- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Culture and Characterization of Differentiated Airway Organoids from Fetal Mouse Lung Proximal Progenitors
(*contributed equally to this work) Published: Vol 14, Iss 23, Dec 5, 2024 DOI: 10.21769/BioProtoc.5129 Views: 1752
Reviewed by: Valeria Fernandez ValloneSamantha HallerAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
A Hybrid 2D/3D Approach for Neural Differentiation Into Telencephalic Organoids and Efficient Modulation of FGF8 Signaling
Michele Bertacchi [...] Michèle Studer
Jun 20, 2025 2777 Views

Protocol for 3D Bioprinting a Co-culture Skin Model Using a Natural Fibrin-Based Bioink as an Infection Model
Giselle Y. Díaz [...] Stephanie M. Willerth
Jul 20, 2025 3706 Views
A Simplified 3D-Plasma Culture Method for Generating Minimally Manipulated Autologous Equine Muscle-Derived Progenitor Cells
Hélène Graide [...] Didier Serteyn
Dec 5, 2025 1235 Views
Abstract
Developing a physiologically relevant in vitro model of the respiratory epithelium is critical for understanding lung development and respiratory diseases. Here, we describe a detailed protocol in which the fetal mouse proximal epithelial progenitors were differentiated into 3D airway organoids, which contain terminal-differentiated ciliated cells and basal stem cells. These differentiated airway organoids could constitute an excellent experimental model to elucidate the molecular mechanisms of airway development and epithelial cell fate determination and offer an important tool for establishing pulmonary dysplasia disease in vitro.
Key features
• Efficient isolation of proximal epithelial progenitors from mouse embryos.
• Differentiation of pulmonary airway organoids differentiated from tracheal progenitors, which recapitulates the process of airway cell differentiation.
• Airway organoids can be used to explore the molecular mechanisms of lung development and respiratory diseases.
Keywords: Airway organoidsGraphical overview
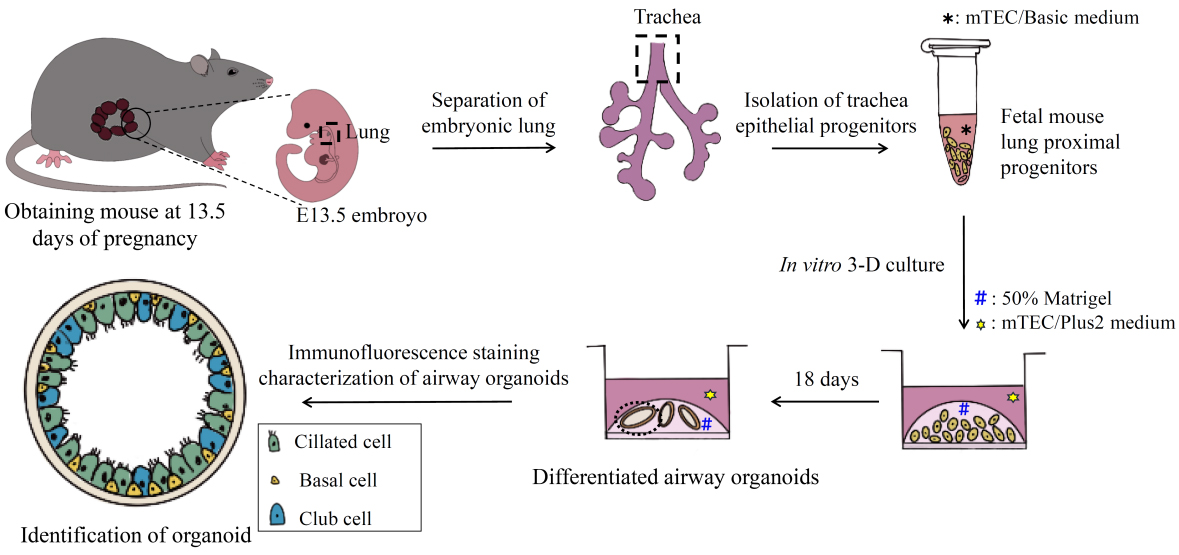
Overview of the culture of airway organoids from isolated E13.5 mouse proximal epithelial cells
Background
The pseudostratified airway epithelium is the first mechanical barrier of the respiratory system and maintains tissue homeostasis after injury. The airway epithelium consists of secretory club cells, terminally differentiated ciliated cells, basal stem cells, and a small population of goblet cells, neuroendocrine cells, and ionocytes [1]. All types of proximal airway epithelial cells differentiate from Sox2+ progenitors during embryonic lung development [2]. Deficiencies in early embryonic development of the airway epithelium are frequently associated with neonatal mortality, as well as a variety of life-threatening lung diseases in adulthood, including bronchopulmonary dysplasia, chronic obstructive pulmonary disease, and lung cancer.
Recent advances in in vitro 3D culture techniques enable the generation of organoids derived from stem or progenitor cells, which functionally and morphologically mimic the airway epithelium at a near-physiological level [3,4]. This approach has allowed researchers to elucidate the molecular mechanisms of lung development and the consequences of genetic alterations associated with various respiratory diseases [5,6]. Additionally, several research groups have developed lung organoid disease models for preclinical drug screening [7–10].
We previously reported a tracheal progenitor isolation and culture system that can generate airway organoids containing differentiated epithelial cells [11]. Here, we provide a detailed protocol to generate 3D airway organoids with hollow structures derived from isolated proximal epithelial progenitors of E13.5 mouse tracheas, the same source for generating airway organoids as previously reported [11]. Compared with the trans-well air-liquid interface (ALI) culture, this protocol requires fewer progenitors and the resulting 3D organoids are more suitable for in vivo transplantation for the study of diseases [12,13]. This robust in vitro 3D culture model provides an opportunity to explore the molecular mechanisms of early lung development and relevant respiratory diseases in an experimentally tractable system.
Materials and reagents
Biological materials
10–12-week-old adult male mice and sexually attracted female mice; obtained from the Animal Experimental Center of Anhui Medical University
Reagents
PBS (Thermo Fisher, catalog number: 20012027), store at RT
Ethanol absolute (Hushi, catalog number: 10009218), store at RT
Sucrose (Sigma-Aldrich, catalog number: V900116), store at RT
Triton X-100 (Sigma-Aldrich, catalog number: T8787), store at RT
Normal goat serum (Gibco, catalog number: 16210-064), shelf-life is 3 months, store at -20 °C
NaN3 (Sigma-Aldrich, catalog number: S2002), dissolve 2 g in 1 mL of sterile Milli-Q-treated water, store at -20 °C.
Caution: NaN3 is hazardous. Avoid inhalation, ingestion, and skin contact.
Tween-20 (Sigma-Aldrich, catalog number: P1379), add 10 µL of Tween-20 in PBS to 10 mL, store at RT
Optimal cutting temperature compound (OCT) (Sakura, catalog number: 4853), store at RT
Chloral hydrate (MACKLIN, catalog number: 302-17-0), dissolve 10 g in 90 mL of sterile Milli-Q-treated water, store at 2–8 °C
Matrigel basement membrane matrix, growth factor reduced (GFR) (Corning, catalog number: 354230), store at -80 °C
Note: Thaw Matrigel on an icebox in a 4 °C refrigerator, aliquot in 200 μL vials upon arrival, and minimize freeze and thaw cycles; it will polymerize at temperatures above 10 °C.
Paraformaldehyde (PFA) (MACKLIN, catalog number: 30525-89-4), dissolve 20 g of PFA in 500 mL of sterile PBS to prepare 4% PFA. Shelf-life is 2 months; store at 2–8 °C
Caution: PFA is hazardous. Avoid inhalation, ingestion, and skin contact. Use protective equipment and work in a fume hood.
Type I rat tail collagen (Corning, catalog number: 354236), dissolve 4 mg of Type I rat tail collagen in 1 mL of 0.02 N acetic acid to prepare 4 mg/mL Type I rat tail collagen, then add 50 μL of 4 mg/mL Type I rat tail collagen in 1.95 mL of 0.02 N acetic acid to prepare 0.1 mg/mL Type I rat tail collagen. Store at -20 °C
Acetic acid (Sigma-Aldrich, catalog number: 695092), dissolve 1 mL in 87 mL of sterile Milli-Q-treated water. Use a syringe filter to filter the acetic acid solution; store at -20 °C
Penicillin/streptomycin (PS) (Life Technologies, catalog number: 15140-163), with 10,000 units penicillin and 10 mg streptomycin per milliliter in 0.9% NaCl; store at -20 °C
DMEM (high glucose, L-glutamine, sodium pyruvate, sodium bicarbonate, phenol red, w/o HEPES) (VivaCell, catalog number: 113-0500), store at 2–8 °C
DMSO (Solarbio, catalog number: D8370), store at RT
Advanced DMEM/F-12 (Gibco, catalog number: C11330500BT), store at 2–8 °C
GlutaMAX, 0.4 M (Gibco, catalog number: 35050061), store at 2–8 °C
HEPES, 1 M (Gibco, catalog number: 15630-080), store at 2–8 °C
PrimocinTM, 50 mg/mL (InvivoGen, catalog number: ant-pm), store at -20 °C aliquoted. Avoid multiple freeze-thaw cycles
NaHCO3 (Sigma-Aldrich, catalog number: S6014), dissolve 0.75 g in 10 mL of sterile Milli-Q-treated water. Use a syringe filter to filter the solution; store at -20 °C
FBS (Biowest, catalog number: S1580-500), store at -20 °C
HBSS (Gibco, catalog number: 14175), store at RT
BSA (Sigma-Aldrich, catalog number: V900933), dissolve 0.1 g in 10 mL of PBS to prepare 1% BSA, store at 2–8 °C
EGF (Corning, catalog number: 354001), dissolve 100 μg in 4 mL of HBSS + 0.1% BSA. Shelf-life is 3 months; store at -20 °C aliquoted
CHIR99021 (Tocris, catalog number: 4423), dissolve 1 mg in 716.3 μL of DMSO. Shelf-life is 3 months; store at -20 °C aliquoted
Y-27632 (Tocris, catalog number: 1254), dissolve 1 mg in 624.5 μL of PBS. Shelf-life is 1 year; store at -20 °C aliquoted
Retinoic acid (Sigma-Aldrich, catalog number: R2625), dissolve 1 mg in 13.31 mL of DMSO. Make fresh each time and store at -20 °C
Note: Vortex retinoic acid stock before making the working solution. This is light sensitive; store in the dark.
Transferrin (Sigma-Aldrich, catalog number: T1147-100 mg), dissolve 100 mg in 20 mL of HBSS + 0.1% BSA. Shelf-life is 2 months; store at -20 °C aliquoted
A-8301 (AbMole, catalog number: M5037), dissolve 5 mg in 23.72 mL of DMSO. Shelf-life is 2 months; store at -20 °C aliquoted
Insulin (Sigma-Aldrich, catalog number: I6634), dissolve 100 mg in 50 mL of 4 mM HCl. Shelf-life is 28 days; store at -20 °C aliquoted
Noggin (Sino Biological, catalog number: 10267), dissolve 1 mg in 50 μL of PBS. Shelf-life is 1 year; store at -20 °C aliquoted
Cholera toxin (Sigma-Aldrich, catalog number: 9012-63-9), dissolve 1 mg in 4 mL of HBSS + 0.1% BSA. Shelf-life is 1 year; store at -20 °C aliquoted
Pronase (Sigma-Aldrich, catalog number: 9036-06-0), dissolve 15 mg of Pronase in 10 mL of Ham’s F12
Note: Use a 0.22 µm sterile filter to filter the Pronase solution and make fresh each time.
Ham’s F12 (Corning, catalog number: 10-080-cv), store at 2–8 °C
Anti-fluorescence quenching agent (Beijing Biotopped Technology, catalog number: Top0702). Shelf-life is 1 year; store at 2–8 °C. This is light sensitive; store in the dark
Antibodies
Anti-mouse Tp63 (Santa Cruz Biotechnology, catalog number: sc-25268), we recommend dilution at 1:400 with dilution buffer and storing at -20 °C aliquoted. Fresh dilution is prepared before staining
Anti-mouse acetylated α Tubulin (Abcam, catalog number: ab-24610), we recommend dilution at 1:400 with dilution buffer and storing at -20 °C aliquoted. Fresh dilution is prepared before staining
Anti-rabbit Krt5 (Abcam, catalog number: ab-64081), we recommend dilution at 1:250 with dilution buffer and storing at -20 °C aliquoted. Fresh dilution is prepared before staining
Goat anti-rabbit 555 (Life Technologies, catalog number: A21430), we recommend dilution at 1:1,000 with dilution buffer and storing at -20 °C aliquoted. This is light sensitive; store in the dark. Fresh dilution is prepared before staining
Goat anti-mouse 488 (Life Technologies, catalog number: A11029), we recommend dilution at 1:1,000 with dilution buffer and storing at -20 °C aliquoted. This is light sensitive; store in the dark. Fresh dilution is prepared before staining
DAPI (Sigma-Aldrich, catalog number: D9542), we recommend dilution at 1:1,000 with dilution buffer and storing at -20 °C aliquoted. This is light sensitive; store in the dark. Fresh dilution is prepared before staining
Solutions
Buffer solution (see Recipes)
Suspension medium (see Recipes)
mTEC/basic medium (see Recipes)
mTEC/Plus1 medium (see Recipes)
mTEC/Plus2 medium (see Recipes)
Blocking buffer (see Recipes)
Dilution buffer (see Recipes)
Recipes
Buffer solution (50 mL)
Shelf-life is 1 month; store at 2–8 °C.
Reagent Final concentration Volume PBS n/a 49.5 mL Penicillin/streptomycin 500 U/mL 500 μL Suspension medium (50 mL)
Make fresh each time and store at 2–8 °C.
Reagent Final concentration Volume DMEM n/a 47 mL FBS 5% 2.5 mL Penicillin/streptomycin 500 U/mL 0.5 mL mTEC/basic medium (50 mL)
Shelf-life is 1 month; store at 2–8 °C.
Reagent Stock concentration Final concentration Volume Advanced DMEM/F-12 n/a n/a 48.05 mL HEPES 1 M 15 mM 0.75 mL Penicillin/streptomycin 500 U/mL 500 U/mL 0.5 mL GlutaMAX 0.4 M 4 mM 0.5 mL NaHCO3 0.9 M 3.6 mM 0.2 mL mTEC/Plus1 medium (3 mL)
Shelf-life is 1 month; store at 2–8 °C.
Reagent Stock concentration Final concentration Volume MTEC/basic medium n/a n/a 2.82 mL FBS 100% 5% 150 μL EGF 25 μg/mL 25 ng/mL 3 μL Insulin 2 mg/mL 10 μg/mL 15 μL Transferrin 5 mg/mL 5 μg/mL 3 μL Cholera toxin 250 μg/mL 0.1 μg/mL 1.2 μL PrimocinTM 50 mg/mL 100 μg/mL 6 μL mTEC/Plus2 medium (1 mL)
Make fresh each time and store at 2–8 °C.
Reagent Stock concentration Final concentration Volume MTEC/Plus1 medium n/a n/a 996.4 μL Retinoic acid 250 μM 50 nM 0.2 μL Y-27632 5 mM 5 μM 1 μL CHIR99021 3 mM 3 μM 1 μL A8301 500 μM 500 nM 1 μL Noggin 20 mg/mL 20 μg/mL 1 μL Blocking buffer (10 mL)
Make fresh each time and store at 2–8 °C.
Reagent Stock concentration Final concentration Volume PBS n/a n/a 7.98 mL Normal goat serum 100% 20% 2 mL Triton X-100 100% 0.1% 10 μL NaN3 2 g/mL 0.2 mg/mL 1 μL Tween-20 100% 0.05% 5 μL Dilution buffer (10 mL)
Make fresh each time and store at 2–8 °C.
Reagent Stock concentration Final concentration Volume PBS n/a n/a 9.78 mL Normal goat serum 100% 2% 0.2 mL Triton X-100 100% 0.1% 10 μL NaN3 2 g/mL 0.2 mg/mL 1 μL Tween-20 100% 0.05% 5 μL
Laboratory supplies
1 mL syringe (Jiufekang, catalog number: 045X15RWLB), containing 4.5-gauge syringe needle
24-well plates (Corning, catalog number: 353047)
35 mm culture dish (Sparkjade, catalog number: GF0006)
60 mm culture dish (Sparkjade, catalog number: GF0007)
1.5 mL centrifuge tube (Sparkjade, catalog number: NZ-86009)
Falcon, 50 mL centrifuge tube (Wocas, catalog number: NZ-86011)
Falcon, 15 mL centrifuge tube (Wocas, catalog number: NZ-86010)
Pipette tip, 10, 200, 1,000 µL (Wocas, catalog numbers: NZ-86001, NZ-86002, NZ-86003)
Parafilm M wrapping film (Thermo Fisher, catalog number: 1337416)
300-mesh cell strainers (Sangon biotech, catalog number: F513453)
Adhesion microscope slides (Citotest, catalog number: 80312-3161)
Syringe filter (SHIGOUYI, catalog number: SFMCE013022NA)
Dyeing tank (Biosharp, catalog number: BS-WB-20B)
Equipment
Cell culture hood (Nuaire, model: NU-425-400S)
Cell culture incubator (Nuaire, model: NU-5510E)
Cell counter (Biosharp, model: BS-QT-1103)
Cryostat (Yidi, model: YD-1900), set from 0 to -40 °C
Surgical scissors (Merck, catalog numbers: S3146)
Surgical forceps (Biolab, catalog numbers: Z168777 and Z225614)
Vortex mixer (Kylin-bell, model: VORTEX-5)
Ice machine (PHCbi, model: SIM-F140AY65-PC)
Autoclave (TOMY, model: SX-500)
Brightfield microscope (Leica, model: DMI3000B)
Cell culture benchtop centrifuge (Merck, model: MGL-16MA)
Micropipettes (Dragon, catalog numbers: 7010101004, 7010101005, 7010101006, 7010101009, 7010101016)
Pure water technology (Zhiang, model: Best-R)
37 °C water bath (Changzhou Yuexin, model: HH-1)
Stereomicroscope (Olympus, model: SZX16)
Multiphoton laser scanning microscope (Leica, model: TCS SP8 DVE)
Procedure
Obtaining mouse at 13.5 days of pregnancy
Cage one 10–12-week-old adult male mouse and two sexually attracted female mice together at about 6 p.m.
Note: House mice in the animal room and control the temperature (21 ± 2 °C) and humidity (50% ± 10%) with a 12/12 h light/dark cycle.
Check female vaginal plugs the next morning; mark the morning of the day when you detect a vaginal plug as E0.5.
Weigh mice every day. Pregnant females generally show a detectable increase in body weight from E6.5 onward, with a 0.5–2 g increase at E13.5 based on the number of embryos in the womb.
Separation of the proximal airways from E13.5 embryos
Before dissecting E13.5 mice, disinfect the stereomicroscope and operating table with 75% ethanol and sterilize the forceps and scissors by autoclaving to minimize the possibility of contamination during the experiment. In addition, prepare an ice box, 35 and 60 mm Petri dishes, and 1.5 and 50 mL tubes containing buffer solution (see Recipes section) (Figure 1).
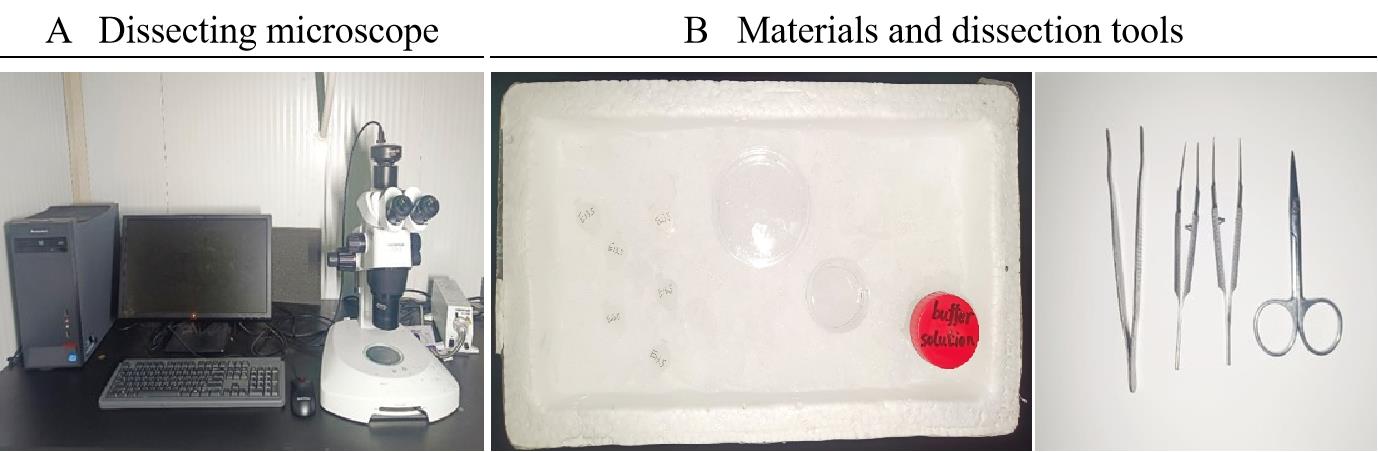
Figure 1. Example of equipment and materials and dissection tools. A. Stereomicroscope. B. Materials and dissection tools needed for proximal airway dissection: tubes (50 mL, 1.5 mL) with buffer solution, Petri dishes (35 mm, 60 mm) with buffer solution, surgical scissors, and straight thin tip forceps.Prepare surgical tools: surgical scissors, surgical forceps, and surgical bed, and select an E13.5 pregnant mouse from the laboratory animal house.
Intraperitoneally inject 0.3 mL of chloral hydrate and wait for 5 min. Clamp the mouse's back foot with forceps; if the mouse does not struggle and twitch, assume that the mouse does not feel pain (Figure 2A).
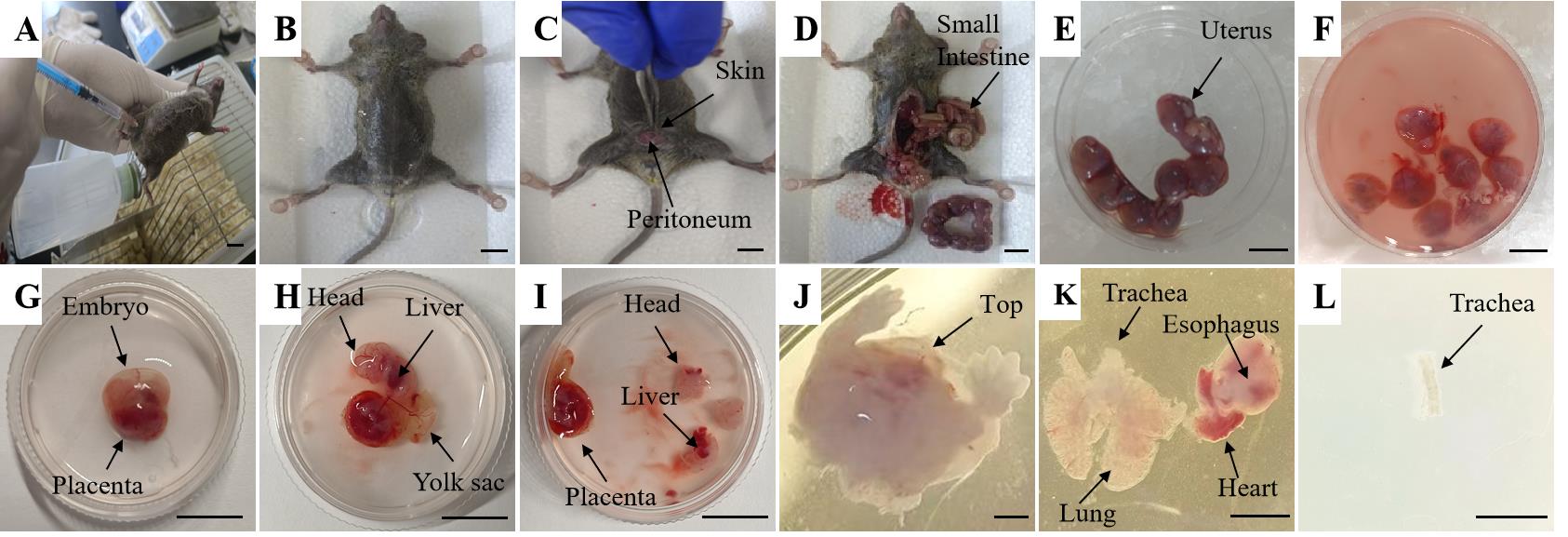
Figure 2. Representative pictures of E13.5 lung and trachea dissection. Each panel illustrates steps B2–B14. Scale bars: 1 cm (A–I); 100 μm (J–L).Fix the pregnant mouse to the surgical bed using 4.5-gauge syringe needles (Figure 2B).
Disinfect the abdomen area with 75% ethanol (Figure 2B).
Pinch and lift the skin with forceps (Figure 2C).
Dissect along the mid-abdominal line with surgical scissors, starting from the pubic area and continuing to the end of the abdominal cavity. Inject PBS into the heart, revealing the abdominal organs (Figure 2D).
Note: This procedure helps to avoid accidental injury to the embryo located below.
Place the uterus with embryos with forceps on the 60 mm dish filled with buffer solution maintained on ice (Figure 2E).
Note: Sacrifice the mouse by cervical dislocation after placing the uterus with embryos on the dish.
Separate each embryo from the uterus with the intact yolk sac using forceps (Figure 2F).
Remove each embryo from the uterus and keep them in the dish with buffer solution on ice using forceps.
Place one embryo in a 35 mm culture dish containing 1 mL of buffer solution using forceps (Figure 2G).
Discard the yolk sac, amniotic membrane, and umbilical cord under the stereomicroscope using forceps. Keep the rest on ice (Figure 2H).
Remove the head and abdomen of the embryo, cut the rest under the liver, pull the chest skin of the embryo open, and open the rib-enclosed part of the chest cavity (Figure 2I–J).
Find the red heart part of the embryo mouse and identify the trachea and lung parts around the heart; then, remove the esophagus from the lung and carefully remove the remaining excess tissues with surgical forceps (Figure 2K).
Note: The stripped airway should remain as intact as possible. Work fast to prevent progenitor cell damage.
Remove the proximal airway with forceps and place in the 35 mm culture dish containing buffer solution at 4 °C (Figure 2L).
Next, dissect the remaining embryos placed on ice.
Isolation of trachea epithelial progenitors
Under the microscope, using fine-tipped forceps, clean off the connective tissue around the trachea in a 35 mm culture dish.
Open the trachea longitudinally using scissors and further dissect the connected tissue using forceps. Wash it three times in a 1.5 mL centrifuge tube containing 200 μL of buffer solution to get off the connected tissue around the proximal airway.
Put all cleaned tracheae into a 1.5 mL centrifuge tube containing 1 mL of Pronase solution using forceps, and digest at 4 °C overnight (approximately 14 h).
Note: Prolonged digestion with Pronase might be harmful for progenitor cells.
Transfer the undigested trachea to a new 1.5 mL tube using forceps and use 200 μL of mTEC/basic medium (see Recipes) to pipette up and down.
Note: After the airway is digested overnight, there is still some tissue around the airway, such as cartilage, and some progenitor cells in the remaining tissue.
Mix the cleaned solution with the mixture of cellular digestive solution. Centrifuge at 800× g for 5 min at 37 °C.
Discard the supernatant and add 1 mL of suspension medium (see Recipes).
Note: When separating the proximal airway progenitor cells of the embryonic lung, pay attention to the placement of the centrifuge tube in the centrifuge to prevent cell loss.
Use a 300-mesh cell strainer (i.e., 300 holes per inch) to filter the cell suspension into a new 15 mL tube and centrifuge at 1,000× g for 3 min.
Discard the supernatant.
Resuspend the cells in 1 mL of prewarmed mTEC/basic medium and transfer into a 1.5 mL tube.
Note: Prewarm the required volume of mTEC/Basic medium in a water bath (37 °C) for approximately 15 min before use.
Prepare type I rat tail collagen coated 35 mm dish.
Note: First, add 1 mL of collagen coating solution in a 35 mm dish, then aspirate the collagen coating solution and allow the membrane to air-dry for at least 5 min. Rinse both apical and basal surfaces with 1 mL of sterile PBS three times to remove free collagen. Allow the 35 mm dish coated surface to dry for 5 min before use and make fresh each time.
Place the cell suspension prepared in step C9 on a 35 mm dish coated type I rat tail collagen. Incubate at 5% CO2 at 37 °C for 3 h.
Collect the nonadherent cells in a new 15 mL tube carefully.
Centrifuge at 1,000× g for 5 min.
Aspirate the supernatant.
Resuspend the cells in 1 mL of mTEC/basic medium.
3D in vitro culture of proximal epithelial progenitors
Thaw Matrigel on ice overnight at 4 °C before use.
Note: When handling Matrigel, always keep it on ice at all times and work quickly. Matrigel will tend to polymerize as it warms up.
Add 100 µL of Matrigel in the bottom of a 24-well plate and place it at 37 °C for 30 min.
Centrifuge the proximal airway progenitor cells at 1,000× g for 10 min at 37 °C and discard the supernatant.
Carefully aspirate the supernatant and resuspend the cells in mTEC/Plus2 medium (see Recipes).
Cell counting: We use a cell counter to calculate the number of cells, and we control 800 cells per 100 µL of cell suspension.
Note: Adjust the cells to 20–50 cells per large grid to minimize counting errors.
Mix Matrigel with the cell suspension on ice at a ratio of 1:1.
Note: Put the resuspended cell–Matrigel mixture on ice to avoid Matrigel solidification.
Inoculate a ratio of 800 cells per well on the center of the Matrigel-coated 24-well plate and ensure cell growth in the 3D droplet shape of Matrigel. Place at 37 °C for 30 min.
After Matrigel has polymerized into a jelly-like texture, add 500 µL of mTEC/Plus2 medium along the wall of the 24-well plate.
Incubate at 5% CO2 at 37 °C for 18 days.
Note: Prewarm the mTEC/Plus2 medium in a 37 °C water bath for 10–20 min before use.
Change the culture medium every two days during the organoid culture and replace half of the mTEC/Plus2 medium each time.
Take pictures on days 1, 2, 3, 6, 9, 12, 15, and 18.
Proximal epithelial progenitor cells start to form lumen-like structures on the second day of culture, which gradually grow larger during subsequent cultures, eventually forming hollow, spherical airway organoids (Figure 3).
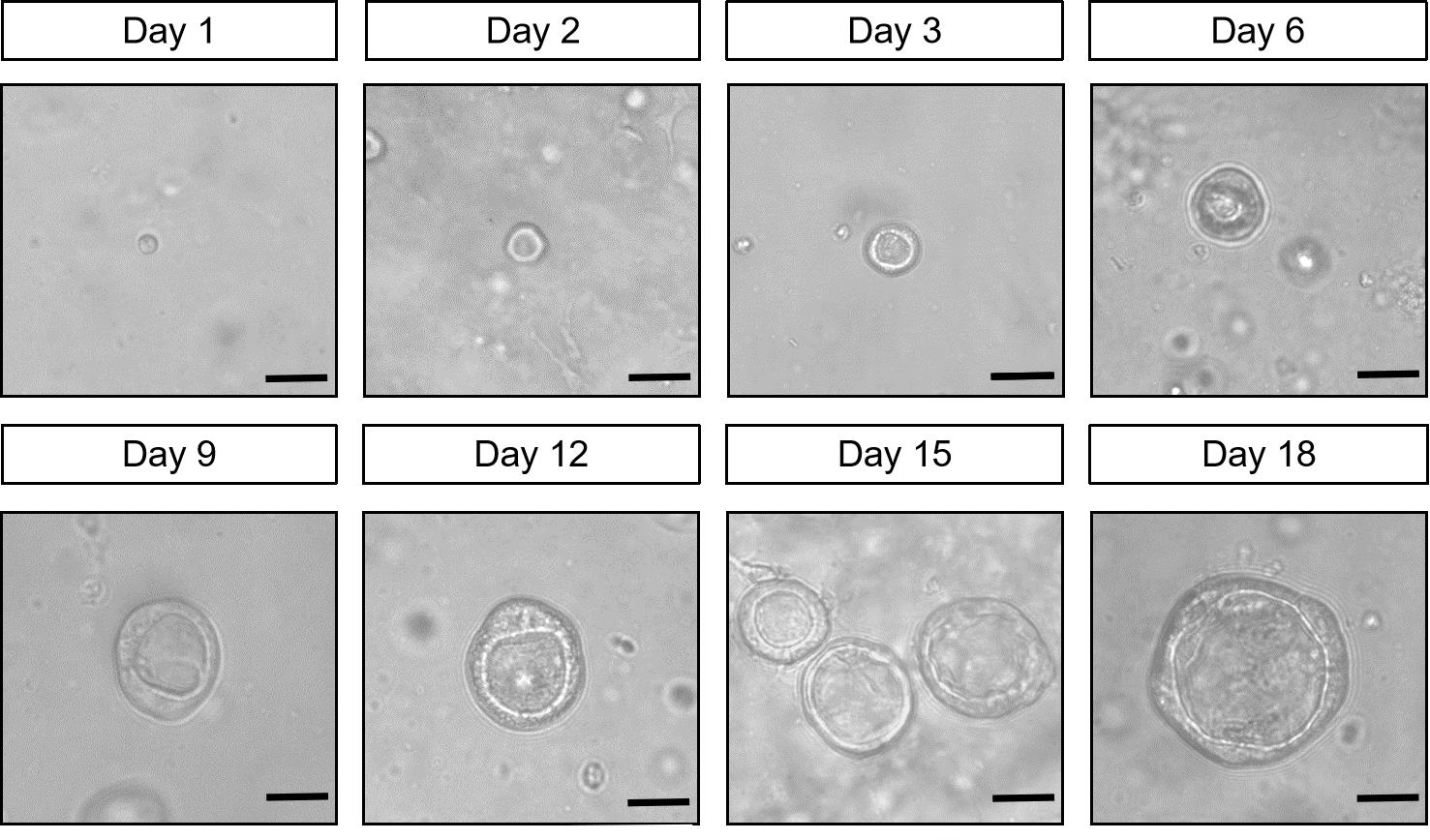
Figure 3. Top view of 3D culture of airway organoids differentiated from mouse lung proximal progenitors. The pictures show a representative picture of organoids culture on days 1, 2, 3, 6, 9, 12, 15, and 18. Scale bars: 25 μm.
Identification of airway organoids by immunofluorescence staining
Organoid fixation
Prepare 1% BSA solution.
Carefully aspirate the mTEC/Plus2 medium.
Add 1 mL of pre-cooled PBS to each well.
Coat the 15 mL tube and pipette tip using 1% BSA solution to wash the tube wall.
Transfer organoids in the 24-well plates to the 15 mL centrifuge tube using the pipette tip that was washed with 1% BSA solution, and then pipette up and down the 24-well plate with 1 mL of 1% BSA solution. Transfer to the 15 mL centrifuge tube.
Add 10 mL of pre-cooled PBS to the 15 mL centrifuge tube, centrifuge at 70× g for 3 min at 4 °C, and carefully aspirate the supernatant. Repeat step E1e.
Add 1 mL of 4% (wt/vol) PFA to fix the organoids at 4 °C for 30 min. Pipette and mix them every 10 min, paying attention to controlling the pipetting force to prevent the organoid structure from being destroyed.
After fixation, add 10 mL of 0.1% Tween-20 and suspend organoids. Then, centrifuge at 70× g for 3 min at 4 °C and carefully aspirate the supernatant.
Add 2 mL of PBS.
Note: Organoids in PBS can be stored at 4 °C for up to a week.
Frozen sections
Centrifuge at 70× g for 3 min at 4 °C before soaking the fixed organoids overnight in PBS with 10% sucrose at 4 °C.
Use a cutting pipette tip to place the organoids on the sample table and add OCT; then, directly freeze in a pre-cooled -20 °C frozen cryostat.
Slice blocks of frozen organoids to a thickness of 8 μm under a cryomicrotome and attach the tissue to an adhesive microscope slide.
Store the frozen slices at -20 °C for immunofluorescence staining.
Immunofluorescence staining
Thaw frozen sections in a sealed environment to avoid damage by frozen water crystals.
Incubate with 0.5% Triton X-100 in PBS for 15 min.
Note: This step helps with permeabilization, especially for sections thicker than 8 μm.
Block with blocking buffer (see Recipes) for 30 min at RT.
Note: This step will help to reduce background staining; a longer incubation may be more effective.
Clean around the tissue after blocking with wiping paper.
Note: The wiping paper should be free of scraps.
Dilute the antibody with dilution buffer (see Recipes) in different proportions and mix well in 1.5 mL centrifuge tubes. Then, add to the tissue drop by drop and cover with parafilm M wrapping film to prevent evaporation. Keep it still during incubation at 4 °C overnight to avoid membrane movement.
Note: When performing immunofluorescence staining, add sterile Milli-Q treated water to the bottom of the dyeing tank to prevent evaporation from drying the tissue. Also, keep it bubble-free when placing the parafilm on the tissue.
On the next day, use the dyeing tank to soak the tissue with 0.5% Triton X-100 in PBS three times for 5 min each time.
Dilute the corresponding fluorescent secondary antibody and DAPI dye with dilution buffer as shown in the antibodies list, then add to the tissue drop by drop and incubate at RT for 2 h.
Note: Avoid exposure to light after the addition of secondary antibody and DAPI.
Soak in the dyeing tank with 0.5% Triton X-100 in PBS three times at RT for 5 min each time.
After adding 10 μL of anti-fluorescence quenching agent, add a coverslip, wrap the samples in tin foil, and scan tissues with a multi-photon laser scanning microscope.
Note: The samples wrapped in tin foil can be stored at 4 °C for up to one week.
As shown in Figure 4, immunofluorescence staining characterization of airway organoids shows that the organoids cultured for 18 days are multilayered cellular structures, which express the terminally differentiated ciliated cell-specific marker Ac-tubulin on the luminal side and the basal cell-specific markers (Tp63 and Krt5) on the peripheral side (Figure 4).
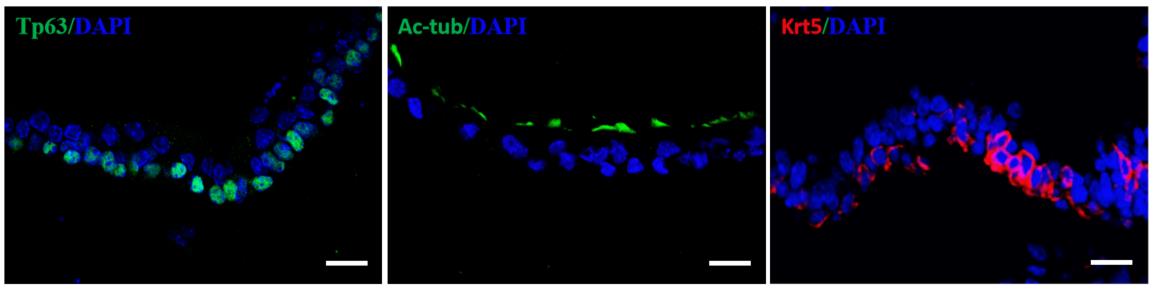
Figure 4. Representative sectional immunofluorescent images showing that organoids express basal cell markers (Krt5, red; Tp63, green) and differentiated ciliated cells (Ac-tub, green). DAPI stains the nuclei (blue). Scale bars: 25 μm.
Validation of protocol
The reproducibility of the protocol has been described within the corresponding article: Li et al. [11].
General notes and troubleshooting
Troubleshooting (Table 1)
Table 1. Troubleshooting suggestions
| Problem | Possible reason | Possible solution |
|---|---|---|
| Few progenitor cells were isolated from a single airway. | The airway is prone to rupturing during the stripping process. | 1. Increase the amount of digested tissues. 2. Constantly shake the digestive fluid that contains the airways. |
| Clogging of mesh. | After overnight digestion, undigested tissues, such as cartilage, remain. | Refilter using a new 300-mesh cell strainer. |
Organoids fail to form lumen-like structures. | 1. The initial seeding density of tracheal progenitors is too high. 2. The various factors added to the medium are not preserved properly and lose potency. | 1. Reduced density of tracheal progenitor cells. 2. If lumen-like structures fail to form, the experiment must be attempted again from the beginning. |
| Trachea epithelial progenitors and organoids appear to drop to the bottom of the Matrigel droplet. | 1. The Matrigel has been repeatedly frozen and thawed. 2. Matrigel and cell mixture placed at 37 °C for too short. | 1. Matrigel must be aliquoted, stored strictly in accordance with the manufacturer's instructions, and used within the expiration date. 2. Extend 20 min of incubation of Matrigel and cell mixture at 37 °C. |
Acknowledgments
We thank the staff of the facility of the Institute of Health Sciences & Technology, Anhui University, for providing technical support. This work was supported by grants from the National Natural Sciences Foundation of China (32270880).
This protocol was derived from the previous publication Li et al. [11].
Competing interests
The authors declare that they have no conflicts of interest with the contents of this article.
Ethical considerations
This study was approved by the animal welfare committee of Anhui University (IACUC(AHU)-2022-012).
References
- Basil, M. C., Katzen, J., Engler, A. E., Guo, M., Herriges, M. J., Kathiriya, J. J., Windmueller, R., Ysasi, A. B., Zacharias, W. J., Chapman, H. A., et al. (2020). The Cellular and Physiological Basis for Lung Repair and Regeneration: Past, Present, and Future. Cell Stem Cell. 26(4): 482–502.
- Que, J., Luo, X., Schwartz, R. J. and Hogan, B. L. M. (2009). Multiple roles for Sox2 in the developing and adult mouse trachea. Development. 136(11): 1899–1907.
- Dye, B. R., Hill, D. R., Ferguson, M. A., Tsai, Y. H., Nagy, M. S., Dyal, R., Wells, J. M., Mayhew, C. N., Nattiv, R., Klein, O. D., et al. (2015). In vitro generation of human pluripotent stem cell derived lung organoids. eLife. 4: e05098.
- Miller, A. J., Dye, B. R., Ferrer-Torres, D., Hill, D. R., Overeem, A. W., Shea, L. D. and Spence, J. R. (2019). Generation of lung organoids from human pluripotent stem cells in vitro. Nat Protoc. 14(2): 518–540.
- Danahay, H., Pessotti, A. D., Coote, J., Montgomery, B. E., Xia, D., Wilson, A., Yang, H., Wang, Z., Bevan, L., Thomas, C., et al. (2015). Notch2 Is Required for Inflammatory Cytokine-Driven Goblet Cell Metaplasia in the Lung. Cell Rep. 10(2): 239–252.
- Lafkas, D., Shelton, A., Chiu, C., de Leon Boenig, G., Chen, Y., Stawicki, S. S., Siltanen, C., Reichelt, M., Zhou, M., Wu, X., et al. (2015). Therapeutic antibodies reveal Notch control of transdifferentiation in the adult lung. Nature. 528(7580): 127–131.
- Shi, R., Radulovich, N., Ng, C., Liu, N., Notsuda, H., Cabanero, M., Martins-Filho, S. N., Raghavan, V., Li, Q., Mer, A. S., et al. (2020). Organoid Cultures as Preclinical Models of Non–Small Cell Lung Cancer. Clin Cancer Res. 26(5): 1162–1174.
- Kim, J. H., An, G. H., Kim, J. Y., Rasaei, R., Kim, W. J., Jin, X., Woo, D. H., Han, C., Yang, S. R., Kim, J. H., et al. (2021). Human pluripotent stem cell-derived alveolar organoids for modeling pulmonary fibrosis and drug testing. Cell Death Discov. 7(1): 48.
- Sachs, N., Papaspyropoulos, A., Zomer‐van Ommen, D. D., Heo, I., Böttinger, L., Klay, D., Weeber, F., Huelsz‐Prince, G., Iakobachvili, N., Amatngalim, G. D., et al. (2019). Long‐term expanding human airway organoids for disease modeling. EMBO J. 38(4): e2018100300.
- Tindle, C., Fuller, M., Fonseca, A., Taheri, S., Ibeawuchi, S. R., Beutler, N., Katkar, G. D., Claire, A., Castillo, V., Hernandez, M., et al. (2021). Adult stem cell-derived complete lung organoid models emulate lung disease in COVID-19. eLife. 10: e66417.
- Li, Q., Jiao, J., Heng, Y., Lu, Q., Zheng, Y., Li, H., Cai, J., Mei, M. and Bao, S. (2023). Prmt5 promotes ciliated cell specification of airway epithelial progenitors via transcriptional inhibition of Tp63. J Biol Chem. 299(8): 104964.
- Bukowy-Bieryłło, Z. (2021). Long-term differentiating primary human airway epithelial cell cultures: how far are we? Cell Commun Signaling. 19(1): 63.
- Barkauskas, C. E., Chung, M. I., Fioret, B., Gao, X., Katsura, H. and Hogan, B. L. M. (2017). Lung organoids: current uses and future promise. Development. 144(6): 986–997.
Article Information
Publication history
Received: Aug 7, 2024
Accepted: Oct 7, 2024
Available online: Oct 22, 2024
Published: Dec 5, 2024
Copyright
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Zhang, Z., Tao, C. and Li, Q. (2024). Culture and Characterization of Differentiated Airway Organoids from Fetal Mouse Lung Proximal Progenitors. Bio-protocol 14(23): e5129. DOI: 10.21769/BioProtoc.5129.
- Li, Q., Jiao, J., Heng, Y., Lu, Q., Zheng, Y., Li, H., Cai, J., Mei, M. and Bao, S. (2023). Prmt5 promotes ciliated cell specification of airway epithelial progenitors via transcriptional inhibition of Tp63. J Biol Chem. 299(8): 104964.
Category
Developmental Biology > Cell growth and fate > Differentiation
Stem Cell > Organoid culture
Cell Biology > Cell isolation and culture > 3D cell culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link