- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Optimized Isolation of Lysosome-Related Organelles from Stationary Phase and Iron-Overloaded Chlamydomonas reinhardtii Cells
Published: Vol 14, Iss 22, Nov 20, 2024 DOI: 10.21769/BioProtoc.5111 Views: 1767
Reviewed by: Ritu GuptaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Sorghum bicolor Extracellular Vesicle Isolation, Labeling, and Correlative Light and Electron Microscopy
Deji Adekanye [...] Jeffrey L. Caplan
Oct 5, 2024 2118 Views

Isolation and Biophysical Characterization of Extracellular Vesicles From Hairy Root Cultures
Marisa Conte [...] Alfredo Ambrosone
Mar 5, 2025 2219 Views

Rapid Miniprep of Intact Chloroplasts from Arabidopsis thaliana Leaves
Brenda A. Carranza-Correa [...] Manuel Gutiérrez-Aguilar
May 20, 2025 2646 Views
Abstract
Lysosome-related organelles (LROs) are a class of heterogeneous subcellular organelles conserved in eukaryotes, performing various functions. An important function of LROs is to mediate phosphorus and metal homeostasis. Chlamydomonas reinhardtii serves as a model organism for investigating metal ion metabolism. Considering that LROs contain polyphosphate and various metal elements, the purification strategy is based on their higher density by fractionating cell lysate through OptiPrep density gradient ultracentrifugation. Here, we optimized a method for purifying LROs from C. reinhardtii cells that have reached stationary phase (sta-LROs) or are overloaded with iron (Fe-LROs). Our protocol provides technical support for further investigations on the biogenesis and function of LROs in C. reinhardtii.
Key features
• This protocol purifies LROs from C. reinhardtii without disrupting the structure of chloroplasts.
• Following the purification of sta-LROs, these can be further fractionated into subgroups with distinct densities through the second iodixanol gradient.
• This protocol is applicable for the purification of LROs from the cell wall–deficient C. reinhardtii strain cw15.
Keywords: sta-LROsGraphical overview
Background
Eukaryotic cells have evolved sophisticated mechanisms to counteract imbalances in environmental metal ions by sequestering excess metal ions (such as iron, copper, manganese, calcium, or zinc) within subcellular compartments to alleviate the toxicity of excessive metals [1–5]. These stored metal ions are subsequently utilized under conditions of deficiency to sustain intracellular metal ion homeostasis. Lysosome-related organelles (LROs) are a group of subcellular organelles involved in the storage of metal ions, including vacuoles in plants and yeast, acidocalcisomes in trypanosomes and algae, and other LROs [2,3,6].
Chlamydomonas reinhardtii is a unicellular green alga, serving as a eukaryotic model organism for studying trace element homeostasis, owing to its ability to grow under conditions of both excess and deficiency of metal elements [7,8]. Moreover, C. reinhardtii exhibits the capability to absorb and transform toxic metals from the environment [9–13], making it a primary producer model for investigating the mechanisms of toxic metal uptake and conversion. Docampo and colleagues isolated polyphosphate bodies from C. reinhardtii and reported that they were similar to acidocalcisomes in terms of their chemical composition and the presence of proton pumps [14]. When there is an excess of iron, copper, and manganese ions in the cultured medium, C. reinhardtii cells sequester the absorbed excess metal ions into LROs, maintaining the homeostasis of metal ions [1,4,5].
Our previous work has demonstrated that C. reinhardtii LROs exhibit heterogeneity in their morphology, elemental content, and protein composition. These LROs are crucial for the storage and homeostasis of trace metals, calcium, and phosphorus [15]. Sta-LROs, which are generated in cells during the stationary phase [15], resemble the protein lytic vacuoles in plants [16] and the digestive vacuoles of the malaria parasite [17]. Additionally, Fe-LROs, which arise in cells overloaded with iron, share similarities with acidocalcisomes [15]. Mg, Ca, Fe, Cu, Zn, and Mn are localized within polyphosphate granules in both sta-LROs and Fe-LROs, which are crucial organelles for the storage of phosphorus and metal ions within cells [15]. We have developed a method to quantify the storage capacity of LROs for phosphorus and metal ions, enabling the assessment of the sequestration of specific trace metals into the LROs under varying conditions [15]. Furthermore, 21 metal transporters have been identified in sta-LROs and Fe-LROs, suggesting their potential roles in mediating the transport of trace metal ions into or out of LROs [15].
Establishing a purification protocol for C. reinhardtii LROs is crucial for studying the formation and function of LROs, including their roles in metal ion metabolism and the transformation of environmentally toxic metals. Here, we introduce the purification steps for sta-LROs and Fe-LROs. The isolation principle relies on the high density of LROs, due to their rich content of phosphorus and metal ions. The cells are squeezed by using a syringe with a needle (diameter of 25G), which preserves the chloroplast structure; sta-LROs and Fe-LROs are then purified via OptiPrep density gradient ultracentrifugation. The limitation of this protocol is that it can only purify LROs from the cell wall–deficient C. reinhardtii strain, as the syringe compression method is insufficient to break wild-type C. reinhardtii cells. In future studies, it would be worth exploring the use of commercial Subtilisin (Alcalase) [18], to remove the cell wall of C. reinhardtii without disrupting other subcellular structures, followed by utilizing this protocol to purify LROs from wild-type C. reinhardtii cells.
Materials and reagents
Reagents
NH4Cl (Sinopharm Chemical Reagent, CAS number: 12125-02-9)
MgSO4·7H2O (Sinopharm Chemical Reagent, CAS number: 10034-99-8)
CaCl2·H2O (Sinopharm Chemical Reagent, CAS number: 10035-04-8)
Tris base (Genview, CAS number: BT350-500G)
KH2PO4 (Sinopharm Chemical Reagent, CAS number: 7778-77-0)
K2HPO4 (Sinopharm Chemical Reagent, CAS number: 7758-11-4)
Na2EDTA·2H2O (Sinopharm Chemical Reagent, CAS number: 6381-92-6)
(NH4)6Mo7O24 (Sinopharm Chemical Reagent, CAS number: 12054-85-2)
ZnSO4·7H2O (Sinopharm Chemical Reagent, CAS number: 7446-20-0)
H3BO3 (Sinopharm Chemical Reagent, CAS number: 10043-35-3)
MnCl2·4H2O (Sinopharm Chemical Reagent, CAS number: 13446-34-9)
CoCl2·6H2O (Sinopharm Chemical Reagent, CAS number: 7791-13-1)
CuSO4·5H2O (Sinopharm Chemical Reagent, CAS number: 7758-99-8)
FeSO4·7H2O (Sinopharm Chemical Reagent, CAS number: 7782-63-0)
KOH (Sinopharm Chemical Reagent, CAS number: 1310-58-3)
Glacial acetic acid (Sinopharm Chemical Reagent, CAS number: 64-19-7)
NH4Cl (MACKLIN, CAS number: 12125-02-9)
CaCl2·2H2O (MACKLIN, CAS number: 10035-04-8)
MgSO4·7H2O (MACKLIN, CAS number: 10034-99-8)
K2HPO4 (MACKLIN, CAS number: 7758-11-4)
KH2PO4 (MACKLIN, CAS number: 7778-77-0)
Acetic acid (MACKLIN, CAS number: 64-19-7)
EDTA Na2·2H2O (Genview, CAS number: 6381-92-6)
(NH4)6Mo7O24·4H2O (MACKLIN, CAS number: 12027-67-7)
ZnSO4·7H2O (MACKLIN, CAS number: 7446-20-0)
MnCl2·4H2O (MACKLIN, CAS number: 13446-34-9)
FeCl3·6H2O (MACKLIN, CAS number: 10025-77-1)
Na2CO3 (MACKLIN, CAS number: 497-19-8)
CuCl2·2H2O (MACKLIN, CAS number: 10125-13-0)
HCl (Sinopharm Chemical Reagent, CAS number: 7647-01-0)
EGTA (Genview, CAS number: 67-42-5)
KCl (Sinopharm Chemical Reagent, CAS number: 7447-40-7)
MgCl2 (Sinopharm Chemical Reagent, CAS number: 7786-30-3)
Sucrose (Biofroxx, CAS number: 57-50-1)
OptiPrep density gradient medium (Sigma-Aldrich, CAS number: 92339-11-2)
DTT (Blotopped, CAS number: 3483-12-3)
Protease inhibitor cocktail (Sigma, CAS number: P9599)
Solutions
TAP medium (see Recipes)
Revised TAP (see Recipes)
IB buffer (see Recipes)
OptiPrep gradient (see Recipes)
2 M DTT (see Recipes)
Recipes
Tris-acetate-phosphate (TAP) medium
Reagent Final concentration Quantity or Volume TAP salts (100×) 1% (v/v) 10 mL Tris base (100×) 1% (v/v) 10 mL Phosphate buffer (1,000×) 0.1% (v/v) 1 mL Hutner trace elements (1,000×) 0.1% (v/v) 1 mL Glacial acetic acid 0.1% (v/v) 1 mL Milli-Q Water Dilute with H2O to a final volume of 1 L Total 1 L Stocks for TAP medium:
TAP salts (100×)
Reagent Final concentration Quantity or Volume NH4Cl 0.7 M 37.5 g MgSO4·7H2O 40 mM 10 g CaCl2·H2O 30 mM 5 g Milli-Q H2O Dilute with H2O to a final volume of 1 L Total 1 L Tris base (100×)
Reagent Final concentration Quantity or Volume Tris base 2 M 242 g Milli-Q H2O Dilute with H2O to a final volume of 1 L Total 1 L Phosphate buffer (1,000×)
Reagent Final concentration Quantity or Volume KH2PO4 0.4 M 27 g K2HPO4 0.62 M 54 g Milli-Q H2O Dilute with H2O to a final volume of 500 mL Total 500 mL Hutner trace elements
Note: Store at 4 °C.
Reagent Quantity or Volume Na2EDTA·2H2O 55.36 g (NH4)6Mo7O24·4H2O 1.108 g ZnSO4·7H2O 22 g H3BO3 11.4 g MnCl2·4H2O 5.06 g CoCl2·6H2O 1.61 g CuSO4·5H2O 1.57 g FeSO4·7H2O 4.99 g Milli-Q H2O Dilute with H2O to a final volume of 1 L Total 1 L Dissolve 55.36 g Na2EDTA·2H2O in 250 mL of H2O and gradually add KOH with heating to accelerate the dissolution. Dissolve 1.108 g of (NH4)6Mo7O24·4H2O in 50 mL of H2O. Dissolve 22 g of ZnSO4·7H2O in 100 mL of H2O. Dissolve 11.4 g of H3BO3 in 200 mL of H2O with heating. Dissolve 5.06 g of MnCl2·4H2O in 50 mL of H2O. Dissolve 1.61 g of CoCl2·6H2O in 50 mL of H2O. Dissolve 1.57 g of CuSO4·5H2O in 50 mL of H2O. Dissolve 4.99 g of FeSO4·7H2O in 50 mL of H2O before mixing to avoid oxidation.
Once all the solutions mentioned above are prepared, mix them, excluding Na2EDTA. Boil the mixture, then add the Na2EDTA solution. The mixture will turn green. Ensure all reagents are completely dissolved, then cool the solution to 70 °C and maintain this temperature. Adjust the pH to 6.7 using KOH (calibrate the pH meter at 70 °C; NaOH is unsuitable for this purpose). After adjusting the pH, dilute the solution to a final volume of 1 L, seal the conical flask with cotton, and let it stand for 1–2 weeks, shaking daily. The solution will eventually turn purple and form a rusty brown precipitate. Filter out the precipitate and store the resulting solution at 4 °C for future use.
Revised TAP medium
Note: Reagents used in the revised TAP medium must be trace element grade. Reagents purchased from MACKLIN are only used in revised TAP.
Reagent Final concentration Quantity or Volume Beijerinck’s solution 1% (v/v) 10 mL Phosphate solution 0.833% (v/v) 8.33 mL Tris-Acetate stock solution 1% (v/v) 10 mL Na2EDTA stock solution 25 µM 1 mL (NH4)6Mo7O24 stock solution 28.5 nM 1 mL Na2SeO3 stock solution 0.1 µM 1 mL ZnEDTA stock solution 2.5 µM 1 mL MnEDTA stock solution 6 µM 1 mL FeEDTA stock solution 20 µM 1 mL CuEDTA stock solution 2 µM 1 mL Milli-Q Water Dilute with H2O to a final volume of 1 L Total 1 L For iron-limited TAP medium, add 0.01 mL of FeEDTA stock solution. For iron-overloading TAP medium, add 10 mL FeEDTA stock solution.
Stocks for revised TAP medium:
Beijerinck’s solutions (100×)
Note: Store at 4 °C.
Reagent Final concentration Quantity or Volume NH4Cl 750 mM 40 g CaCl2·2H2O 34 mM 5 g MgSO4·7H2O 40 mM 10 g Milli-Q Water Dilute with H2O to a final volume of 1 L Total 1 L Phosphate solution (120×)
Note: Store at 4 °C.
Reagent Final concentration Quantity or Volume K2HPO4 82 mM 14.34 g KH2PO4 54 mM 7.26 g Milli-Q Water Dilute with H2O to a final volume of 1 L Total 1 L Tris-Acetate stock solution (100×)
Note: Store at 4 °C.
Reagent Final concentration Quantity or Volume Tris base 2 M 242 g Acetic acid 1.7 M 100 mL Milli-Q Water Dilute with H2O to a final volume of 1 L Total 1 L Na2EDTA concentrate (Pre 1)
Note: Store at 4 °C.
Reagent Final concentration Quantity or Volume Na2EDTA·2H2O 125 mM 13.959 g Milli-Q Water Dilute with H2O to a final volume of 300 mL Total 300 mL Dissolve 13.959 g of Na2EDTA·2H2O in about 250 mL of H2O and titrate to pH 8.0 with trace element grade KOH (about 1.7 g).
(NH4)6Mo7O24 concentrate (Pre 2)
Note: Store at 4 °C.
Reagent Final concentration Quantity or Volume (NH4)6Mo7O24·4H2O 285 µM 0.088 g Milli-Q Water Dilute with H2O to a final volume of 250 mL Total 250 mL Na2SeO3 concentrate (Pre 3)
Note: Store at 4 °C.
Reagent Final concentration Quantity or Volume Na2SeO3 1 mM 0.043 g Milli-Q Water Dilute with H2O to a final volume of 250 mL Total 250 mL Individual stock solutions (1,000×) of trace elements
Note: Store at 4 °C.
i. Na2EDTA stock solution
Reagent Final concentration Quantity or Volume Na2EDTA 25 mM 50 mL of Pre 1 Milli-Q Water 200 mL Total 250 mL ii. (NH4)6Mo7O24 stock solution
Reagent Final concentration Quantity or Volume (NH4)6Mo7O24 28.5 µM 25 mL of Pre 2 Milli-Q Water 225 mL Total 250 mL iii. Na2SeO3 stock solution
Reagent Final concentration Quantity or Volume Na2SeO3 0.1 mM 25 mL of Pre 3 Milli-Q Water 225 mL Total 250 mL iv. ZnEDTA stock solution
Reagent Final concentration Quantity or Volume ZnSO4·7H2O 2.5 mM 0.18 g Na2EDTA 2.75 mM 5.5 mL of Pre1 Milli-Q Water Dilute with H2O to a final volume of 250 mL Total 250 mL v. MnEDTA stock solution
Reagent Final concentration Quantity or Volume MnCl2·4H2O 6 mM 0.297 g Na2EDTA 6 mM 12 mL of Pre1 Milli-Q Water Dilute with H2O to a final volume of 250 mL Total 250 mL vi. FeEDTA stock solution
Reagent Final concentration Quantity or Volume FeCl3·6H2O 20 mM 1.35 g Na2EDTA 22 mM 2.05 g Na2CO3 22 mM 0.58 g Milli-Q Water Dilute with H2O to a final volume of 250 mL Total 250 mL Note: Mix Na2EDTA with Na2CO3 in Milli-Q water. Add FeCl3·6H2O after the first two components are dissolved. Do not use Pre 1.
vii. CuEDTA stock solution
Reagent Final concentration Quantity or Volume CuCl2·2H2O 2 mM 0.085 g Na2EDTA 2 mM 4 mL of Pre1 Milli-Q Water Dilute with H2O to a final volume of 250 mL Total 250 mL
Isolation buffer (IB buffer)
Note: Store at 4 °C.
Reagent Final concentration Quantity or Volume 1 M Tris-HCl 20 mM 4 mL 0.5 M EGTA 5 mM 2 mL 0.5 M KCl 5 mM 2 mL 0.2 M MgCl2 2 mM 2 mL Sucrose 6% (W/V) 12 g Milli-Q Water Dilute with H2O to a final volume of 200 mL Total 200 mL Stocks for IB buffer:
1 M Tris-Cl
Reagent Final concentration Quantity or Volume Tris base 1 M 12.114 g Milli-Q Water Dilute with H2O to a final volume of 100 mL Total 100 mL Dissolve 12.114 g of Tris base in 50 mL of H2O, adjust the pH to 7.6 with HCl, and adjust to 100 mL.
0.5 M EGTA
Reagent Final concentration Quantity or Volume EGTA 0.5 M 19 g Milli-Q Water Dilute with H2O to a final volume of 100 mL Total 100 mL 0.5 M KCl
Reagent Final concentration Quantity or Volume KCl 0.5 M 3.725 g Milli-Q Water Dilute with H2O to a final volume of 100 mL Total 100 mL 0.2 M MgCl2
Reagent Final concentration Quantity or Volume MgCl2 0.2 M 1.9 g Milli-Q Water Dilute with H2O to a final volume of 100 mL Total 100 mL
OptiPrep gradient
Note: OptiPrep density gradient medium needs to be dried into iodixanol powder at 50 °C before use. Store at 4 °C.
Reagent Final concentration Quantity or Volume OptiPrep density gradient 80% (w/v) 3.2 g of iodixanol powder in 4 mL of IB buffer 60% (w/v) 2.4 g of iodixanol powder in 4 mL of IB buffer 40% (w/v) 1.6 g of iodixanol powder in 4 mL of IB buffer 37% (w/v) 1.48 g of iodixanol powder in 4 mL of IB buffer 34% (w/v) 1.36 g of iodixanol powder in 4 mL of IB buffer 27% (w/v) 1.08 g of iodixanol powder in 4 mL of IB buffer 24% (w/v) 0.96 g of iodixanol powder in 4 mL of IB buffer 15% (w/v) 0.6 g of iodixanol powder in 4 mL of IB buffer 2 M DTT
Note: Store at -20 °C.
Reagent Final concentration Quantity or Volume DTT 2 M 0.3085 g Milli-Q Water Dilute with H2O to a final volume of 1 mL Total 1 mL
Laboratory supplies
Blue cap bottles, 1 L (Beyotime, catalog number: FBT008), 500 mL (Beyotime, catalog number: FBT006), 250 mL (Beyotime, catalog number: FBT002)
1 L beaker, 500 mL beaker, 250 mL beaker
500 mL glass conical flask
Volumetric flasks of different volumes
50 mL centrifuge tube
BD Precision Glide needle 25G×5/8 (Becton Dickinson Medical, catalog number: 301805)
Open-top thin wall ultra-clear tube, 5 mL (Beckman Coulter, catalog number: 344057)
1.5 mL Eppendorf tubes
Equipment
Avanti® J-E centrifuge (Beckman Coulter, model: 369001)
96 mm diameter polypropylene conical bottle adapter (Beckman Coulter, model: 392078)
JS-5.3 AllSpin swinging-bucket rotor and buckets (Beckman Coulter, model: 368690)
JA-20 fixed-angle aluminum rotor (Beckman Coulter, model: 334831)
Desktop micro speed freezing centrifuge Optima MAX-XP (Beckman Coulter, model: 393315)
MLS-50 swinging-bucket rotor (Beckman Coulter, model: 367280)
Microcentrifuge (Eppendorf, model: 5424R)
Procedure
Cell cultures
Inoculate cell wall–deficient strain cw15 cells grown on a TAP plate into two flasks containing 300 mL of TAP medium. Culture the cells at 24 °C with shaking (180 rpm) under continuous illumination (25 E·m-2·s-1) for 6 days. These cells can then be used to purify sta-LROs.
For the iron overloading experiment, inoculate 5–6 mL of 2-day-old cw15 cells, cultured in revised TAP medium, into two flasks containing 300 mL of iron-limited TAP medium (0.2 µM Fe). Culture the cells under continuous light for 5 days with shaking (180 rpm) until the cell density reaches 3–5 × 106 cells mL−1. Subsequently, culture cells for another 26–28 h in iron-overloading TAP medium (200 µM Fe). These cells can then be used to isolate Fe-LROs.
Note: It is recommended to purify LROs from freshly cultured cells. Storing cell pellets at -80 °C is not advised, as freeze-thaw cycles can cause partial cell lysis and chloroplast disruption, leading to impure LROs extraction.
Cell disruption
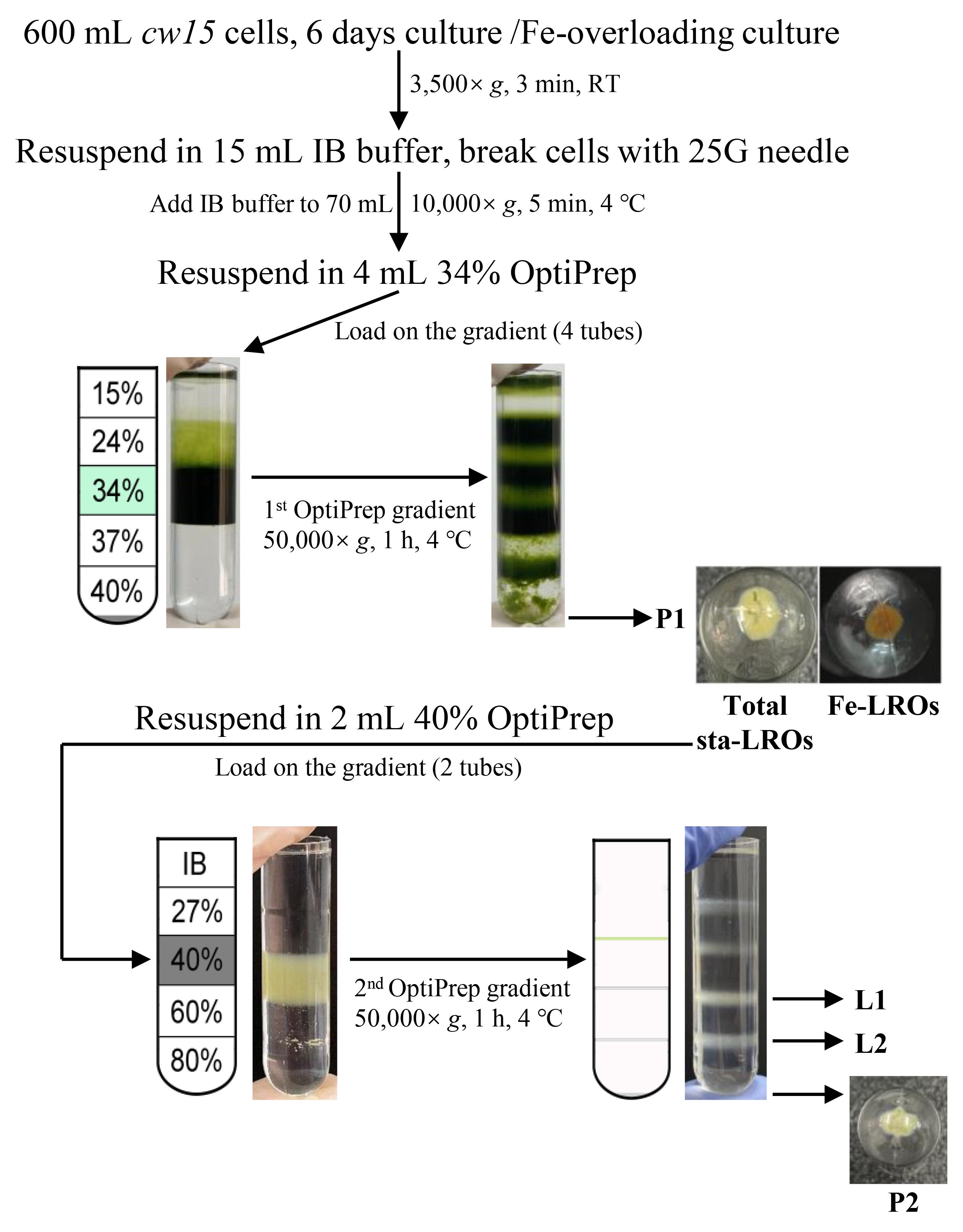
Collect the cells from each flask (600 mL in total) by centrifugation at 3,500× g for 3 min.
Discard the supernatant and wash the pellets once with 100 mL of IB buffer by resuspending with a pipette.
Discard the supernatant again and resuspend the total pellets in 15 mL of IB buffer supplemented with a complete protease inhibitor cocktail and 2 mM DTT.
Disrupt the cells by squeezing them through a syringe with a 25 G needle three times, ensuring most cells are opened without disrupting the chloroplast structure. During the squeeze process, ensure that the cell suspension flows out of the syringe needle in a continuous stream rather than intermittently dripping.
First OptiPrep gradient ultracentrifugation
Adjust the volume of each sample to 70 mL with IB buffer.
Centrifuge the samples at 10,000× g for 10 min at 4 °C.
Discard the supernatant and resuspend the pellets in 4 mL of 34% OptiPrep (add 8 μL of DTT and 40 μL of protein inhibitor cocktail before use).
Prepare a discontinuous OptiPrep gradient with 1 mL each of 15%, 24%, 34% (with samples), 37%, and 40% OptiPrep. It is recommended to prepare the OptiPrep gradient solution fresh for each use. The gradient should be constructed from the bottom up, starting with the highest density gradient at the bottom of the ultracentrifuge tube. Using a 1 mL pipette, carefully layer each subsequent gradient along the inner wall of the centrifuge tube at the slowest possible speed. During the gradient preparation process, distinct boundaries between different density layers can be clearly observed when held up to the light. There is no need for pause time between the different density layers.
Centrifuge the gradient at 50,000× g in a Beckman MLS-50 rotor for 60 min at 4 °C, setting the acceleration to the maximum level and the deceleration to coast. The pellet below the 40% layer (P1) is the purified total LROs. Purified LROs can be stored at -80 °C for subsequent protein identification and related experiments, with a recommended storage duration of no more than three months. For localization experiments such as staining or immunofluorescence, it is recommended to use the purified LROs immediately after preparation.
Note: The above steps are applicable for the purification of both sta-LROs and Fe-LROs from cw15 cells.
Second OptiPrep gradient ultracentrifugation
Discard the supernatant and resuspend the total sta-LROs with 2 mL of 40% OptiPrep (add 4 μL of DTT and 20 μL of protein inhibitor cocktail before use).
Prepare a discontinuous OptiPrep gradient with 1 mL each of IB buffer, 27%, 40% (with samples), 60%, and 80% OptiPrep.
Centrifuge the gradient at 50,000× g in a Beckman MLS-50 rotor for 60 min at 4 °C, setting the acceleration to the maximum level and the deceleration to coast.
Collect each layer (L1: layer between 40% and 60%, L2: layer between 60% and 80%) and the pellet below 80% (P2) in 1.5 mL Eppendorf tubes, fill to 1.5 mL with IB buffer, and mix by repeated pipetting.
Centrifuge at 15,000× g for 10 min at 4 °C.
Discard the supernatant; pellets are subgroups of sta-LROs separated by different densities.
Note: The second OptiPrep gradient ultracentrifugation is used to separate total sta-LROs into subgroups, as most Fe-LROs accumulate in the pellet below 80% OptiPrep due to their high density.
Validation of protocol
This protocol has been used and validated in the following research article:
Long et al. [15]. Structural and functional regulation of Chlamydomonas lysosome-related organelles during environmental changes. Plant Physiology. DOI: 10.1093/plphys/kiad189
General notes and troubleshooting
General notes
Set the acceleration to the maximum level and the deceleration to coast during ultracentrifugation.
Vacuum must be applied prior to ultracentrifugation.
Acknowledgments
This study was supported by the National Key R&D Program of China (2020YFA0907400). This protocol was described and validated in the following research article: Long et al. [15]. Structural and functional regulation of Chlamydomonas lysosome-related organelles during environmental changes. Plant Physiology. DOI: 10.1093/plphys/kiad189.
Competing interests
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Hong-Hermesdorf, A., Miethke, M., Gallaher, S. D., Kropat, J., Dodani, S. C., Chan, J., Barupala, D., Domaille, D. W., Shirasaki, D. I. and Loo, J. A. (2014). Subcellular metal imaging identifies dynamic sites of Cu accumulation in Chlamydomonas. Nat Chem Biol. 10(12): 1034–1042.
- Docampo, R. and Huang, G. (2016). Acidocalcisomes of eukaryotes. Curr Opin Cell Biol. 4166–72.
- Docampo, R., (2024). Advances in the cellular biology, biochemistry, and molecular biology of acidocalcisomes. Microbiol Mol Biol Rev. 88(1): e00042–23.
- Tsednee, M., Castruita, M., Salomé, P. A., Sharma, A., Lewis, B. E., Schmollinger, S. R., Strenkert, D., Holbrook, K., Otegui, M. S. and Khatua, K. (2019). Manganese co-localizes with calcium and phosphorus in Chlamydomonas acidocalcisomes and is mobilized in manganese-deficient conditions. J Biol Chem. 294(46): 17626–17641.
- Schmollinger, S., Chen, S., Strenkert, D., Hui, C., Ralle, M. and Merchant, S. S. (2021). Single-cell visualization and quantification of trace metals in Chlamydomonas lysosome-related organelles. Proc Natl Acad Sci USA. 118(16): e2026811118.
- Blaby-Haas, C. E. and Merchant, S. S. (2014). Lysosome-related Organelles as Mediators of Metal Homeostasis. J Biol Chem. 289(41): 28129–28136.
- Blaby-Haas, C. E. and Merchant, S. S. (2017). Regulating cellular trace metal economy in algae. Curr Opin Plant Biol. 3988–96.
- Salomé, P. A. and Merchant, S. S. (2019). A Series of Fortunate Events: Introducing Chlamydomonas as a Reference Organism. Plant Cell. 31(8): 1682–1707.
- Hanikenne, M., (2003). Chlamydomonas reinhardtii as a eukaryotic photosynthetic model for studies of heavy metal homeostasis and tolerance. New Phytol. 159(2): 331–340.
- Beauvais-Flück, R., Slaveykova, V. I. and Cosio, C. (2017). Cellular toxicity pathways of inorganic and methyl mercury in the green microalga Chlamydomonas reinhardtii. Sci Rep. 7(1): 8034.
- Samadani, M., Perreault, F., Oukarroum, A. and Dewez, D. (2018). Effect of cadmium accumulation on green algae Chlamydomonas reinhardtii and acid-tolerant Chlamydomonas CPCC 121. Chemosphere. 191174–182.
- Samadani, M. and Dewez, D. (2018). Cadmium accumulation and toxicity affect the extracytoplasmic polyphosphate level in Chlamydomonas reinhardtii. Ecotoxicol Environ Saf. 166200–206.
- Thiriet-Rupert, S., Gain, G., Jadoul, A., Vigneron, A., Bosman, B., Carnol, M., Motte, P., Cardol, P., Nouet, C. and Hanikenne, M. (2021). Long-term acclimation to cadmium exposure reveals extensive phenotypic plasticity in Chlamydomonas. Plant Physiol. 187(3): 1653–1678.
- Ruiz, F. A., Marchesini, N., Seufferheld, M. and Govindjee. and Docampo, R. (2001). The Polyphosphate Bodies of Chlamydomonas reinhardtii Possess a Proton-pumping Pyrophosphatase and Are Similar to Acidocalcisomes. J Biol Chem. 276(49): 46196–46203.
- Long, H., Fang, J., Ye, L., Zhang, B., Hui, C., Deng, X., Merchant, S. S. and Huang, K. (2023). Structural and functional regulation of Chlamydomonas lysosome-related organelles during environmental changes. Plant Physiol. 192(2): 927–944.
- Jiang, L., Phillips, T.E., Hamm, C.A., Drozdowicz, Y.M., Rea, P.A., Maeshima, M., Rogers, S.W. and Rogers, J.C. (2001). The protein storage vacuole: a unique compound organelle. J Cell Biol. 155991–1002.
- Biagini, G. A., Bray, P. G., Spiller, D. G., White, M. R. and Ward, S. A. (2003). The Digestive Food Vacuole of the Malaria Parasite Is a Dynamic Intracellular Ca2+ Store. J Biol Chem. 278(30): 27910–27915.
- Hwang, H. J., Kim, Y. T., Kang, N. S. and Han, J. W. (2018). A Simple Method for Removal of the Chlamydomonas reinhardtii Cell Wall Using a Commercially Available Subtilisin(Alcalase). Microb Physiol. 28(4): 169–178.
Article Information
Publication history
Received: Jul 9, 2024
Accepted: Sep 13, 2024
Available online: Oct 15, 2024
Published: Nov 20, 2024
Copyright
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Li, J. and Long, H. (2024). Optimized Isolation of Lysosome-Related Organelles from Stationary Phase and Iron-Overloaded Chlamydomonas reinhardtii Cells. Bio-protocol 14(22): e5111. DOI: 10.21769/BioProtoc.5111.
- Long, H., Fang, J., Ye, L., Zhang, B., Hui, C., Deng, X., Merchant, S. S. and Huang, K. (2023). Structural and functional regulation of Chlamydomonas lysosome-related organelles during environmental changes. Plant Physiol. 192(2): 927–944.
Category
Plant Science > Plant cell biology > Organelle isolation
Cell Biology > Organelle isolation > Lysosome
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









