- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Vegetative Propagation of Cannabis sativa and Resin Obtained From its Female Inflorescences
Published: Vol 15, Iss 4, Feb 20, 2025 DOI: 10.21769/BioProtoc.5204 Views: 1746
Reviewed by: Noelia ForesiSimab KanwalAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Fluorescent Biosensor Imaging of Nitrate in Arabidopsis thaliana
Yen-Ning Chen and Cheng-Hsun Ho
Aug 20, 2023 3640 Views

A New Approach to Detect and Semi-quantify All Molecular Species and Classes of Anionic Phospholipids Simultaneously in Plant Samples
Manon Genva [...] Laetitia Fouillen
Apr 20, 2025 1730 Views
Abstract
Cannabis (Cannabis sativa L.) derivatives are of great importance in the medical, cosmetic, and pharmaceutical industries. This relevance is mainly due to the active principles (cannabinoids) found mainly in the trichomes of the female inflorescences. One of the most commonly used methods to propagate cannabis is by vegetative stem cuttings. This low-cost technique produces genetically uniform plants, ensuring consistent growth rates and cannabinoid production. The extraction of cannabinoids and other active compounds from the resin of the flowers is the main limitation of cannabis processing. Here, we describe a step-by-step protocol for propagating female cannabis plants from vegetative stem cuttings, inducing flower development, and obtaining high-quality cannabinoid-enriched resin.
Key features
• The propagation of cannabis is done by vegetative cuttings.
• It takes 4–5 months to cultivate the plant and obtain female inflorescences.
• Resin is the end product of the alcoholic extraction of flowers, and the obtained resin is a full-spectrum mixture of active compounds.
Keywords: Cannabis sativaGraphical overview
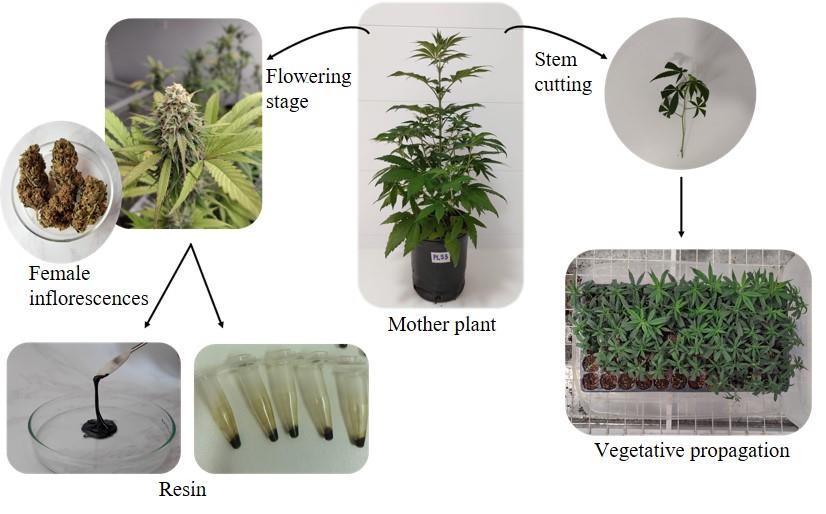
Main steps for the vegetative propagation of cannabis and extraction of resin from female inflorescences. The figure highlights the characteristics of the cutting and its arrangement in the corresponding tray. Additionally, it shows female inflorescences both in vivo and after harvesting and subsequent drying. From this point, different resin yields can be obtained depending on the protocol used.
Background
Cannabis sativa L. is an annual dicotyledonous herbaceous plant of the Cannabaceae family [1]. Nowadays, the female plant is mainly used in the medical, cosmetic, and pharmaceutical industries. The development of new products in these health-related fields is mainly due to the therapeutic effects associated with various cannabinoids [2]. These compounds are present in trichomes, glandular structures located mainly in female inflorescences [3,4]. Currently, several subclasses of cannabinoids have been identified and categorized into 11 families, each containing a variable number of compounds. The best-known cannabinoids include tetrahydrocannabinol, cannabidiol, cannabinol, cannabichromene, cannabicyclol, and cannabigerol. In addition, there is a wide variety of terpenes, sugars, steroids, flavonoids, and nitrogenous compounds that give flavor and aroma to different strains and have synergistic effects with cannabinoids [5]. The ratios of active compounds in flowers are influenced by a number of factors, including plant genetics, growing and storage conditions, maturity at harvest, and the methods used to process and formulate the material [6].
To maintain the genetic stability of a plant with a specific chemical profile, asexual propagation by vegetative stem cutting is a very suitable method [7]. In this technique, a section of the plant is taken to create a clone that is genetically identical to the parent plant. It is then placed on a moist substrate to allow the roots to develop. When the root system is thriving, it is transplanted to its final location where the plant will grow vegetatively until it reaches flowering and subsequent cannabinoid production. Although this technique is simple, the success of this method depends on many variables such as plant genetics, explant characteristics, hormones, and growing conditions such as light, humidity, and temperature [8].
Once harvested, flowers should be trimmed and then dried (in the absence of sunlight to prevent photochemical transformation) before beginning extraction of the active compounds. Cannabinoids are located in the trichomes on the surface of the bud, where the concentration gradient between the solvent and the trichome surface acts as the primary barrier to chemical transfer. Ethanol is the solvent of choice because of its solubility, relatively low price, and more importantly, its boiling point (for recovery purposes). For medical or dietary applications, ethanol is preferred due to its lower toxicity [9]. After extraction, the solvent is removed to yield a resin consisting of cannabinoids, terpenes, and other accompanying compounds. Depending on the final use of the resin, additional steps for purification and/or isolation are required. In this paper, we specifically detail the procedure to effectively generate cuttings and then list the steps to achieve an efficient resin yield from an alcoholic extraction of cannabinoids.
Materials and reagents
Biological materials
1. Cannabis sativa L. plants
Reagents
1. Substrate GrowMix Multipro (Terrafertil)
2. Substrate enriched with worm castings, peat, river sand, pine needles, and perlite (Pura Pacha)
3. Root promoter, alpha naphthalene acetic acid 0.1 g/100 mL (Fertifox)
4. Ethanol 96% and 70% (Porta, CAS 64-17-5)
5. Sodium hypochlorite 30% (Odex, CAS 7681-52-9)
6. Fertilizer NPK 5-9-12 Top Bloom (Top Crop)
Laboratory supplies
1. Bootstrap farmer 72-cell tray (Carluccio)
2. Transparent plastic box 60 cm × 50 cm × 30 cm (Colombraro, catalog number: 257)
3. Pots (1 and 5 L) (Carluccio)
4. Falcon tubes 50 mL (Deltalab, catalog number: 429931)
5. Eppendorf tubes 1.5 mL (Deltalab, catalog number: 4092.2AM)
6. Pipette tips 1,000 μL (Deltalab, catalog number: 200070 309)
7. Airtight bags (Ziploc)
8. Beaker 250 mL (Borosil, catalog number: 1000D21)
9. Laboratory gloves (Fisher Scientific, catalog number: 19-130-1597B)
10. Qualitative filter paper Grade 1 (Whatman, catalog number: WHA1001125)
11. Scissors
Equipment
1. Cultivation room at 25 °C, 18/6 h light/dark photoperiod for vegetative stages and 12/12 h for floral stages. Led hell panels 400 W (Led Osram HyperRED 660 nm, Led duris E2836 4000K and 3000K, Cri80, Drivers Philips)
2. Homogenizer (IKA, model: T10 basic ULTRA-TURRAX)
3. PIPETMAN P1000L 100–1,000 μL (Gilson, model: F167370)
4. Analytical balance (Shimadzu, model: AUY220)
5. Automatic environmental SpeedVac system (Savant, model: AES1010)
6. Rotary evaporator (Dragon Lab, model: RE-100 PRO)
7. Centrifuge (Thermo Scientific, model: Sorvall ST16R)
Procedure
A. Obtaining cuttings
To obtain cuttings, start with a healthy Cannabis sativa L. plant in the vegetative phase of growth, with green branches. The plant must be free of visible pathogens and without symptoms of nutrient deficiency or excess (Figure 1).

Figure 1. Vegetative propagation of cannabis by stem cuttings procedure. The diagram illustrates the process of taking a cutting from a mother plant in the vegetative stage, highlighting the area where the rooting hormone is applied. The cuttings are then placed in a tray with substrate within a wet chamber, where they remain until visible roots form.
1. Select a green branch with at least three nodes and 6 cm in length.
2. Sterilize a pair of scissors with 70% alcohol.
3. Make a 45° cut just below the third node.
4. Completely remove the petiole and the leaf that emerges from the third node by making a cut through the petiole.
5. Remove a portion of the leaf tip (50%) of fully expanded leaves to reduce water loss through evaporation.
6. Submerge the stem of the cutting in tap water while making new cuttings.
7. Apply a rooting agent to the basal area of the cutting containing the third node.
B. Substrate preparation
Once a good-quality substrate is available, follow these steps:
1. Clean a tray with the required number of wells with 30% sodium hypochlorite and rinse with tap water.
2. Fill the required number of wells with substrate.
3. Moisten the substrate with potable water.
4. Make a hole in the center of the substrate.
5. Insert the cut into the substrate, making sure the third knot is below the substrate surface.
6. Gently press down with your fingertips to secure the cut.
7. Add more substrate until it reaches the edge of the tray.
8. Place the tray in a transparent plastic box or tub.
9. Close the lid of the transparent plastic box to create a wet chamber.
10. Place the “wet chamber” with cuttings in the cultivation room to allow root development.
C. Root development and growth of cutting
The rooting conditions should be as follows: 18/6 h (light/dark) photoperiod, light intensity 50 W/m2, temperature 24 °C, relative humidity 80%–100% inside the humid chamber. After three days, press the substrate with your fingertip to check if it remains moist. If the substrate is dry, add potable water until moisture is restored. After 14 days, check for the presence of roots. These can be visualized by observing the drainage surface of the seed tray. Alternatively, remove the block of substrate from the germination tray and observe root development in the substrate directly. Once the roots have developed, transplant the cutting into a 1 L pot pre-filled with organic substrate (Pura Pacha) to facilitate root expansion and growth. This process should be repeated with larger pots as needed. Rooted cuttings should be watered every two days and kept in vegetative growth under the following conditions: 18/6 h (light/dark) photoperiod, light intensity 100 W/m2, temperature 24 °C, relative humidity 75%, and continuous airflow. When the plants have reached an adequate vegetative development (3–5 weeks), transplant them to a 5 L pot; the flowering stage begins.
D. Flowering plants
To initiate the flowering stage, switch to a photoperiod of 12/12 h light/dark and maintain the following conditions: light intensity 200 W/m2, temperature 24 °C, relative humidity 60%–70%, and continuous airflow. Within a period of 7–14 days, the plants will begin to develop sexual characteristics or flowers. The development and maturation of the female flowers will take approximately two to three months, depending on the variety of cannabis.
Throughout this process, the plants should be watered with potable water and the substrate should be kept at a pH around 6 with adequate nutrients (NPK 5-9-12), free of pesticides or heavy metal residues. It is important to have airflow around the leaf surface by installing fans in the growing environment. Good air circulation also helps prevent the establishment of plant diseases.
E. Harvesting, drying, and storing flowers
At the end of the growing season, when the pistils begin to turn brown, label and harvest the plants. Cut the main stem at the bottom and the main branches if the plant has developed a large biomass. Remove larger leaves to allow airflow between the branches, then hang the plants/branches upside down to dry in the dark for 10 days. Then, separate the female flowers from the plants with scissors and manually remove the remaining leaves between the flowers. Wear gloves when handling the plant.
At this point, it is necessary to have an additional room or drying tent. This room should be kept dark and cool to prevent contamination of the flowers with fungi or other microorganisms. Once the flowers are dry, store them in airtight bags and place them in darkness. Open them for 1 min during the first 10 days to further remove any remaining moisture. The harvested material must be protected from air, temperature, and light to prevent processes such as oxidation and decarboxylation of cannabinoids. These dried flowers are now ready to be processed for resin extraction as described below.
F. Obtaining resin from female flowers
The most appropriate extraction method should be selected based on the characteristics of the desired product. Conventional extraction using organic solvents involves macerating the plant material and then removing the solvent under reduced pressure. It is important to exercise caution during extraction, as the constituents of the original plant compounds are exposed to heat, air, and pressure, which can result in chemical changes (Figure 2).
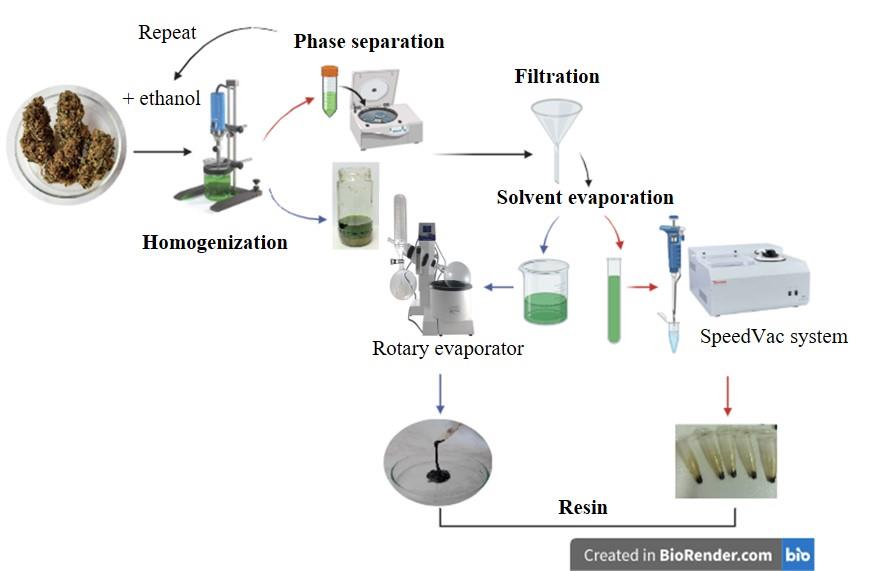
Figure 2. Procedure for resin extraction from female inflorescences. The procedure for working with small volumes (red arrows) involves the use of a centrifuge and SpeedVac equipment. For larger extraction volumes (blue arrows), the extract is decanted prior to filtration, and the solvent is removed using a rotary evaporator.
1. Weigh the dried female flowers on a precision balance.
2. Place the flowers in a sterilized container and add cold 96% ethanol at a 1/15 w/v ratio.
3. Process the samples by mechanical disruption using a homogenizer until a fine suspension is formed.
4. Separate the plant material from the liquid phase by centrifugation at 800× g for 5 min in Falcon tubes or allow it to decant at room temperature.
5. Retain the liquid phase, collect the solid material, and repeat steps F2–4.
6. Filter the liquid phase through grade 1 filter paper and save the filtered extract.
7. Remove solvent under vacuum. Depending on the volume obtained, different equipment may be required. For evaporating large volumes, a rotary evaporator may be required under the following conditions: 40 °C, 60× g, and 20 MPa for the vacuum pump until ethanol recovery is complete. For small volumes, the sample can be evaporated using a SpeedVac concentrator under the following conditions: radiant cover off, cryopumping, and low drying rate. Samples should be fractionated into pre-weighed Eppendorf tubes. For example, 1 mL of extract should be evaporated for approximately 4 h.
8. Store the obtained resin at -20 °C.
Validation of protocol
This protocol or parts of it has been used and validated in the following research article:
• Vozza Berardo et al. [10]. Antifungal and antibacterial activities of Cannabis sativa L. resins. J Ethnopharmacology 318:116839. https://doi.org/10.1016/j.jep.2023.116839
General notes and troubleshooting
General notes
1. High relative humidity and adequate temperature (22–26 °C) are critical for successful explanting.
2. If no roots have appeared by day 15, discard the plants.
3. This resin can also be suspended in vegetable oil to obtain cannabis oil, a highly useful product in the medical, cosmetic, and pharmaceutical industries.
4. This protocol is also applicable to other research objectives (e.g., in vitro detached leaf/fruit inoculation) and flowering plants with appropriate modifications.
Troubleshooting
Problem 1: There is no root development.
Possible cause: The temperature in the chamber is too low. The stem is too thick.
Solution: Keep the temperature inside the chamber between 22 and 26 °C. Use green branches.
Problem 2: The cutting dries up.
Possible cause: The humidity inside the chamber is not adequate.
Solution: Keep the humidity inside the chamber between 80% and 100%.
Problem 3: The cutting rots before developing roots.
Possible cause: The substrate is too wet.
Solution: Be careful when wetting the substrate to avoid overdoing it.
Problem 4: There is remaining solvent in the resin.
Possible cause: The evaporation time was not enough.
Solution: Evaporate until no visible traces of solvent remain in the resin.
Acknowledgments
We are grateful to the following science and technology entities for technical support: Universidad Nacional de Mar del Plata and Comisión de Investigaciones Científicas.
This paper was supported by Agencia Nacional de Investigaciones Científicas y Técnicas (ANPCyT, PICT Serie A 03535) and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) PIP 0422.
This protocol was described and validated in the original research article Vozza Berardo et al. [10]. Antifungal and antibacterial activities of Cannabis sativa L. resins. J Ethnopharmacology 318:116839.
Competing interests
The authors declare no competing interests.
References
- Bremer, K. (1998). An Ordinal Classification for the Families of Flowering Plants. Ann Mo Bot Gard. 85(4): 531.
- Abot, A., Bonnafous, C., Touchard, F., Thibault, F., Chocinski-Arnault, L., Lemoine, R. and Dédaldéchamp, F. (2013). Effects of cultural conditions on the hemp (Cannabis sativa) phloem fibres: Biological development and mechanical properties. J Compos Mater. 47(8): 1067–1077.
- Schilling, S., Dowling, C. A., Shi, J., Ryan, L., Hunt, D., OReilly, E., Perry, A. S., Kinnane, O., McCabe, P. F., Melzer, R., et al. (2020). The Cream of the Crop: Biology, Breeding and Applications of Cannabis sativa. 10.22541/au.160139712.25104053/v2
- Aizpurua-Olaizola, O., Soydaner, U., Öztürk, E., Schibano, D., Simsir, Y., Navarro, P., Etxebarria, N. and Usobiaga, A. (2016). Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes. J Nat Prod. 79(2): 324–331.
- Chandra, S., Lata, H., Mehmedic, Z., Khan, I. A. and ElSohly, M. A. (2015). Light dependence of photosynthesis and water vapor exchange characteristics in different high Δ9-THC yielding varieties of Cannabis sativa L. J Appl Res Med Aromat Plants. 2(2): 39–47.
- Potter, D. J. (2014). A review of the cultivation and processing of cannabis (Cannabis sativa L.) for production of prescription medicines in the UK. Drug Test Anal. 6: 31–38.
- de Meijer, E. P. M., Bagatta, M., Carboni, A., Crucitti, P., Moliterni, V. M. C., Ranalli, P. and Mandolino, G. (2003). The Inheritance of Chemical Phenotype in Cannabis sativa L. Genetics. 163(1): 335–346.
- Caplan, D., Stemeroff, J., Dixon, M. and Zheng, Y. (2018). Vegetative propagation of cannabis by stem cuttings: effects of leaf number, cutting position, rooting hormone, and leaf tip removal. Can J Plant Sci. 98(5): 1126–1132.
- Valizadehderakhshan, M., Shahbazi, A., Kazem-Rostami, M., Todd, M. S., Bhowmik, A. and Wang, L. (2021). Extraction of Cannabinoids from Cannabis sativa L. (Hemp)—Review. Agriculture. 11(5): 384.
- Vozza Berardo, M. E., Mendieta, J. R., Villamonte, M. D., Colman, S. L. and Nercessian, D. (2024). Antifungal and antibacterial activities of Cannabis sativa L. resins. J Ethnopharmacol. 318: 116839.
Article Information
Publication history
Received: Sep 14, 2024
Accepted: Dec 16, 2024
Available online: Jan 19, 2025
Published: Feb 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
D´Ippolito, S., Landaburu, M., Vozza Berardo, M. E., Villamonte, M. D., Mendieta, J. R., Nercessian, D. and Colman, S. L. (2025). Vegetative Propagation of Cannabis sativa and Resin Obtained From its Female Inflorescences. Bio-protocol 15(4): e5204. DOI: 10.21769/BioProtoc.5204.
Category
Plant Science > Plant biochemistry > Metabolite
Plant Science > Plant breeding > Cultivation technique
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










