end of the 1 h period, return the mice to their home cages.
7. Remove the marked Whatman paper, clean any feces, and hang to dry overnight to allow for the fixation of urinary marks.
Note: In its entirety, the urine-countermarking assay takes approximately 1.25 h per 20 mice with two observers. This does not include quantification time.
Urine-countermarking quantification: details on quantification and analysis can be found in section G.
G. Urine marking and countermarking quantification and analysis
1. Before conducting the following procedure, ensure that the marked Whatman paper is completely dry and as flat as possible.
2. UV scanning
Caution: Prior to beginning scanning, take appropriate personal safety measures to protect yourself from the UV light emitting from the transilluminator.
a. Turn off the lights in the room.
b. Place marked Whatman paper as flat as possible on your UV transilluminator. The paper should be completely in the illuminated area.
c. Using a high-resolution camera, take a picture of the illuminated paper (Figures 5a and 6a).
Critical: Prior to taking a picture, ensure that the photo will be saved in .jpeg format. This is especially important if you are using a phone camera that may have unique file types for pictures.
Critical: Ensure that the highest degree of consistency is maintained in the distance between the camera and the Whatman paper as well as the angle of the camera between subsequent photos. Both factors dictate how many pixels the illuminated paper and markings take up within the dimensions of the captured photo. Later analysis steps can correct for reasonable amounts of variability in pixel dimension ratios between photos, but large variability may result in pervasive bias in results.
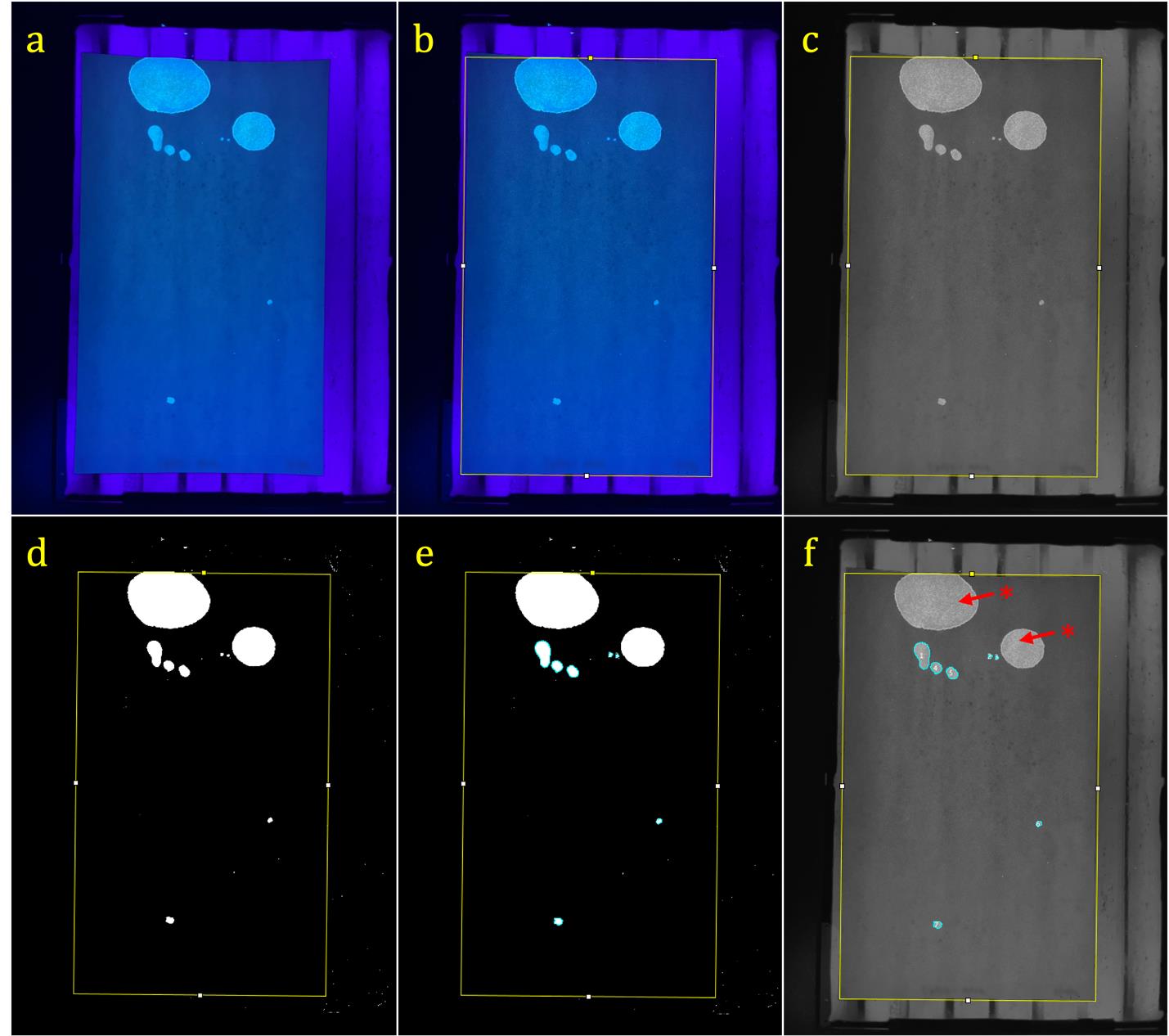
Figure 5. Item 2: Urine marking exemplar quantification and analysis steps. (a) Photo of marked Whatman paper from urine-marking test over UV transilluminator imported to ImageJ (steps G1–3b); the paper is laid flat on the surface of the transilluminator. The field of view includes the entire sheet of paper, and the camera angle is close to parallel with the sheet of paper. (b) Rotated rectangle tool outlining the paper to specify the area of analysis (step G3c). (c) Rectangle outlining the paper after conversion to greyscale (step G3d). (d) Threshold applied to create contrast highlighting illuminated marks; noise (i.e., light not produced by markings) is present outside the bounds of the rectangle outline; no noise inside the bounds of the rectangle (step G3e). (e) Analyze particles function applied within the bounds of the analysis area, highlighting (in light blue) the markings that were counted (step G3f). (f) Analyzed photo of Whatman paper in greyscale after the threshold was removed to exhibit which markings were quantified within the specified bounds. *: large urinary spots that fell outside of the specified size range (100–40,000 pixels) and were not counted in the analysis. Note: This example was obtained from a 16-month-old C57BL6 male mouse. Labels on the Whatman paper pertaining to experimental information have been blurred.
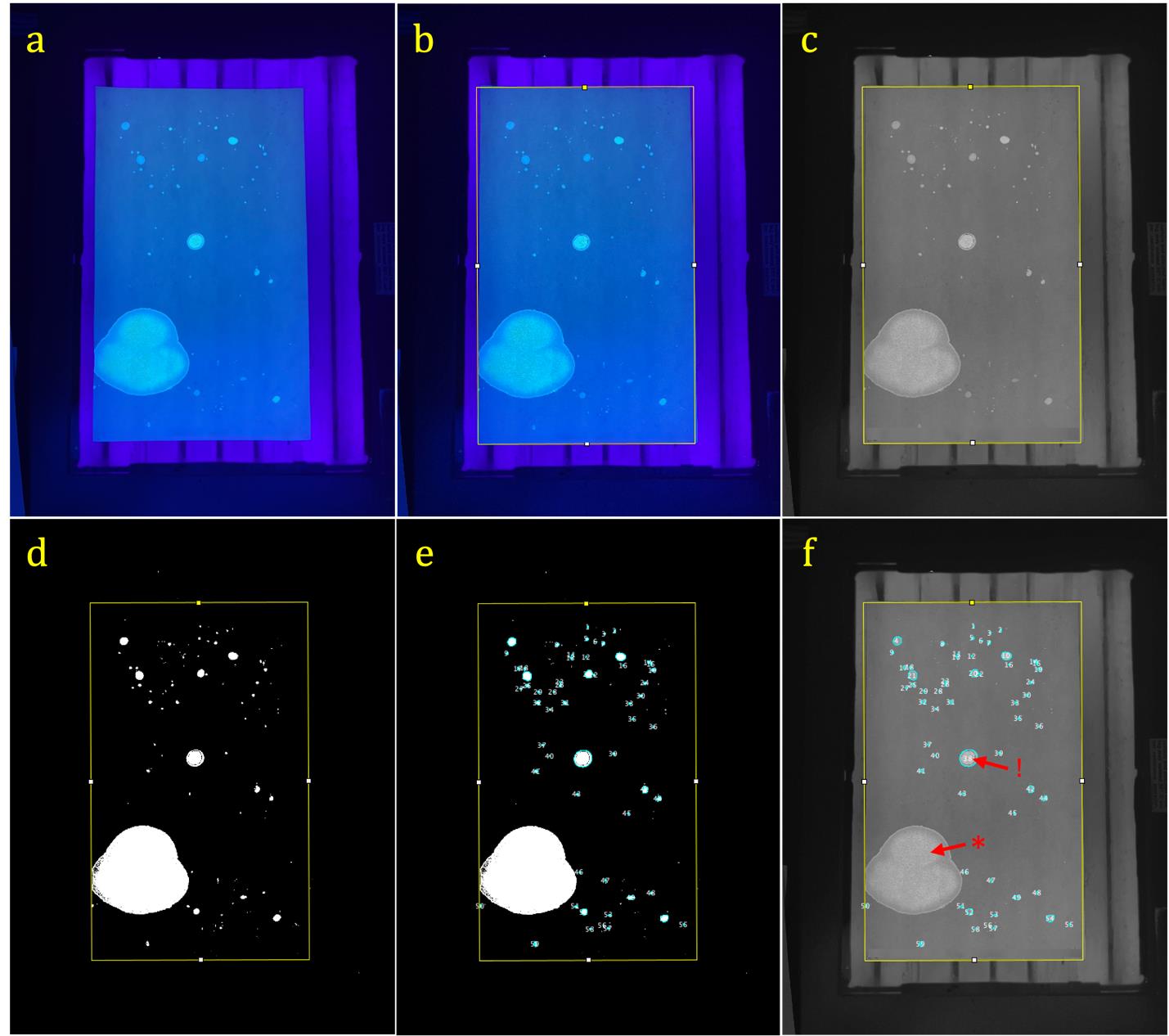
Figure 6. Item 3: Urine countermarking exemplar quantification and analysis steps. (a) Photo of marked Whatman paper from the urine-countermarking test over UV transilluminator imported to ImageJ (steps G1–3b); the paper is laid flat on the surface of the transilluminator, the field of view includes the entire sheet of paper, and the camera angle is close to parallel with the sheet of paper. (b) Rotated rectangle tool outlining the paper to specify the area of analysis (step G3c). (c) Rectangle outlining the paper after conversion to greyscale (step G3d). (d) Threshold applied to create contrast highlighting illuminated marks; noise (i.e., light not produced by markings) is present outside the bounds of the rectangle outline; no noise inside the bounds of the rectangle (step G3e). (e) Analyze particles function applied within the bounds of the analysis area, highlighting (in light blue) the markings that were counted (step G3f). (f) Analyzed photo of Whatman paper in greyscale after the threshold was removed to exhibit which markings were quantified within the specified bounds. *: large urinary spot that fell outside of the specified size range (100–40,000 pixels) and was not counted in the analysis. !: 10 µL stimulus aliquot; this mark will fall within the specified size range and will be included in the count of markings, hence why the formula for calculating the number of markings in the countermarking test is number of markings - 1. Note: This example was obtained from a 16-month-old C57BL6 male mouse. Labels on the Whatman paper pertaining to experimental information have been blurred.
3. Example quantification steps using ImageJ software
a. Import images into your machine.
b. Drag and drop the image into the open ImageJ window.
c. Left-click on the rectangle tool to select the rotated rectangle tool. Using the rotated rectangle tool, outline the paper (Figures 5b and 6b).
d. Select Image, Type, and 8-bit to convert the image to greyscale (Figures 5c and 6c).
e. Under Image, select Threshold; threshold the image so that urine-marked spots within the paper are starkly contrasted against the background of the paper, eliminating as much noise (coloration due to lighting) as possible (Figures 5d and 6d).
f. Once the optimal threshold is achieved, select Analyze and Analyze particles.
i. Under Size (pixel^2), enter 100 as a minimum limit and 40,000 as a maximum. The minimum ensures that no noise from lighting is detected, and the maximum ensures that no large urine spots are detected, as these are not a display of marking behavior but rather pure urination.
ii. Check Display results, Overlay, and Summarize.
iii. Select OK.
iv. A summary window will pop up displaying some values. The only ones that pertain to behavioral quantification in these two assays are count, which is the number of markings present, and the statistic of interest for both assays (Figures 5e/f and 6e/f).
Critical: Double-check that i) you are not quantifying any noise from lighting, ii) you are only capturing pixels that are on the quantified paper, and iii) all large urinary spots have been omitted.
Note: The quantification time is dependent on the experience of the observer.
Urine-marking quantification: The measure of interest in the urine-marking assay is the number of markings deposited on the paper.
Formula: number of markings = total number of markings (“count” value in summary window of ImageJ)
Urine-countermarking quantification: The measure of interest in the urine-countermarking assay is the number of markings deposited on the paper.
Formula: number of markings = total number of markings (‘count’ value in summary window of ImageJ) – 1
Note for countermarking: “Count” will display one redundant mark due to the 10 μL of pooled urine added as a stimulus. You will need to subtract this single mark (-1) from the number of markings reported (Figure 6f).
H. Item 4: Juvenile social interaction, home environment
1. Place the home cages (Figure 7) on the working surface where the observations will be conducted (up to five cages per observer), aligning them in a semi-circle with the long sides of the cages facing the centrally located observer.

Figure 7. Items 4–5: Social-interaction test apparatus. Sagittal view. Depending on the item, this test should either be conducted in the experimental mouse’s home cage (item 4) or a novel cage (item 5). In each case, the cage should be configured as depicted above. Food and water are provided in both items. Some nesting material can be removed for ease of observation. Note: There are no mice in this figure.
2. Place stimulus juvenile mice in the home environment of the experimental mice with the experimental mice, closing the cage afterward.
Note: If not all stimulus mice can be placed in experimental home cages simultaneously, place female stimulus mice first and male stimulus mice second. This will minimize the time in which aggressive behavior by the male mice might manifest before the observation period begins.
3. Allow mice to freely roam the cage for 5 min before removing the stimulus mouse from the cage and ending instantaneous observation. The procedure for instantaneous sampling and behavioral characterizations during the juvenile social-interaction tests are described in Section J.
4. In the circumstance that aggressive behavior between experimental and stimulus mice escalates to the point where there is a threat of wounding for either mouse, the interaction should be immediately terminated. A general heuristic for interrupting due to escalated aggression is after a maximum of three consecutive observation intervals of aggressive behavior.
Notes:
1. See J4, note 2 for scoring after termination due to escalated aggressive behavior.
2. One observer should score five mice per round at the most. Sampling rate should be adjusted for fewer mice being observed by each observer to ensure that a consistent number of intervals is scored. In its entirety, the social-interaction test in the home environment takes approximately 45 min per 20 mice with two observers.
Juvenile social interaction in home environment quantification: details on quantification and analysis can be found in Section J.
I. Item 5: Juvenile social interaction, novel environment
1. On the working surface where observations will be conducted, align up to five novel static cages (Figure 7) in a semi-circle with the long sides of the cages facing the centrally located observer.
2. Place stimulus juvenile mice in each cage and close the cage.
3. Remove experimental mice from their home cage and place them into the novel cage with the stimulus juvenile mice.
Critical: Experimental mice must be placed into the novel cage last to ensure that all exploratory behavior that is observed is done in the presence of the stimulus juvenile mouse.
3. Allow mice to freely roam the cage for 5 min before ending instantaneous observation and removing the stimulus mouse. The procedure for instantaneous sampling and behavioral characterizations during the juvenile social-interaction tests are described in section J.
4. In the circumstance that aggressive behavior between experimental and stimulus mice escalates to the point where there is a threat of wounding for either mouse, the interaction should be immediately terminated. Best judgment should be used on a case-by-case basis, but a general heuristic for interrupting due to escalated aggression is after a maximum of three consecutive observation intervals of aggressive behavior.
Note: See J4, note 2 for scoring after termination due to escalated aggressive behavior.
Critical: Experimental mice should never be exposed to the same juvenile twice to prevent social recognition and decreased interaction [47,48].
Note: One observer should score five mice per round at the most. Sampling rate should be adjusted for fewer mice being observed by each observer to ensure that a consistent number of intervals is scored. In its entirety, the social-interaction test in the home environment takes approximately 45 min per 20 mice with two observers.
Juvenile social interaction in novel environment quantification: details on quantification and analysis can be found in section J.
J. Observation, instantaneous sampling, and quantification: Juvenile social interaction, home and novel environments
1. During the 5-min interaction, observe experimental mice for 1 s sequentially (i.e., each mouse is observed every 5 s), and instances of social behavior initiated by the experimental mouse are recorded on a printed coding sheet for instantaneous sampling (Supplemental Appendix 1). The use of a clipboard will facilitate ease of writing.
2. The observer should scan left to right along the semi-circle of cages, spending 1 s observing each mouse while simultaneously recording behavior.
3. Social behaviors measured in this test span categories of sniffing, aggression, and allogrooming, and are displayed in Table 1.
Table 1. Mouse social behavior ethogram for use in social-interaction tests (adapted from Winslow, 2003) [49]
| Category | Behavior | Description |
|---|---|---|
| Sniffing | Pursuit | Closely follows and chases stimulus animal. |
| Olfactory investigation | Uses nose to inspect any portion of the stimulus mouse’s body, including the tail. Initially, the area of interest is typically the anogenital region of the stimulus animal. | |
| Aggression | Attack bite | Bites are usually focused on the rump or hind of the stimulus mouse, typically associated with an escape/leap. |
| Sideways offensive posture | The subject mouse positions its body perpendicular to the front of the stimulus animal. While having its side facing the head of the stimulus mouse, the subject mouse orients its head toward the stimulus animal's hindquarters. The body of the subject mouse may appear U-shaped. It must occur with a physical attack. | |
| Mount | Appears to attempt to copulate with the stimulus animal. The observed animal may climb and perform pelvic thrusts onto the rump, side, or head of the stimulus animal. | |
| Allogrooming | Allogrooming | Licks, cleans, and/or physically probes the fur of the stimulus mouse. This usually occurs close to the facial, neck, or upper back regions. |
4. Juvenile social interaction in home environment and novel environment quantification. The measure of interest in both the juvenile social interaction in the home environment and novel environment is the same: the percentage of social interaction.
Formula: % social interaction = (# sniffing intervals + # aggression intervals + # allogrooming intervals) / total # intervals × 100,
Notes:
1. Theoretically, the number of intervals scored over 5 min should be 60; however, this may vary slightly between observers due to experience or general speed of observation. Every attempt should be made to train observers to achieve 60 scoring intervals to maximize the reliability of the assessment.
2. If an interaction requires termination due to escalated aggressive behavior, the test should be scored as is (i.e., total # of intervals observed = how many intervals occurred before interaction termination).
K. Item 6: Social/novel object preference test, novel environment
1. On the working surface where the observations will be conducted, align up to five novel large static cages (257 mm × 483 mm × 152 mm) with bedding but without nesting material, food, water bottle, or metal wire lids, in a semi-circle with the long sides of the cages facing the centrally located observer.
2. To assemble the social/novel object preference apparatus in each cage, place a wire mesh cup containing the stimulus same-sex juvenile on one side of the cage and a wire mesh cup containing the novel object on the opposite side (Figure 8). The side on which each is placed should alternate in subsequent cages.
Critical: The cups should be far enough from each other so that the experimental mouse must choose to approach them (i.e., not sit in the middle and have access to both) while still allowing enough room for the mouse to maneuver behind them.
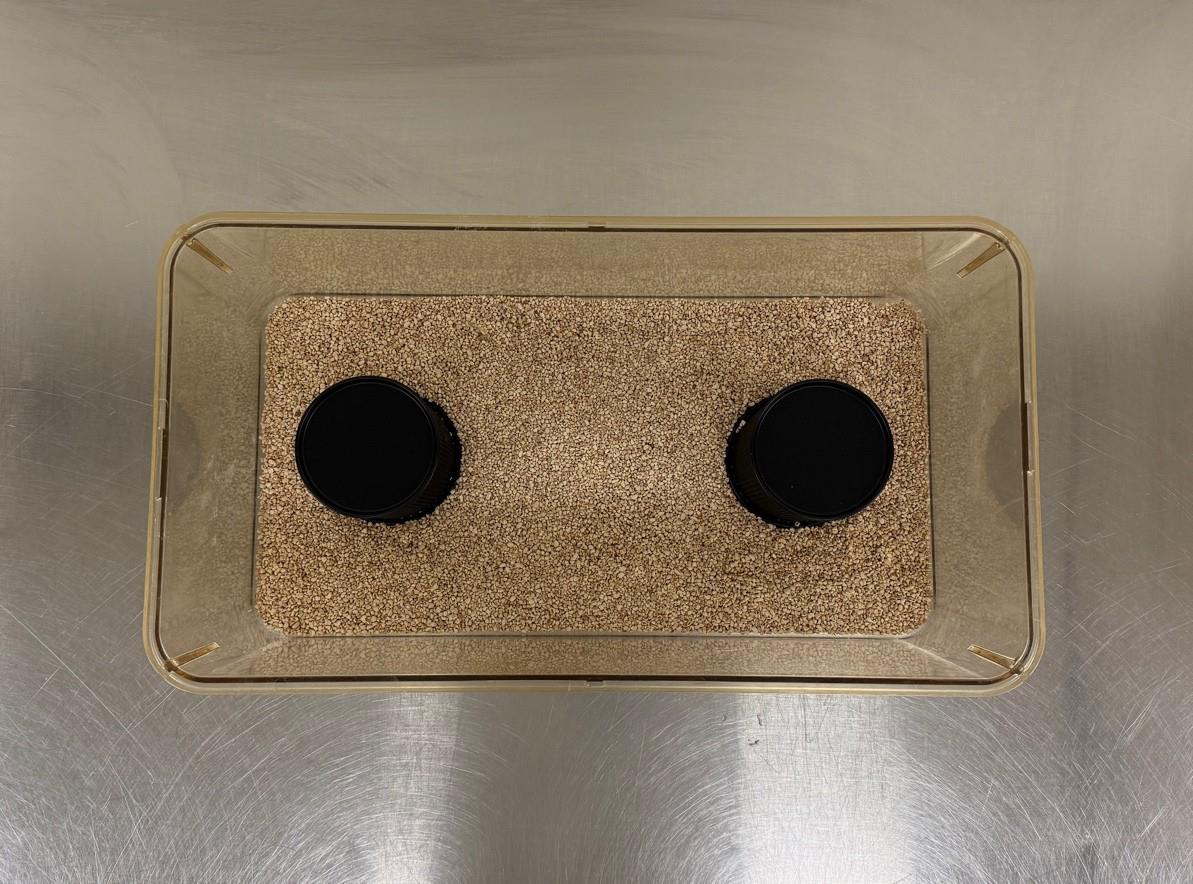
Figure 8. Item 6: Social/novel object preference test apparatus. Transverse view. This test should be conducted in a novel large static cage (257 mm × 483 mm × 152 mm) with bedding but without nesting material, food, water bottle, or metal wire lids. Depicted above is the testing apparatus configuration without the weighted container on top of the wire mesh cups and without a lid. This transverse view above exhibits the spacing between wire mesh cups within the cage; they should be far enough from each other so that the experimental mouse must choose to approach them (i.e., not sit in the middle with access to both) while still allowing enough room for the mouse to maneuver behind them.
3. Place the cup or other container filled with water on each wire mesh cup.
4. Place the experimental mice into the assembled cages and close the filter top (Figure 9).

Figure 9. Item 6: Social/novel object preference test apparatus. Sagittal view. This test should be conducted in a novel large static cage (257 mm × 483 mm × 152 mm) with bedding and the necessary objects to assemble the full testing apparatus. The cage should be configured as depicted above for testing. Note: There are no mice in this picture. The juvenile stimulus mouse would be placed in the wire mesh cup on the left, opposite the wire mesh cup with the novel object.
5. Allow mice to freely roam the cage for 5 min before ending instantaneous observation and removing the experimental mouse. The procedure for instantaneous sampling and behavioral characterizations during the social/novel object preference test is described in Section L.
6. If doing another round of testing: after removing the experimental mice, remove the stimulus mice, then remove the objects, wire mesh cups, and water-filled containers. Clean the objects, wire mesh cups, and water-filled containers with 70% ethanol and allow them to dry for 1 min before reusing them.
Critical: Experimental mice should never be exposed to the same juvenile twice to prevent social recognition and decreased interaction [47,48].
Note: One observer should score five mice per round at the most. The sampling rate should be adjusted for fewer mice being observed by each observer to ensure that a consistent number of intervals is scored. In its entirety, the social/novel object preference test takes approximately 1.25 h per 20 mice with two observers.
L. Observation, instantaneous sampling, and quantification: social/novel object preference test
1. The instantaneous sampling method used for this test is similar to that described in the juvenile social interaction tests; however, the behaviors recorded are different.
a. Behaviors measured in the social/novel object preference test are simply interactions with the stimulus mouse and interactions with the object.
b. Experimental mice are considered to be interacting with either the stimulus mouse or the object when actively sniffing or digging around the wire mesh cup but also when they are oriented toward it (i.e., stretch attend posture, rearing or climbing on it) in close proximity to the wire mesh cup.
2. Each experimental mouse should be observed for 1 s, every 5 s, via the aforementioned scanning method. The behaviors of the experimental mouse are recorded on a printed coding sheet for instantaneous sampling (Supplemental Appendix 1). The use of a clipboard will facilitate ease of writing.
3. Social/novel object preference test quantification: The measure of interest in the social/novel object preference test is the percentage of social approach.
Formula: % social approach = (# intervals spent interacting with stimulus mouse - # intervals spent interacting with object) / total # intervals spent interacting × 100.
Note: Theoretically, the number of intervals scored over 5 min should be 60; however, this may vary slightly between observers due to experience or general speed of observation. Every attempt should be made to train observers to achieve 60 scoring intervals to maximize the reliability of the assessment.
M. Item 7: Nest building, home environment
1. Approximately 1 h before the dark phase, remove all nesting material from the experimental mouse home cage. If testing mice that are group-housed or in another housing paradigm, experimental mice should be transferred along with bedding from their home cage into novel small static cages (186 mm × 298 mm × 128 mm) with food and water but without nesting material. Mice are returned to their home cage immediately after assessment.
2. Place one new intact cotton nestlet weighing 3.0 g in the mouse cage and allow mice to build their nests overnight.
Note: Nestlets are typically not made to a quantitative weight standard. To standardize the amount of nesting material provided, weigh nestlets and provide exactly 3.0 g of nesting material [50].
3. The following morning, while the mouse is still in the cage and making sure not to disturb the natural structure of the built nest, assess the nest visually using the published scale provided below [50,51].
Note: The assessment of nests the following morning does not preclude scheduling another test of the mSFI in the AM session following a PM nest-building test. The assessment of nests can occur alongside another test of the mSFI.
4. Nest-building quantification: Nests are assessed visually and subsequently rated based on the 5-point scale detailed in Table 2. Examples of what a nest of each score looks like have been described in detail elsewhere [50,51].
Table 2. Nest-building test scoring criteria (adapted from Deacon, 2006) [50]
| Nest score | Criteria |
|---|---|
| 1 | Nestlet not noticeably touched: more than 90% intact. |
| 2 | Nestlet partially torn: 50%–90% intact. |
| 3 | Nestlet mostly shredded but no identifiable nest site present: 50%–10% intact. |
| 4 | Mouse has constructed an identifiable but flat nest: nestlet more than 90% shredded. |
| 5 | A near-perfect nest. More than 90% of the nestlet is shredded and formed into a crater-like structure with walls higher than the mouse body height. |
Note: In case mice need to be transferred to new cages, the placement of nests takes 30 min maximum per 20 mice in its entirety with two observers. If no mice need to be transferred, it takes less time. Visual assessment of nests the following morning with two observers takes 15 min maximum per 20 mice.
Data analysis
Each item produces a quantitative score that represents behavioral performance in its respective dimension of social behavior (e.g., a percentage urine preference is calculated from the olfactory test). Similar to the standard procedure in deficit accumulation-based frailty indices [22,28], an mSFI from 0 to 1 is calculated for each mouse based on the extent of deviation from mean quantitative scores obtained in an initial sample of young adult mice (3–4 months of age) that undergoes mSFI testing. Thus, before using this index in mice beyond four months of age, it first needs to be applied in a reference sample of sex- and strain-matched young adult mice to obtain reference values (see General Note 5). In the case that you plan to make a cross-sectional comparison between groups of same-strain mice, your reference sample should be sex-matched. Reference samples can be used across experiments but must match the comparison group in sex and strain. An mSFI of 0 represents a mouse with no impairment in social functioning, and an mSFI of 1 represents a mouse with maximal impairment in social functioning. Between these minimal and maximal scores exists a gradient of impairment levels with greater values representing greater social frailty.
To calculate the comprehensive mSFI score, continuous quantitative scores in each item are assigned a scaled score based on the following cutoffs: scores less than ±1 standard deviation (SD) of the reference sample mean are assigned a 0, ≤ 2 SD = 0.25, ≤ 3 SD = 0.5, ≤ 4 SD = 0.75, > 4 SD = 1. It should be noted that this methodology is not used in the case of the nest-building assessment because it is scored on a discrete 5-point scale. Instead, scaled scores were assigned so that nest scores of 5 receive a score of 0, 4 = 0.25, 3 = 0.5, 2 = 0.75, and 1 = 5. Scaled index scores for each mouse in all seven items are summed and divided by seven, the total number of items, to obtain a final mSFI score (see General Note 6).
The predefined spreadsheet template included as a supplement to this protocol automates the mSFI calculation described above (Supplementary Appendix 2). After collection, data from all items of the mSFI should be manually entered and stored in the appropriate location within the “msfi” sheet. Quantitative scores demarcating the standard deviation cutoffs for each item of the mSFI based on an initial sample of sex- and strain-matched young adult mice should be entered into the appropriate location within the “reference” sheet. Once data is entered into the “msfi” sheet, predefined formulas will automatically calculate quantitative scores for the measure of interest in each item of the mSFI as well as their scaled scores based on the reference sample values in the “reference” sheet.
Validation of protocol
This protocol has been used and validated in the following open-access research article:
Collinge et al. [44]. The mouse social frailty index: A novel behavioral assessment for impaired social functioning in mice. GeroScience.
Reliability
The degree of reliability between multiple trained observers needs to be assessed. Prior to administering the mSFI, observers should undergo rigorous observational training by being exposed to live behavioral sessions, shadowing experienced observers, and scoring behavior from recorded video sessions (see General Note 7). Statistically, reliability between observers can be ascertained by using the intra-class correlation coefficient (ICC) [52]. For example, Table 3 shows data from our lab showing ICC in the good-to-excellent range obtained between experienced and inexperienced observers in all items, after shadowing administrations of the mSFI.
Table 3. Intraclass correlation coefficients (ICC) for items of the mSFI.
ICC (A,2) estimates and 95% confidence intervals (CI) between experienced and inexperienced observers based on an average rating (k = 2), absolute agreement two-way random-effects model. All obtained estimates are in the good-to-excellent range [52], although lower bound estimates for SNoP ICC descend into the fair range. Data was obtained for each item from samples of C57BL6 mice (N = 15–36). Note for significance testing: H0: ICC = 0; H1: ICC > 0. Analyses conducted using R statistical software (v4.4.1[53]) and the irr R package (v0.84.1[54]). S.I.: Social interaction. SNoP: Social/novel object preference.
| Item | ICC [95% CI] | F | df1 | df2 | p |
|---|---|---|---|---|---|
| 1. Olfactory | 0.927 [0.724 – 0.975] | 19.10 | 20 | 7.91 | <0.001 |
| 2. Marking | 0.999 [0.998 – 1.000] | 1245.24 | 14 | 14.10 | <0.001 |
| 3. Countermarking | 0.998 [0.994 – 0.999] | 530.60 | 14 | 14.80 | <0.001 |
| 4. S.I., home | 0.921 [0.643 – 0.976] | 18.59 | 16 | 6.30 | <0.001 |
| 5. S.I., novel | 0.957 [0.827 – 0.985] | 34.40 | 25 | 7.29 | <0.001 |
| 6. SNoP | 0.704 [0.414 – 0.850] | 3.31 | 35 | 35.00 | <0.001 |
| 7. Nest building | 0.986 [0.962 – 0.995] | 76.47 | 16 | 16.02 | <0.001 |
Validity
Multiple aspects of the validity [55] of the mSFI were demonstrated by Collinge et al. [44].
1. Content validity: As a construct, social frailty has been broadly defined as an age-related lack of social skills and resources necessary to achieve well-being [38]. To measure this construct in mice, the mSFI was designed as an assessment of age-related impairments in ethologically relevant behaviors for the adaptive social functioning of mice of both sexes. Mice are a highly social species that naturally adopt a despotic social hierarchy maintained by the territorial aggression of males [56]. They socially interact through odorant communication via scent marking and production of pheromones, direct social interaction and fixed action patterns, and functional behaviors such as communal nesting, courtship, and parenting [56–59]. Accordingly, we created three sub-domains of mouse social behavior—social communication, social interaction, and social functional ability—and sampled from commonly used assays in each domain. From social communication, the mSFI evaluates spontaneous urine marking behavior, urine marking in responses to the urinary marks of a conspecific, and olfactory discrimination of a social stimulus. Social interaction is evaluated in spontaneous social interactions in a home environment, the tradeoff between social interaction and exploring a novel environment, and the preference for social stimuli. Social functional ability is evaluated through nest-building ability. The ethological repertoire of mice is exceedingly complex, and this sampling technique creates an index that is representative of each sub-domain of mouse social behavior, supporting its content validity—the extent to which the items of a test theoretically represent all possible items relevant to the construct the test attempts to measure—while also maintaining the feasibility and wieldiness of the mSFI.
2. Criterion validity: The mSFI was designed to detect age-related changes in social behavior in order to quantify social dimensions of the aging process. Therefore, it should be sensitive to changes in biological age. In practice, the mSFI was found to be sensitive to increases in the chronological age of C57BL6 group-housed mice, showing significant progressive increases that parallelled increases in chronological age in both sexes of mice (see Collinge et al. [44], Figure 1B, C). The mSFI also demonstrated sensitivity to the accelerated biological aging characteristic of progeroid syndromes in two distinct bona fide mouse models of segmental progeroid syndromes (Ercc1-/Δ [60] and Xpg-/- [61]) compared to wild-type mice of the same chronological age in both sexes (see Collinge et al. [44], Figure 4B, C). Further, to support the criterion validity of the mSFI, it should be associated with other measures of biological aging in mice. We demonstrated a strong positive correlation between the mSFI and a widely used and validated measure of biological aging in mice, the 31-item Clinical Frailty Index (CFI) [28], in both male and female group-housed C57BL6 mice (see Collinge et al. [44], Figure 2B). Additionally, we found parallel increases in mSFI and CFI scores in both Ercc1-/Δ and Xpg-/- mice compared to wild types in both sexes (see Collinge et al. [44], Figure 4D, E).
3. Construct validity: As a construct, social frailty is still relatively new. Before our work [44], it had only been formally defined in human literature [38]. Relating to the current definition of social frailty as a construct, we demonstrated that the mSFI measures age-related impairment of social skills that would impact a mouse’s well-being, and therefore decrease its survival given the reliance of this species on social functions. The construct validity of the mSFI will be further informed by longitudinal studies examining the mSFI’s relationship with aging-related health outcomes and mortality.
Reproducibility
We demonstrated reproducibility by conducting the experiments in Collinge et al. [44] at multiple sites with unique research animal services departments and resources. Specifically, the mSFI was performed in multiple testing sites at the University of Minnesota (Bartolomucci, Niedernhofer, and Pacak Labs) and at the University of Wisconsin-Madison (Lamming Lab).
General notes and troubleshooting
General notes
1. The mSFI was initially validated for application in C57BL6 mice, the most commonly used inbred laboratory mouse strain [44]. We have demonstrated the validity of the mSFI for application in other strains through our usage of Ercc-/Δ, Xpg-/-, and their wild-type strains [44].
2. In case pooled intact male and female strain-matched urine cannot be obtained from a vendor, fresh urine can be obtained in-house (see [62] for a review on urine collection methods), with the urine in a 1:1 female-to-male ratio. Proper storage practices should be followed to ensure the viability of urinary stimuli.
3. Although the mSFI requires behavioral testing environments constructed using caging materials common to most animal research facilities, the demand for fulfilling the caging needs of the mSFI varies with the number of mice being tested. If the number of mice being tested is high, we recommend setting up cage arrangements far in advance.
4. There is no specific number of observers to conduct the mSFI, and an absolute optimal number of observers depends on a plethora of factors that differ between laboratories and experiments. However, we have found that, in our laboratory, a maximum of 15 mice per observer works well to balance efficiency with practicality in the administration of the mSFI. Execution time will vary depending on resources, expertise, and number of mice. We have provided examples of administration times for each item of the mSFI assuming two experienced observers testing 20 mice.
5. mSFI scores are based on the extent of deviation from mean quantitative scores obtained in an initial normative sample of young adult mice. Due to this quantitative design, the mSFI will only be as good as the normative sample used to derive it. Therefore, it is imperative that the normative sample used to derive mSFI scores matches the experimental sample in sex and strain [63]. Just as for the widely used 31-item Clinical Frailty Index [28], there is no gold standard for the size of the reference sample for the mSFI. Previous work [44] used sample sizes between 4 and 14.
6. The deficit accumulation model of frailty posits that the more health deficits individuals accumulate, the more at risk they are of adverse outcomes [64]. Deficit accumulation-based indices (including the mSFI) quantify a proportion of deficits present out of those measured to account for the number of deficits examined. As such, the more items included, i) the better an index distinguishes between graded levels of deficit accumulation, ii) the less susceptible it is to ceiling effects whereby high levels of frailty are reached by the presence of a few deficits, and iii) the more useful it will be in capturing meaningful differences in aging [28,45,65]. Deficit accumulation-based indices of physical frailty in both humans and mice commonly include 20–30 items [22,28,45,65]. Social behavior can be very time intensive and disruptive to measure in mice. Those concerns are only amplified in longitudinal experiments relevant to aging research that involve large cohorts and repeated measurements. Including many items (e.g., > 30) would render the mSFI unwieldy. The mSFI is optimized with seven commonly used behavioral tests across multiple domains of mouse social behavior to balance parsimony with high-throughput data and minimize the behavioral and physiological impact of the index while ensuring its large-scale application. Notably, i) a graded increase in social frailty was seen across the lifespan, ii) ceiling effects were not observed in application, with a submaximal limit in the mSFI at 0.57, below the 0.7 limit commonly observed in human frailty indices [66,67], and iii) the mSFI was sensitive to progeria-induced acceleration of biological aging [44].
7. To facilitate interrater reliability and accurate use of the mSFI, it is recommended that observers i) be well-versed in standard mouse behavioral testing practices, and ii) practice observing and scoring each item of the mSFI in a low-stakes environment prior to administration. Such standard mouse behavioral testing practices include but are not limited to limiting sudden noises, lighting changes, and vibrations, avoiding the use of fragrances, use of proper PPE, habituating mice to testing environments, and conducting proper handling practices. To practice observing and scoring items of the mSFI, new observers can be trained during a live administration of the mSFI (i.e., coding observations “over the shoulder” of an experienced observer) or via behavioral recordings of the mSFI outside of live administration that have already been observed and scored by an experienced observer. In case mSFI items will be recorded for practice purposes, adequate permission should be obtained from institutional ethics committees. In either method, results from new observers should be compared with those of experienced observers via ICC and reach a good-to-excellent agreement in all items of the mSFI (for reading on ICC analysis, see [68]; for commonly cited ICC cutoffs, see [52]).
Supplementary information
The following supporting information can be downloaded here:
Behavioral coding sheet for instantaneous sampling.
Spreadsheet template for automated mSFI computation.
Acknowledgments
Supported by NIH/NIA R61 AG078520, MN Partnership for Biotechnology and Molecular Genomics #18.4, and IBP Grant Accelerator Program to A.B. Drs. Dudley W. Lamming, Christina A. Pacak, Ingrid van der Pluijm, and Laura Niedernhofer are acknowledged for providing mouse models and/or resources used in the original publication in GeroScience. Amisha Undavia, Vanessa Lozano Wun, and Adam Haag are acknowledged for their help with the study. Patricia Smith and Diana Creswell and the staff of the Research Animal Resources at the University of Minnesota are also acknowledged for their instrumental role in animal care. This protocol was originally described and validated in the following open-access research article: Collinge et al. [44].
Competing interests
The authors declare no competing interests.
Ethical considerations
All mice used in the development of this protocol were cared for and maintained according to ethical guidelines set by the US National Institutes of Health. Experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Minnesota-Twin Cities (Minneapolis, MN, USA) or the William S. Middleton Memorial Veterans Hospital (Madison, WI, USA).
References
- He, W., Goodkind, D. and Kowal, P. (2016). An aging world: 2015 (No. P95/16-1; International Population Reports). U.S. Census Bureau.
- Niccoli, T. and Partridge, L. (2012). Ageing as a Risk Factor for Disease. Curr Biol. 22(17): R741–R752. https://doi.org/10.1016/j.cub.2012.07.024
- Vos, T., Flaxman, A. D., Naghavi, M., Lozano, R., Michaud, C., Ezzati, M., Shibuya, K., Salomon, J. A., Abdalla, S., Aboyans, V., et al. (2012). Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 380(9859): 2163–2196. https://doi.org/10.1016/s0140-6736(12)61729-2
- Kirkwood, T. B. (2005). Understanding the Odd Science of Aging. Cell. 120(4): 437–447. https://doi.org/10.1016/j.cell.2005.01.027
- Kuo, P. L., Schrack, J. A., Levine, M. E., Shardell, M. D., Simonsick, E. M., Chia, C. W., Moore, A. Z., Tanaka, T., An, Y., Karikkineth, A., et al. (2022). Longitudinal phenotypic aging metrics in the Baltimore Longitudinal Study of Aging. Nat Aging. 2(7): 635–643. https://doi.org/10.1038/s43587-022-00243-7
- Elliott, M. L., Caspi, A., Houts, R. M., Ambler, A., Broadbent, J. M., Hancox, R. J., Harrington, H., Hogan, S., Keenan, R., Knodt, A., et al. (2021). Disparities in the pace of biological aging among midlife adults of the same chronological age have implications for future frailty risk and policy. Nat Aging. 1(3): 295–308. https://doi.org/10.1038/s43587-021-00044-4
- Horvath, S. and Raj, K. (2018). DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. 19(6): 371–384. https://doi.org/10.1038/s41576-018-0004-3
- Jaul, E. and Barron, J. (2017). Age-Related Diseases and Clinical and Public Health Implications for the 85 Years Old and Over Population. Front Public Health. 5: e00335. https://doi.org/10.3389/fpubh.2017.00335
- Rowe, J. W. and Kahn, R. L. (1987). Human Aging: Usual and Successful. Science (1979). 237(4811): 143–149. https://doi.org/10.1126/science.3299702
- Lowsky, D. J., Olshansky, S. J., Bhattacharya, J. and Goldman, D. P. (2013). Heterogeneity in Healthy Aging. J Gerontol A Biol Sci Med Sci. 69(6): 640–649. https://doi.org/10.1093/gerona/glt162
- Barzilai, N., Cuervo, A. M. and Austad, S. (2018). Aging as a Biological Target for Prevention and Therapy. JAMA. 320(13): 1321. https://doi.org/10.1001/jama.2018.9562
- Kennedy, B. K., Berger, S. L., Brunet, A., Campisi, J., Cuervo, A. M., Epel, E. S., Franceschi, C., Lithgow, G. J., Morimoto, R. I., Pessin, J. E., et al. (2014). Geroscience: Linking Aging to Chronic Disease. Cell. 159(4): 709–713. https://doi.org/10.1016/j.cell.2014.10.039
- Sierra, F. (2016). The Emergence of Geroscience as an Interdisciplinary Approach to the Enhancement of Health Span and Life Span. Cold Spring Harb Perspect Med. 6(4): a025163. https://doi.org/10.1101/cshperspect.a025163
- Olshansky, S. J. (2018). From Lifespan to Healthspan. JAMA. 320(13): 1323. https://doi.org/10.1001/jama.2018.12621
- Belsky, D. W., Caspi, A., Corcoran, D. L., Sugden, K., Poulton, R., Arseneault, L., Baccarelli, A., Chamarti, K., Gao, X., Hannon, E., et al. (2022). DunedinPACE, a DNA methylation biomarker of the pace of aging. eLife. 11: e73420. https://doi.org/10.7554/elife.73420
- Crimmins, E. M., Thyagarajan, B., Kim, J. K., Weir, D. and Faul, J. (2021). Quest for a summary measure of biological age: the health and retirement study. Geroscience. 43(1): 395–408. https://doi.org/10.1007/s11357-021-00325-1
- Diebel, L. W. M. and Rockwood, K. (2021). Determination of Biological Age: Geriatric Assessment vs Biological Biomarkers. Curr Oncol Rep. 23(9): 104. https://doi.org/10.1007/s11912-021-01097-9
- Clegg, A., Young, J., Iliffe, S., Rikkert, M. O. and Rockwood, K. (2013). Frailty in elderly people. Lancet. 381(9868): 752–762. https://doi.org/10.1016/s0140-6736(12)62167-9
- Rockwood, K., Fox, R. A., Stolee, P., Robertson, D., & Beattie, B. L. (1994). Frailty in elderly people: An evolving concept. CMAJ. 150(4): 489–495. https://pubmed.ncbi.nlm.nih.gov/8313261/
- Mitnitski, A. B., Mogilner, A. J. and Rockwood, K. (2001). Accumulation of Deficits as a Proxy Measure of Aging. Sci World. 1: 323–336. https://doi.org/10.1100/tsw.2001.58
- Fried, L. P., Tangen, C. M., Walston, J., Newman, A. B., Hirsch, C., Gottdiener, J., Seeman, T., Tracy, R., Kop, W. J., Burke, G., et al. (2001). Frailty in Older Adults: Evidence for a Phenotype. J Gerontol A Biol Sci Med Sci. 56(3): M146–M157. https://doi.org/10.1093/gerona/56.3.m146
- Searle, S. D., Mitnitski, A., Gahbauer, E. A., Gill, T. M. and Rockwood, K. (2008). A standard procedure for creating a frailty index. BMC Geriatr. 8(1): 24. https://doi.org/10.1186/1471-2318-8-24
- Farhat, J. S., Velanovich, V., Falvo, A. J., Horst, H. M., Swartz, A., Patton, J. H. and Rubinfeld, I. S. (2012). Are the frail destined to fail? Frailty index as predictor of surgical morbidity and mortality in the elderly. J Trauma Acute Care Surg. 72(6): 1526–1531. https://doi.org/10.1097/ta.0b013e3182542fab
- Dent, E., Chapman, I., Howell, S., Piantadosi, C. and Visvanathan, R. (2013). Frailty and functional decline indices predict poor outcomes in hospitalised older people. Age Ageing. 43(4): 477–484. https://doi.org/10.1093/ageing/aft181
- Clegg, A., Bates, C., Young, J., Ryan, R., Nichols, L., Ann Teale, E., Mohammed, M. A., Parry, J. and Marshall, T. (2016). Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 45(3): 353–360. https://doi.org/10.1093/ageing/afw039
- Kim, S., Myers, L., Wyckoff, J., Cherry, K. E. and Jazwinski, S. M. (2017). The frailty index outperforms DNA methylation age and its derivatives as an indicator of biological age. Geroscience. 39(1): 83–92. https://doi.org/10.1007/s11357-017-9960-3
- Kojima, G., Iliffe, S. and Walters, K. (2017). Frailty index as a predictor of mortality: a systematic review and meta-analysis. Age Ageing. 47(2): 193–200. https://doi.org/10.1093/ageing/afx162
- Whitehead, J. C., Hildebrand, B. A., Sun, M., Rockwood, M. R., Rose, R. A., Rockwood, K. and Howlett, S. E. (2013). A Clinical Frailty Index in Aging Mice: Comparisons With Frailty Index Data in Humans. J Gerontol A Biol Sci Med Sci. 69(6): 621–632. https://doi.org/10.1093/gerona/glt136
- Rockwood, K., Blodgett, J. M., Theou, O., Sun, M. H., Feridooni, H. A., Mitnitski, A., Rose, R. A., Godin, J., Gregson, E., Howlett, S. E., et al. (2017). A Frailty Index Based On Deficit Accumulation Quantifies Mortality Risk in Humans and in Mice. Sci Rep. 7(1): e1038/srep43068. https://doi.org/10.1038/srep43068
- Schultz, M. B., Kane, A. E., Mitchell, S. J., MacArthur, M. R., Warner, E., Vogel, D. S., Mitchell, J. R., Howlett, S. E., Bonkowski, M. S., Sinclair, D. A., et al. (2020). Age and life expectancy clocks based on machine learning analysis of mouse frailty. Nat Commun. 11(1): 4618. https://doi.org/10.1038/s41467-020-18446-0
- Snyder-Mackler, N., Burger, J. R., Gaydosh, L., Belsky, D. W., Noppert, G. A., Campos, F. A., Bartolomucci, A., Yang, Y. C., Aiello, A. E., O’Rand, A., et al. (2020). Social determinants of health and survival in humans and other animals. Science. 368(6493): eaax9553. https://doi.org/10.1126/science.aax9553
- Kivimäki, M., Bartolomucci, A. and Kawachi, I. (2022). The multiple roles of life stress in metabolic disorders. Nat Rev Endocrinol. 19(1): 10–27. https://doi.org/10.1038/s41574-022-00746-8
- Schrempft, S., Belsky, D. W., Draganski, B., Kliegel, M., Vollenweider, P., Marques-Vidal, P., Preisig, M. and Stringhini, S. (2021). Associations Between Life-Course Socioeconomic Conditions and the Pace of Aging. J Gerontol A Biol Sci Med Sci. 77(11): 2257–2264. https://doi.org/10.1093/gerona/glab383
- Stringhini, S., Carmeli, C., Jokela, M., Avendaño, M., Muennig, P., Guida, F., Ricceri, F., d'Errico, A., Barros, H., Bochud, M., et al. (2017). Socioeconomic status and the 25 × 25 risk factors as determinants of premature mortality: a multicohort study and meta-analysis of 1·7 million men and women. Lancet. 389(10075): 1229–1237. https://doi.org/10.1016/s0140-6736(16)32380-7
- Lyons, C. E., Razzoli, M. and Bartolomucci, A. (2023). The impact of life stress on hallmarks of aging and accelerated senescence: Connections in sickness and in health. Neurosci Biobehav Rev. 153: 105359. https://doi.org/10.1016/j.neubiorev.2023.105359
- Razzoli, M., Nyuyki-Dufe, K., Chen, B. H. and Bartolomucci, A. (2023). Contextual modifiers of healthspan, lifespan, and epigenome in mice under chronic social stress. Proc Natl Acad Sci USA. 120(16): e2211755120. https://doi.org/10.1073/pnas.2211755120
- Razzoli, M., Nyuyki‐Dufe, K., Gurney, A., Erickson, C., McCallum, J., Spielman, N., Marzullo, M., Patricelli, J., Kurata, M., Pope, E. A., et al. (2018). Social stress shortens lifespan in mice. Aging Cell. 17(4): e12778. https://doi.org/10.1111/acel.12778
- Bunt, S., Steverink, N., Olthof, J., van der Schans, C. P. and Hobbelen, J. S. M. (2017). Social frailty in older adults: a scoping review. Eur J Ageing. 14(3): 323–334. https://doi.org/10.1007/s10433-017-0414-7
- Shah, S. J., Oreper, S., Jeon, S. Y., Boscardin, W. J., Fang, M. C. and Covinsky, K. E. (2023). Social Frailty Index: Development and validation of an index of social attributes predictive of mortality in older adults. Proc Natl Acad Sci USA. 120(7): e2209414120. https://doi.org/10.1073/pnas.2209414120
- Bartolomucci, A., Kane, A. E., Gaydosh, L., Razzoli, M., McCoy, B. M., Ehninger, D., Chen, B. H., Howlett, S. E. and Snyder-Mackler, N. (2024). Animal Models Relevant for Geroscience: Current Trends and Future Perspectives in Biomarkers, and Measures of Biological Aging. J Gerontol A Biol Sci Med Sci. 79(9): e1093/gerona/glae135. https://doi.org/10.1093/gerona/glae135
- Boyer, F., Jaouen, F., Ibrahim, E. C. and Gascon, E. (2019). Deficits in Social Behavior Precede Cognitive Decline in Middle-Aged Mice. Front Behav Neurosci. 13: e00055. https://doi.org/10.3389/fnbeh.2019.00055
- Moran, J. M., Jolly, E. and Mitchell, J. P. (2012). Social-Cognitive Deficits in Normal Aging. J Neurosci. 32(16): 5553–5561. https://doi.org/10.1523/jneurosci.5511-11.2012
- Prediger, R. D., Batista, L. C. and Takahashi, R. N. (2005). Caffeine reverses age-related deficits in olfactory discrimination and social recognition memory in rats. Neurobiol Aging. 26(6): 957–964. https://doi.org/10.1016/j.neurobiolaging.2004.08.012
- Collinge, C. W., Razzoli, M., Mansk, R., McGonigle, S., Lamming, D. W., Pacak, C. A., van der Pluijm, I., Niedernhofer, L. and Bartolomucci, A. (2024). The mouse Social Frailty Index (mSFI): a novel behavioral assessment for impaired social functioning in aging mice. Geroscience. 47(1): 85–107. https://doi.org/10.1007/s11357-024-01263-4
- Rockwood, K. and Mitnitski, A. (2007). Frailty in Relation to the Accumulation of Deficits. J Gerontol A. 62(7): 722–727. https://doi.org/10.1093/gerona/62.7.722
- Schneider, C. A., Rasband, W. S. and Eliceiri, K. W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 9(7): 671–675. https://doi.org/10.1038/nmeth.2089
- Bluthé, R. M., Gheusi, G. and Dantzer, R. (1993). Gonadal steroids influence the involvement of arginine vasopressin in social recognition in mice. Psychoneuroendocrinology. 18(4): 323–335. https://doi.org/10.1016/0306-4530(93)90028-j
- Dantzer, R. (1999). Vasopressin, gonadal steroids and social recognition. Prog Brain Res. 119: 409–414. https://doi.org/10.1016/s0079-6123(08)61584-8
- Winslow, J. T. (2003). Mouse social recognition and preference. Curr. Protoc. Neurosci. 22(1):8.16.1-8.16.16. https://doi.org/10.1002/0471142301.ns0816s22
- Deacon, R. M. (2006). Assessing nest building in mice. Nat Protoc. 1(3): 1117–1119. https://doi.org/10.1038/nprot.2006.170
- Deacon, R. (2012). Assessing Burrowing, Nest Construction, and Hoarding in Mice. J Visualized Exp. 59: e2607. https://doi.org/10.3791/2607
- Cicchetti, D. V. (1994). Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess. 6(4): 284–290. https://doi.org/10.1037//1040-3590.6.4.284
- R Core Team. (2024). R: A langauge and Environment for Statistical Computing (Version 4.4.1) [Computer software]. R Foundation for Statistical Computing. https://www.R-project.org/
- Gamer, M., Lemon, J. and Singh, I. F. P. (2019). irr: Various Coefficients of Interrater Reliability and Agreement (Version 0.84.1) [Computer software]. https://cran.r-project.org/web/packages/irr/index.html
- Cronbach, L. J. and Meehl, P. E. (1955). Construct validity in psychological tests.. Psychol Bull. 52(4): 281–302. https://doi.org/10.1037/h0040957
- Miczek, K. A., Maxson, S. C., Fish, E. W. and Faccidomo, S. (2001). Aggressive behavioral phenotypes in mice. Behav Brain Res. 125: 167–181. https://doi.org/10.1016/s0166-4328(01)00298-4
- Arakawa, H., Blanchard, D. C., Arakawa, K., Dunlap, C. and Blanchard, R. J. (2008). Scent marking behavior as an odorant communication in mice. Neurosci Biobehav Rev. 32(7): 1236–1248. https://doi.org/10.1016/j.neubiorev.2008.05.012
- Van de Weerd, H. A., Van Loo, P. L. P., Van Zutphen, L. F. M., Koolhaas, J. M. and Baumans, V. (1997). Preferences for nesting material as environmental enrichment for laboratory mice. Lab Anim. 31(2): 133–143. https://doi.org/10.1258/002367797780600152
- Silverman, J. L., Yang, M., Lord, C. and Crawley, J. N. (2010). Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 11(7): 490–502. https://doi.org/10.1038/nrn2851
- Weeda, G., Donker, I., de Wit, J., Morreau, H., Janssens, R., Vissers, C., Nigg, A., van Steeg, H., Bootsma, D., Hoeijmakers, J., et al. (1997). Disruption of mouse ERCC1 results in a novel repair syndrome with growth failure, nuclear abnormalities and senescence. Curr Biol. 7(6): 427–439. https://doi.org/10.1016/s0960-9822(06)00190-4
- Barnhoorn, S., Uittenboogaard, L. M., Jaarsma, D., Vermeij, W. P., Tresini, M., Weymaere, M., Menoni, H., Brandt, R. M. C., de Waard, M. C., Botter, S. M., et al. (2014). Cell-Autonomous Progeroid Changes in Conditional Mouse Models for Repair Endonuclease XPG Deficiency. PLos Genet. 10(10): e1004686. https://doi.org/10.1371/journal.pgen.1004686
- Kurien, B. T., Everds, N. E. and Scofield, R. H. (2004). Experimental animal urine collection: a review. Lab Anim. 38(4): 333–361. https://doi.org/10.1258/0023677041958945
- Kopachev, N., Netser, S. and Wagner, S. (2022). Sex-dependent features of social behavior differ between distinct laboratory mouse strains and their mixed offspring. iScience. 25(2): 103735. https://doi.org/10.1016/j.isci.2022.103735
- Rockwood, K. and Mitnitski, A. (2011). Frailty Defined by Deficit Accumulation and Geriatric Medicine Defined by Frailty. Clin Geriatr Med. 27(1): 17–26. https://doi.org/10.1016/j.cger.2010.08.008
- Parks, R. J., Fares, E., MacDonald, J. K., Ernst, M. C., Sinal, C. J., Rockwood, K. and Howlett, S. E. (2011). A Procedure for Creating a Frailty Index Based on Deficit Accumulation in Aging Mice. J Gerontol A. 67A(3): 217–227. https://doi.org/10.1093/gerona/glr193
- Rockwood, K. and Mitnitski, A. (2006). Limits to deficit accumulation in elderly people. Mech Ageing Dev. 127(5): 494–496. https://doi.org/10.1016/j.mad.2006.01.002
- Howlett, S. E. and Rockwood, K. (2013). New horizons in frailty: ageing and the deficit-scaling problem. Age Ageing. 42(4): 416–423. https://doi.org/10.1093/ageing/aft059
- ten Hove, D., Jorgensen, T. D. and van der Ark, L. A. (2024). Updated guidelines on selecting an intraclass correlation coefficient for interrater reliability, with applications to incomplete observational designs. Psychol Methods. 29(5): 967–979. https://doi.org/10.1037/met0000516
Article Information
Publication history
Received: Dec 4, 2024
Accepted: Mar 11, 2025
Available online: Mar 25, 2025
Published: Apr 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Collinge, C. W., Razzoli, M. and Bartolomucci, A. (2025). The Mouse Social Frailty Index (mSFI): A Standardized Protocol. Bio-protocol 15(8): e5272. DOI: 10.21769/BioProtoc.5272.
Category
Neuroscience > Behavioral neuroscience > Cognition
Neuroscience > Behavioral neuroscience > Animal model
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










