- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Development of a Rapid and Efficient Protocol for Seed Germination and Seedling Establishment of Oryza coarctata
Published: Vol 15, Iss 4, Feb 20, 2025 DOI: 10.21769/BioProtoc.5203 Views: 1825
Reviewed by: Samik BhattacharyaArsheed Hussain SheikhAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Non-invasive Imaging of Rice Roots in Non-compacted and Compacted Soil
Bipin K. Pandey [...] Craig J. Sturrock
Dec 20, 2021 4534 Views

Vegetative Propagation of Cannabis sativa and Resin Obtained From its Female Inflorescences
Sebastián D´Ippolito [...] Silvana L. Colman
Feb 20, 2025 1708 Views
Abstract
Seed germination is a critical and challenging process in the propagation of Oryza coarctata, a wild halophytic rice species. This protocol outlines the seed germination procedure for O. coarctata. All steps required for optimal germination and seedling establishment of O. coarctata in both sterile and soil-based systems are described in detail. Additionally, the protocol includes an analysis of the primary hormones, abscisic acid (ABA) and gibberellin (GA), involved in regulating seed dormancy and germination.
Key features
• This protocol provides detailed instructions for germinating Oryza coarctata and improving its germination rate.
• The protocol enables the successful vegetative and reproductive growth of O. coarctata in soil.
• Additionally, the protocol outlines the analysis of hormones (abscisic acid and gibberellin) that play key roles in the germination process.
Keywords: Oryza coarctataGraphical overview
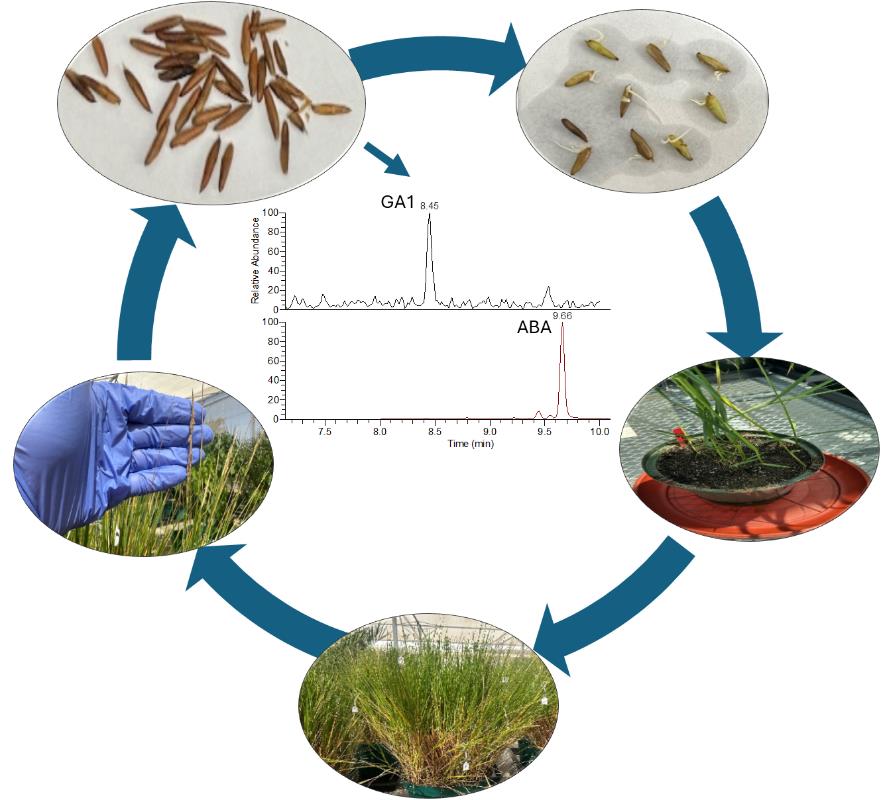
Protocol for germination, seedling development, and hormone analysis of Oryza coarctata seeds. Step-by-step instructions include germinating seeds on filter papers or sterile growing medium; the established seedlings can be grown in controlled environments or a greenhouse. Extraction and analysis of endogenous key hormones offer insights into their roles in controlling dormancy and germination.
Background
Oryza coarctata is a perennial wild species of rice and the only halophytic species in the genus Oryza (family Poaceae) [1]. It has unique morphological features such as thicker waxy succulent leaves and salt glands, allowing the plant to survive under harsh environmental conditions such as high salinity and submersion [2]. Additionally, O. coarctata leaves have a unique leaf anatomical feature exhibited by C4 plants, the Kranz anatomy (enlarged bundle sheath cells encircling vascular bundles) [3,4]. O. coarctata is primarily found in coastal areas across Southeast Asian countries [1]. O. coarctata offers a wealth of genetic resources for rice breeding research, making it a good model system for investigating mechanisms of salinity and submersion tolerance in rice [5,6]. The germination rate of wild rice seeds is very low, posing significant challenges for research on its conservation. The low germination rate of wild rice seeds leads to inconsistent seedling emergence, causing a reduction in their yield [7]. Dormancy is a limiting factor for the breeding of wild rice, which is regulated by several plant hormones, mainly abscisic acid and gibberellin.
Biotic and abiotic stresses significantly challenge environmental stability and influence the survival of living organisms, including plants. For instance, salinity stress poses significant challenges to global agriculture, affecting production yield, which is essential for human consumption. In addition, salt stress affects around 6% of the world’s cultivable land, making it a significant threat to food security [8,9]. Salt accumulation has various effects, including reduced plant expansion, accelerated wilting, and disrupted photosynthesis. While changing climate intensifies these obstacles, addressing approaches to agriculture, such as genetic engineering to improve crop resilience in response to climatic stresses, is imperative [10,11]. Amongst various climatic stresses, salt concentration remarkably affects rice (Oryza sativa), one of the essential crops. Under these circumstances, the wild rice relative, O. coarctata, has become increasingly significant due to its resistance under salt stress compared to O. sativa. Moreover, cultivation efforts are directed toward producing saline-tolerant crops [12,13]. Therefore, the salt-tolerant characteristics of O. coarctata establish resources for cultivation programs intended to improve the adaptability of rice varieties to high salt levels, thus promoting food stability in regions affected by elevated salinity [4]. Additionally, O. coarctata is a promising candidate for neo-domestication.
Despite its potential as a promising crop for nutritional security, the germination of O. coarctata under experimental and laboratory conditions is challenging. Seed germination is controlled by phytohormones such as gibberellins (GAs) and abscisic acid (ABA) [14]. These hormones are also of particular significance given their role in the regulation of growth and development as well as responses to environmental stresses [15]. For instance, ABA controls plant development from germination to seed dormancy and modulates plant responses to challenging abiotic stresses [16,17]. Apart from their role in inducing seed germination, GAs modulate stem elongation, flower development, fruit ripening, and leaf senescence [18]. The quantitative analysis of these phytohormones is frequently required in both basic plant research and agricultural studies.
Taken together, developing a fast, reproducible, and efficient germination protocol for O. coarctata is crucial for both fundamental research and breeding programs. Establishing this protocol is critical for studying and investigating O. coarctata characteristics, such as biological traits and genetic features, to enhance crop resilience against elevated salt levels in multiple regions worldwide. In this protocol, we outline detailed steps for seed germination and seedling development of O. coarctata. Additionally, we determine the content of key hormones, offering new insights into their roles in controlling dormancy, germination, seedling establishment, and responses to environmental stresses.
Materials and reagents
Biological materials
1. Oryza coarctata seeds; original seeds were obtained from the International Rice Research Institute (IRRI), Philippines. For germination tests, seeds were harvested in our laboratory from plants that were grown at King Abdullah University of Science and Technology (KAUST) greenhouse facility
Reagents
1. Commercial bleach, sodium hypochlorite solution (NaClO) Sigma-Aldrich, CAS number: 7681-52-9)
2. Murashige and Skoog basal medium (MS) (Sigma-Aldrich, catalog number: M5519)
3. Sucrose (Sigma-Aldrich, CAS number: 57-50-1)
4. Agar (Sigma-Aldrich, CAS number: 9002-18-0)
5. LC-grade methanol (VWR, CAS number: 67-56-1)
6. LC-grade water (VWR, CAS number: 7732-18-5)
7. LC-grade acetonitrile (Fisher ChemicalTM, CAS number: 75-05-8)
8. Formic acid (Thermo ScientificTM, CAS number: 64-18-6)
9. Gibberellin A1 (GA1) (Sigma-Aldrich, CAS number: 545-97-1)
10. Abscisic acid (ABA) (Sigma-Aldrich, CAS number: 21293-29-8)
Solutions
1. 1% NaClO (100 mL) (see Recipes)
2. 0.5× MS media (100 mL) (see Recipes)
3. 50% acetonitrile:water (see Recipes)
4. Abscisic acid (ABA) stock solution (1 mg/mL) (see Recipes)
5. Gibberellin A1 (GA1) stock solution (1 mg/mL) (see Recipes)
6. Water containing 0.1% formic acid (1,000 mL) (see Recipes)
7. Acetonitrile containing 0.1% formic acid (1,000 mL) (see Recipes)
Recipes
1. 1% NaClO (100 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaClO (5% w/v) | 1% (w/v) | 20 mL |
| Water (ddH2O) | - | 100 mL* |
*Note: Water should be added in increments to reach 100 mL final volume. This solution can be prepared and stored protected from light at room temperature for up to one month.
2. 0.5× MS media (100 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Sucrose | 2% (w/v) | 2 g |
| MS powder | 0.22% (w/v) | 0.22 g |
| Water (ddH2O) | n/a | 100 mL* |
Adjust pH to 5.8 with 3N HCl or 3N KOH. Add agar to 0.8% and autoclave at 121 °C for 15 min. The medium should be freshly prepared.
*Note: Water should be added in increments to reach 100 mL final volume.
3. 50% acetonitrile:water
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| LC-grade water | 50% | 50 mL |
| LC-grade acetonitrile | 50% | 50 mL |
| Total | n/a | 100 mL* |
*Note: This solution can be prepared and stored protected from light at room temperature for up to three months.
4. Abscisic acid (ABA) stock solution (1 mg/mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Abscisic acid | 1 mg/mL | 1 mg |
| LC-grade methanol | - | 1 mL* |
*Note: Working solution (10 µg/mL) from this stock can be prepared and stored protected from light at −20 °C for up to six months.
5. Gibberellin A1 (GA1) stock solution (1 mg/mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Gibberellin A1 | 1 mg/mL | 1 mg |
| LC-grade methanol | - | 1 mL* |
*Note: Working solution (10 µg/mL) from this stock can be prepared and stored protected from light at −20 °C for up to six months.
6. Water containing 0.1% formic acid (1,000 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Formic acid | 0.1% | 1 mL |
| LC-grade water | 100% | Up to 1,000 mL* |
*Note: Water should be added in increments to reach 1000 mL final volume. This solution should be freshly prepared before analysis.
7. Acetonitrile containing 0.1% formic acid (1,000 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Formic acid | 0.1% | 1 mL |
| LC-grade acetonitrile | 100% | Up to 1,000 mL* |
*Note: Acetonitrile should be added in increments to reach 1,000 mL final solution.
This solution should be freshly prepared before analysis.
Laboratory supplies
1. Sterile deionized water, room temperature
2. 50 mL tubes (FalconTM, catalog number: 14-432-22)
3. Filter paper (Whatman®, Grade 1, model number: WHA1001090)
4. Parafilm (Fisher Scientific, catalog number: S37440)
5. Laminar flow hood (AIREGARD ES Horizontal Laminar Flow, model number: NU-201/E)
6. Petri dishes (FisherbrandTM, catalog number: AS4052)
7. Tissue culture boxes (e.g., Magenta Vessel with cover) (Sigma-Aldrich, model number: C0542)
8. 5″ diameter round black pots (with holes at the base for drainage)
9. Mixture of soil (Stender) and local sand (3:1)
10. 0.2 µm filter (Target2TM PTFE Syringe Filters, Thermo Scientific™, catalog number: F2504-4)
11. Steel beads (3.2 mm chrome-steel beads, BioSpec Products, Inc., catalog number: 11079132c)
12. 2 mL safe-lock microcentrifuge tubes (Eppendorf, catalog number: 0030123620)
13. 1.5 mL safe-lock microcentrifuge tubes (Eppendorf, catalog number: 0030123611)
14. Autosampler vials (LC screw-thread vials with caps and inserts, VWR®, catalog number: 82030-974A)
15. HPLC column (Hypersil GOLD C18 Selectivity, 150 × 4.6 mm; 3 µm; Thermo ScientificTM, catalog number: 25003-154630)
Equipment
1. pH meter (FisherbrandTM accumetTM AB150 pH benchtop meter, catalog number: 13-636-AB150)
2. 30 °C incubator (FisherbrandTM IsotempTM Incubator, catalog number: 15-103-0515)
3. Culture room/growth chamber (e.g., Conviron, BioChambers, or Percival) [with controlled LED light and humidity controller with humidity range from 60% to 90% relative humidity (RH) and temperature range from 10 °C to 40 °C]
4. Weighing balance (Sartorius M-Power Analytical Balance, model number: AZ214)
5. Tissue grinder (Mixer Mill, RETSCH®, model number: 20.746.0001)
6. Vortexer (VWR® Fixed Speed Vortex Mixer, catalog number: 10153-834)
7. Sonicator (Bransonic® Ultrasonic Cleaner, model: 5510)
8. Centrifuge (EppendorfTM 5424R Microcentrifuge, catalog number: 05-401-205)
9. Vacuum centrifugal concentrator (Eppendorf® Concentrator Plus, catalog number: 5305000568)
10. Liquid chromatography system (Thermo Scientific, model: VanquishTM Duo UHPLC System)
11. Mass spectrometer system (Thermo Scientific, model: TSQ AltisTM triple quadrupole)
Software and datasets
1. Statistical software for data analysis software (Microsoft Excel, Microsoft Corporation, v. 2410, 2024)
2. LC–MS/MS software (Xcalibur 4.1, Thermo Fisher Scientific Inc., USA)
Procedure
A. Seed germination
1. Before germination, remove the husks from the seeds.
2. Put the de-husked seeds in a 50 mL Falcon tube.
3. Proceed to sterilize them with 1% bleach solution on a vortex shaker (100 rpm) for 5 min.
4. Thoroughly rinse the seeds with 40 mL of sterile water at least five times. See General Note 1.
5. Prepare magenta boxes containing 0.5× MS media for section B or a Petri dish by placing two filter papers inside with 5 mL of sterile water for section C. For submerged germination, see General Note 2. The germination can be scored daily for one week. For dormant or non-germinated seeds, continue scoring for 12 days. The germination process requires a constant humid environment with temperature control for 7–10 days.
B. Growing O. coarctata in controlled environments
1. Place 5–8 disinfected seeds on magenta boxes (tissue culture boxes) containing 0.5× MS media.
2. Keep the boxes at 30 °C in the dark for 24–48 h.
3. Transfer the box containing germinated seeds to a controlled environment for growth.
4. Maintain a day/night temperature of 28/22 °C with a 12/12 h photoperiod, 200 µmol photons m−2 s−1, and 85% relative humidity (RH). The germination and seedling establishment can be scored for 7–10 days.
C. Growing O. coarctata in the greenhouse
1. Transfer 5–8 disinfected seeds onto the filter papers.
2. Seal the petri dish with parafilm to maintain seed moisture.
3. Incubate the Petri dish at 30 °C for 24–48 h in the dark.
4. Transfer the plates containing germinated seeds to a controlled environment for growth.
5. Maintain a day/night temperature of 28/22 °C with a 12/12 h photoperiod and 200 µmol photons m−2 s−1.
6. Fill 5″ diameter pots with a mixture of sand and soil (3:1).
7. Plant two seedlings per pot.
8. Transfer the pots containing seedlings to the greenhouse chamber. The temperature in our greenhouse ranges from 25 to 29 °C during the day and 22 to 25 °C at night. RH varies from 60% to 90%. The greenhouse is supplemented with artificial lights from 6 am to 7 pm. The entire reproductive growth is reached within three months.
9. After two weeks in the greenhouse, plants can also be retransferred to a shade house.
10. The plants can be irrigated on a daily basis and fertilized weekly with nitrogen, phosphorus, and potassium (NPK) fertilizer (20:20:20, Folicat Plantifol) at 2 g/L water.
D. Seed harvesting and storage
1. Seed collection: Harvest the seeds when they begin to turn brown, typically about two weeks after flowering.
2. Preparation: Place the collected seeds into tubes or suitable containers.
3. Storage: Store the seeds in a tightly sealed container at a cold temperature (around 4 °C) to preserve their viability. After the seed reaches maturity, wild rice goes into a dormant state. To break dormancy, seeds need to be kept in low temperatures for a few months.
E. Extraction and analysis of endogenous GA and ABA by LC/MS
1. Aliquot (50 mg) seeds in safe-lock 2.0 mL Eppendorf tubes.
2. Grind and homogenize seeds for 1 min at a frequency of 25 Hz.
3. Add 1.5 mL of LC-grade methanol to each tube.
4. Vortex samples at 1,000 rpm for 1 min.
5. Sonicate the samples at 40 kHz for 15 min.
6. Centrifuge samples at 10,000× g for 10 min at room temperature.
7. Transfer the supernatant to a new 1.5 mL Eppendorf tube.
8. Dry the supernatant by vacuum concentration for 1.5 h.
9. Resuspend the dry extracts in 120 µL of 50% acetonitrile:water followed by 1 min sonication at 40 kHz.
10. Filter the solution through a 0.22 µm filter into a LC glass vial.
11. Analyze samples by LC–MS/MS. See General Note 3.
12. Include standards for LC–MS/MS analysis in the running sequence. Dilute the standard hormones with methanol to 10 μg/mL (working solution) from the stock solution of 1 mg/mL ABA and the stock solution of 1 mg/mL GA1.
Data analysis
Germination rate estimation is based on scoring the percentage of germinated seeds every 24 h for 7 days. Use the following formula: Germination % = seeds germinated/total seeds × 100. Microsoft Excel or any other software can be used. Consider that radicle protrusion is the germination event.
The obtained LC/MS raw chromatograms from phytohormone analysis can be manually inspected using Xcalibur 4.1 (Thermo Fisher Scientific Inc., USA) or any other software. The retention time and peak areas can be recorded. For absolute quantification of endogenous hormones, use isotope-labeled standards as internal references during analysis [19].
Validation of protocol
In this protocol, we detail a rapid and efficient method for the germination of Oryza coarctata seeds. The protocol is validated through the figures and results demonstrating the functionality of the experimental setup. Using this method, radicle emergence was observed within 24–48 h and allowed us to obtain established seedlings in 10 days (Figure 1). Additionally, we analyzed the tolerance of O. coarctata seeds to submersion in water. We observed that seedling vigor was not affected.

Figure 1. Representative photographs of Oryza coarctata seeds at different days of germination initiation. Photographs were taken for dry seeds (A), 2 days after germination (B), and 10 days after sowing (C and D). Germination after submersion in water for 7 days is shown in (E).
Scoring the seed germination rate after the radical emergence, which is defined as the first sign of seed germination, revealed that around 40% of seeds successfully germinate after two days (Figure 2). Seeds are scored every day until seed germination rate reaches over 80% after five days.
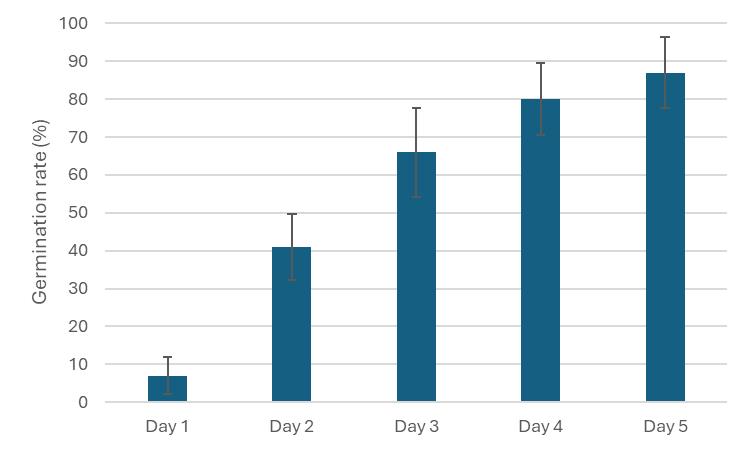
Figure 2. Germination percentage of Oryza coarctata scored five days after sowing. Data are shown as mean ± SE (n = 10). In each replicate, 10 seeds were subjected to germination.
Optimizing the yield of O. coarctata depends on precise harvest timing, ideally during the green, milky stage (the stage when the grains are being filled) or approximately two weeks after flowering. The flowering period for O. coarctata typically spans under our growth conditions from December to January (Figure 3).

Figure 3. Representative photographs of Oryza coarctata plants vegetatively grown in the greenhouse. Photographs of two-week-old (A), one-month-old (B), and three-month-old (C–E) plants.
Simultaneous determination of GA and ABA from dry seeds was achieved by liquid chromatography–tandem mass spectrometry (LC–MS/MS) method. The extraction method with a single solvent (methanol) allows the detection of both hormones without the solid–phase extraction (SPE) pretreatment method. The chromatographic separation was carried out on a reversed-phase (C18) column, using acetonitrile/water containing 0.1% formic acid as mobile phases. Both phytohormones were eluted within 10 min. The full mass spectrum of ABA showed that [M−H]− ion was the most intensive at m/z 263.20 with a retention time of 9.67 min (Figure 4A). Multiple reaction monitoring (MRM) transitions (precursor ion/ product ion) are 263.20/153.10 and 263.20/219.10 (Figure 4B).
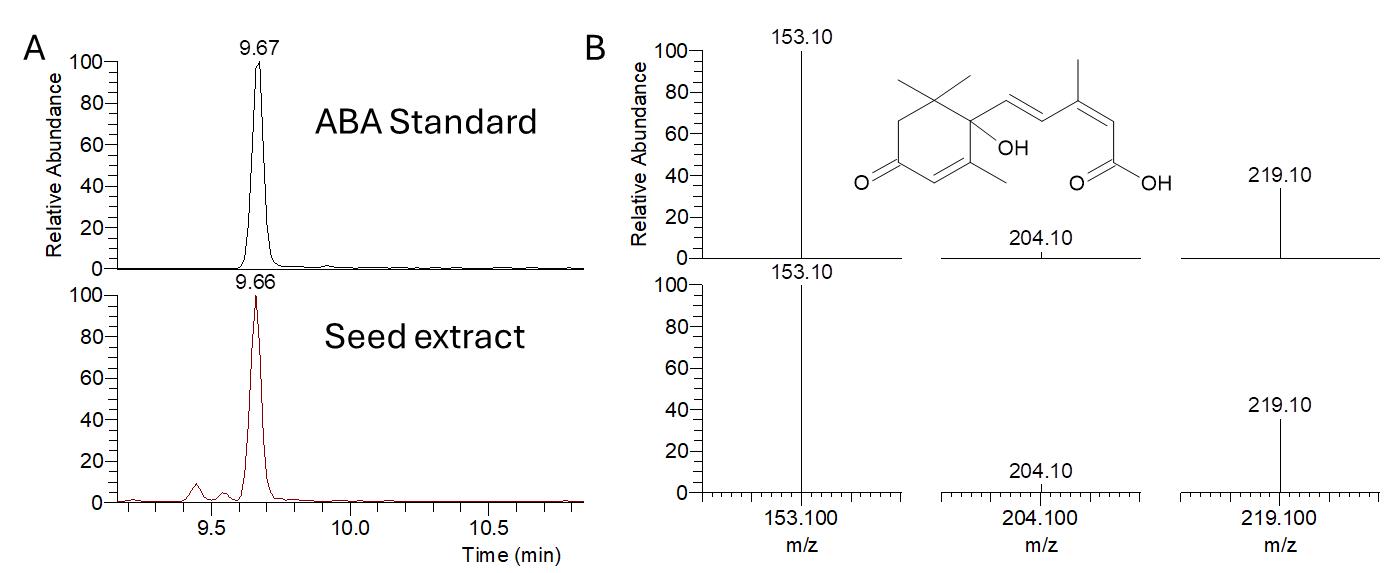
Figure 4. Typical LC chromatogram (A) and negative ion MS/MS spectrum (B) from LC–ESI-MS/MS analysis of abscisic acid (ABA) standard solution (upper panel) and Oryza coarctata seed extract (lower panel). The MRM transition for ABA, 263.20→ 219.10, 153.10. The chemical structure of ABA is shown.
The full mass spectrum of GA1 showed that the [M−H]− ion was the most intensive at m/z 347.20 with a retention time of 8.47 min (Figure 5A). MRM transitions are 347.20/259.10 and 347.20/273.00 (Figure 5B).
In conclusion, we have developed an efficient protocol for seed germination and seedling development of the wild rice species (Oryza coarctata) under sterile conditions for tissue culture and other agricultural applications.
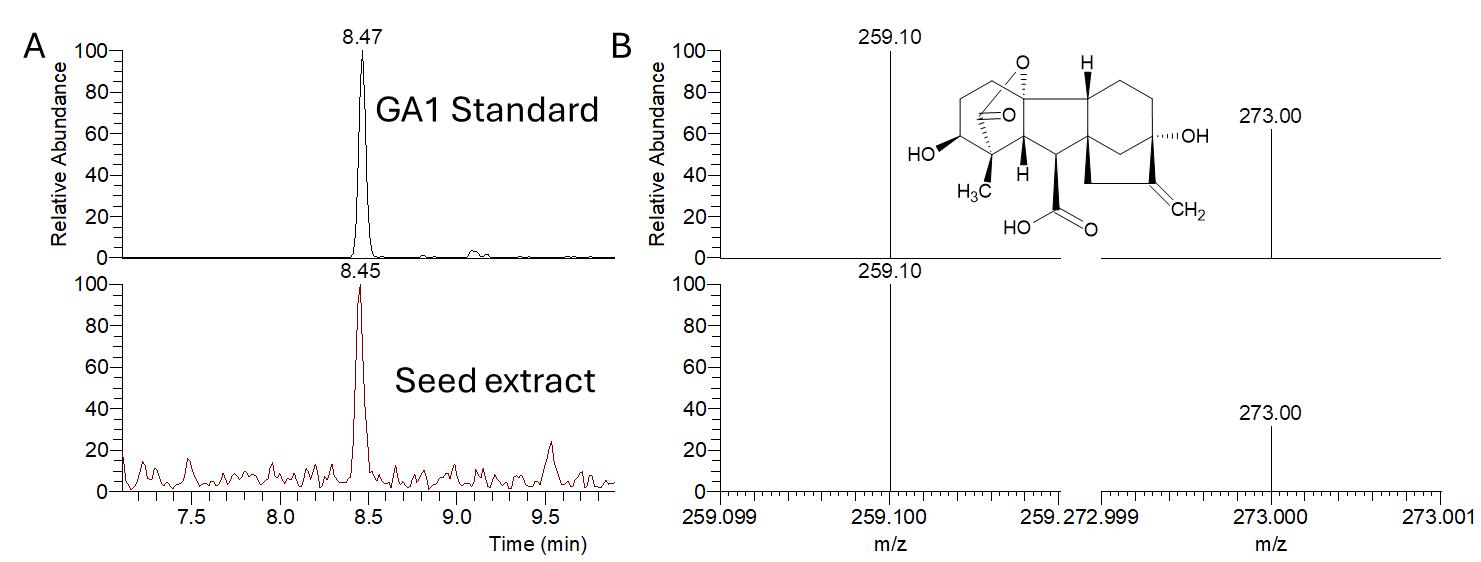
Figure 5. Typical LC chromatogram (A) and negative ion MS/MS spectrum (B) from LC–ESI-MS/MS analysis of gibberellin A1 (GA1) standard solution (upper panel) and Oryza coarctata seed extract (lower panel). The MRM transitions for GA1, 347.20→ 273.00, 259.10. The chemical structure of GA1 is shown.
General notes and troubleshooting
General notes
1. Perform steps in a laminar flow hood under sterile conditions.
2. For the submerged germination, place the seeds in a sterile tube filled to 90% capacity with sterile water, positioning them diagonally. Incubate the tubes in a 30 °C incubator for 24–48 h in the dark. Transfer the tubes containing germinated seeds to a controlled environment for growth. Maintain a day/night temperature of 28/22 °C with a 12/12 h photoperiod and 200 µmol photons m−2 s−1.
3. The LC–MS analysis was performed following our previous studies [20,21]. Chromatographic separation was carried out on a C18 HPLC column maintained at 35 °C. Mobile phases consist of water containing 0.1% formic acid (A) and acetonitrile containing 0.1% formic acid (B). The gradient program was as follows: 0–10 min, 15% B to 100% B; 10–15 min, 100% B; 15–17 min, 15% B at 0.5 mL/min flow rate. The MS parameters were as follows: negative ion, 3000 V; sheath gas, 40 Arb; aux gas, 15 Arb; collision energy of 20 eV; ion transfer tube temperature, 350 °C; vaporizer temperature, 350 °C; cycle time, 1 s; Q1/Q3 resolution (FWHM), 0.4; CID gas (mTorr), 2; and chromatographic peak width (s), 6.
Troubleshooting
Problem 1: No germinations after three days.
Possible cause: Seeds are dormant.
Solution: Wait for at least seven days. Sometimes, seeds need more time to start germination (up to 10 days in the dark).
Problem 2: Fungal growth in culture boxes.
Possible cause: Contamination during seed sterilization or media preparation.
Solution: Discard the culture boxes and begin again under controlled sterile conditions. Perform steps in a laminar flow hood under sterile conditions.
Problem 3: No detection of ABA or GA signal from tissues but clear signal for standard.
Possible cause: The endogenous level is below the detection level.
Solution: Start extraction with ~100 mg of dry tissues.
Acknowledgments
This work was supported by Neo-domestication funding (CDA-neodom) and baseline funding given to S. A-B from King Abdullah University of Science and Technology (KAUST).
Competing interests
The authors declare that they have no competing interests.
References
- Chowrasia, S., Rawal, H. C., Mazumder, A., Gaikwad, K., Sharma, T. R., Singh, N. K. and Mondal, T. K. (2018). Oryza coarctata Roxb. Mondal, T. K. and Henry, R. J. (Eds.) The Wild Oryza Genomes. Springer International Publishing. 87–104. https://doi.org/10.1007/978-3-319-71997-9_8
- Bansal, J., Gupta, K., Rajkumar, M. S., Garg, R. and Jain, M. (2020). Draft genome and transcriptome analyses of halophyte rice Oryza coarctata provide resources for salinity and submergence stress response factors. Physiol Plant. 173(4): 1309–1322. https://doi.org/10.1111/ppl.13284
- Zhao, H., Wang, W., Yang, Y., Wang, Z., Sun, J., Yuan, K., Rabbi, S. M. H. A., Khanam, M., Kabir, M. S., Seraj, Z. I., et al. (2023). A high-quality chromosome-level wild rice genome of Oryza coarctata. Sci Data. 10(1): 701. https://doi.org/10.1038/s41597-023-02594-1
- Chowrasia, S., Nishad, J., Pandey, R. and Mondal, T. K. (2021). Oryza coarctata is a triploid plant with initial events of C4 photosynthesis evolution. Plant Sci. 308: 110878. https://doi.org/10.1016/j.plantsci.2021.110878
- Mondal, T. K., Rawal, H. C., Gaikwad, K., Sharma, T. R. and Singh, N. K. (2017). First de novo draft genome sequence of Oryza coarctata, the only halophytic species in the genus Oryza. F1000Research. 6: 1750. https://doi.org/10.12688/f1000research.12414.1
- Bansal, J., Gupta, K., Rajkumar, M. S., Garg, R. and Jain, M. (2020). Draft genome and transcriptome analyses of halophyte rice Oryza coarctata provide resources for salinity and submergence stress response factors. Physiol Plant. 173(4): 1309–1322. https://doi.org/10.1111/ppl.13284
- Yao, Q., Zheng, X., Zhou, G. and Zhang, J. (2024). SGR-YOLO: a method for detecting seed germination rate in wild rice. Front Plant Sci. 14: e1305081. https://doi.org/10.3389/fpls.2023.1305081
- Rodríguez Coca, L. I., García González, M. T., Gil Unday, Z., Jiménez Hernández, J., Rodríguez Jáuregui, M. M. and Fernández Cancio, Y. (2023). Effects of Sodium Salinity on Rice (Oryza sativa L.) Cultivation: A Review. Sustainability. 15(3): 1804. https://doi.org/10.3390/su15031804
- Teshome, D. T., Zharare, G. E. and Naidoo, S. (2020). The Threat of the Combined Effect of Biotic and Abiotic Stress Factors in Forestry Under a Changing Climate. Front Plant Sci. 11: e601009. https://doi.org/10.3389/fpls.2020.601009
- Liu, X., Feike, T., Shao, L., Sun, H., Chen, S. and Zhang, X. (2016). Effects of saline irrigation on soil salt accumulation and grain yield in the winter wheat-summer maize double cropping system in the low plain of North China. J Integr Agric. 15(12): 2886–2898. https://doi.org/10.1016/s2095-3119(15)61328-4
- Khan, M. S., Akther, T., Mubarak Ali, D. and Hemalatha, S. (2019). An investigation on the role of salicylic acid alleviate the saline stress in rice crop (Oryza sativa (L)). Biocatal Agric Biotechnol. 18: 101027. https://doi.org/10.1016/j.bcab.2019.101027
- Tamanna, N., Mojumder, A., Azim, T., Iqbal, M. I., Alam, M. N. U., Rahman, A. and Seraj, Z. I. (2024). Comparative metabolite profiling of salt sensitive Oryza sativa and the halophytic wild rice Oryza coarctata under salt stress. Plant-Environ Interact. 5(3): e10155. https://doi.org/10.1002/pei3.10155
- Mukherjee, S., Mukherjee, A., Das, P., Bandyopadhyay, S., Chattopadhyay, D., Chatterjee, J. and Majumder, A. L. (2021). A salt‐tolerant chloroplastic FBPase from Oryza coarctata confers improved photosynthesis with higher yield and multi‐stress tolerance to indica rice. Plant Cell, Tissue Organ Cult. 145(3): 561–578. https://doi.org/10.1007/s11240-021-02026-1
- Zhang, Y., Berman, A. and Shani, E. (2023). Plant Hormone Transport and Localization: Signaling Molecules on the Move. Annu Rev Plant Biol. 74(1): 453–479. https://doi.org/10.1146/annurev-arplant-070722-015329
- Waadt, R., Seller, C. A., Hsu, P. K., Takahashi, Y., Munemasa, S. and Schroeder, J. I. (2022). Plant hormone regulation of abiotic stress responses. Nat Rev Mol Cell Biol. 23(10): 680–694. https://doi.org/10.1038/s41580-022-00479-6
- Wang, J. Y., Lin, P. Y. and Al-Babili, S. (2021). On the biosynthesis and evolution of apocarotenoid plant growth regulators. Semin Cell Dev Biol. 109: 3–11. https://doi.org/10.1016/j.semcdb.2020.07.007
- Jamil, M., Alagoz, Y., Wang, J. Y., Chen, G. E., Berqdar, L., Kharbatia, N. M., Moreno, J. C., Kuijer, H. N. J. and Al‐Babili, S. (2024). Abscisic acid inhibits germination of Striga seeds and is released by them likely as a rhizospheric signal supporting host infestation. Plant J. 117(5): 1305–1316. https://doi.org/10.1111/tpj.16610
- Shani, E., Hedden, P. and Sun, T. P. (2024). Highlights in gibberellin research: A tale of the dwarf and the slender. Plant Physiol. 195(1): 111–134. https://doi.org/10.1093/plphys/kiae044
- Salem, M. A., Yoshida, T., Perez de Souza, L., Alseekh, S., Bajdzienko, K., Fernie, A. R. and Giavalisco, P. (2020). An improved extraction method enables the comprehensive analysis of lipids, proteins, metabolites and phytohormones from a single sample of leaf tissue under water‐deficit stress. Plant J. 103(4): 1614–1632. https://doi.org/10.1111/tpj.14800
- Wang, J. Y., Alseekh, S., Xiao, T., Ablazov, A., Perez de Souza, L., Fiorilli, V., Anggarani, M., Lin, P. Y., Votta, C., Novero, M., et al. (2021). Multi-omics approaches explain the growth-promoting effect of the apocarotenoid growth regulator zaxinone in rice. Commun Biol. 4(1): 1222. https://doi.org/10.1038/s42003-021-02740-8
- Chen, G. T., Wang, J. Y., Votta, C., Braguy, J., Jamil, M., Kirschner, G. K., Fiorilli, V., Berqdar, L., Balakrishna, A., Blilou, I., et al. (2023). Disruption of the rice 4-DEOXYOROBANCHOL HYDROXYLASE unravels specific functions of canonical strigolactones. Proc Natl Acad Sci USA. 120(42): e2306263120. https://doi.org/10.1073/pnas.2306263120
Article Information
Publication history
Received: Oct 15, 2024
Accepted: Dec 23, 2024
Available online: Jan 9, 2025
Published: Feb 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Berqdar, L., Salem, M. A., Wang, J. Y., Alrudayan, A., Jamil, M. and Al-Babili, S. (2025). Development of a Rapid and Efficient Protocol for Seed Germination and Seedling Establishment of Oryza coarctata. Bio-protocol 15(4): e5203. DOI: 10.21769/BioProtoc.5203.
Category
Plant Science > Plant breeding > Cultivation technique
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









