- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Method for Large-scale Production of hIPSC Spheroids
Published: Vol 14, Iss 7, Apr 5, 2024 DOI: 10.21769/BioProtoc.4965 Views: 1944
Reviewed by: Alessandro DidonnaLionel SchiavolinAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
A Hybrid 2D/3D Approach for Neural Differentiation Into Telencephalic Organoids and Efficient Modulation of FGF8 Signaling
Michele Bertacchi [...] Michèle Studer
Jun 20, 2025 2777 Views

Protocol for 3D Bioprinting a Co-culture Skin Model Using a Natural Fibrin-Based Bioink as an Infection Model
Giselle Y. Díaz [...] Stephanie M. Willerth
Jul 20, 2025 3706 Views
A Simplified 3D-Plasma Culture Method for Generating Minimally Manipulated Autologous Equine Muscle-Derived Progenitor Cells
Hélène Graide [...] Didier Serteyn
Dec 5, 2025 1236 Views
Abstract
Stem cell spheroids are rapidly becoming essential tools for a diverse array of applications ranging from tissue engineering to 3D cell models and fundamental biology. Given the increasing prominence of biotechnology, there is a pressing need to develop more accessible, efficient, and reproducible methods for producing these models. Various techniques such as hanging drop, rotating wall vessel, magnetic levitation, or microfluidics have been employed to generate spheroids. However, none of these methods facilitate the easy and efficient production of a large number of spheroids using a standard 6-well plate. Here, we present a novel method based on pellet culture (utilizing U-shaped microstructures) using a silicon mold produced through 3D printing, along with a detailed and illustrated manufacturing protocol. This technique enables the rapid production of reproducible and controlled spheroids (for 1 × 106 cells, spheroids = 130 ± 10 μm) from human induced pluripotent stem cells (hIPSCs) within a short time frame (24 h). Importantly, the method allows the production of large quantities (2 × 104 spheroids for 1 × 106 cells) in an accessible and cost-effective manner, thanks to the use of a reusable mold. The protocols outlined herein are easily implementable, and all the necessary files for the method replication are freely available.
Key features
• Provision of 3D mold files (STL) to produce silicone induction device of spheroids using 3D printing.
• Cost-effective, reusable, and autoclavable device capable of generating up to 1.2× 104 spheroids of tunable diameters in a 6-well plate.
• Spheroids induction with multiple hIPSC cell lines.
• Robust and reproducible production method suitable for routine laboratory use.
Graphical overview
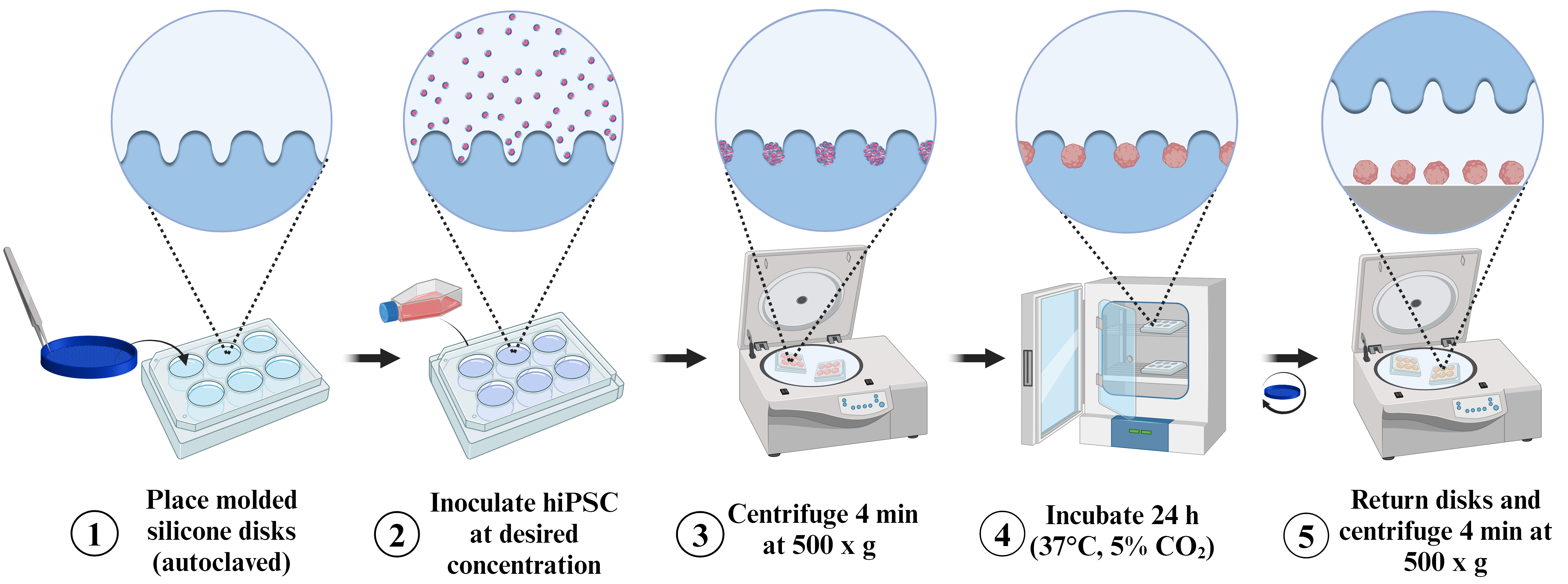
Spheroid induction process following the pellet method on molded silicon discs
Background
Stem cell spheroids ideally mimic the physiological 3D environment of tissues, making them increasingly indispensable in tissue engineering [1]. Since the inception of cellular aggregates in the 1930s, spheroids have become a model of choice across various fields of fundamental biology. Their applications span from drug testing to disease modeling, encompassing oncology and regenerative medicine [2,3]. Despite the growing use of spheroids in the past two decades, the demand for spheroids as a raw material remains substantial [4]. This development has given rise to numerous spheroid production methods, tailored to specific applications.
Concurrently, the use of human induced stem cells (hiPSCs) has surged since their discovery in 2007 [5]. This model, based on the reprogramming of unipotent cells into pluripotent ones, proves valuable from practical and ethical perspectives, offering an excellent substitute for embryonic stem cells. Today, hIPSCs play a pivotal role in both basic research and applied biology [6,7]. Simultaneously, spheroids represent a highly promising tool for both tissue engineering [8] and the study of fundamental biological phenomena [9] and have been recently used to assess the impact of the viscoelastic properties of the 3D environment on their growth [10]. The most employed techniques to form spheroids from stem cells, including hiPSCs, are rotary cell culture, hanging drop, cell culture in non-adhesive hydrogel, pellet method, and microfluidics [11]. However, these methods share drawbacks related to low productivity, time inefficiency, high cost, and very limited reusability.
The technique presented here is built upon the pellet method (U-shaped), offering a compromise between existing methods. Optimized for rapid and cost-effective production of well-defined spheroids from hiPSCs, this solution is easily transferable for integration into a routine production pipeline. Exhibiting the ability to tune the diameter of spheroids (50–150 μm) by modulating the number of cells used (from 1.0 × 105 to 1.5 × 106), its adaptability extends to other cell lines, demonstrating robust aggregation capabilities.
Materials and reagents
Biological materials
hiPSCs AG08C5 cell line (European hPSCreg as PGNMi001-A) ( https://hpscreg.eu/cell-line/PGNMi001-A) [12]
Healthy Control Human iPSC Line, Female, SCTi003-A (STEMCELL, catalog number: 200-0511)
Reagents
Maintenance culture media mTeSRTM Plus kit, cGMP (STEMCELL, catalog number: 100-0276)
Vitronectin XFTM (STEMCELL, catalog number: 100-0763)
TrypLETM (Thermo Fisher, catalog number: 12604013)
Y-27632 (Dihydrochloride), RHO/ROCK pathway inhibitor (STEMCELL, catalog number: 72302)
Fetal bovine serum (FBS) (Sigma Aldrich, catalog number:341506)
Gibco Dulbecco’s phosphate buffered saline (DPBS) (Thermo Scientific, catalog number: 14190)
Soap (e.g., Enzypin, Distrimed, catalog number: 201261)
Ethanol 70% (e.g., Fisher Scientific, catalog number: 16320638)
Solutions
hiPSC spheroids induction medium (see Recipes)
TrypLETM inhibition medium (see Recipes)
Recipes
hiPSC spheroids induction medium
Composition Final concentration Volume mTeSRTM Plus - 50 mL Y-27632 (10 mM) 10 μM 50 μL TrypLETM inhibition medium
Composition Final concentration Volume mTeSRTM Plus - 9.8 mL FBS 2% 200 μL The solutions should be freshly prepared immediately before each experiment, with volumes adjusted according to the specific requirements. Prior to contact with the cells, these solutions must be prewarmed to 37 °C. Antibiotics can be supplemented to the medium according to your culture protocol.
Laboratory supplies
P1,000 pipette (e.g., Pipetman 100–1,000 µL, Gilson, catalog number: F144059M).
Tips 1,000 µL, (e.g., AmpliPur Expert Tips 100–1,000 µL, Gilson, catalog number: F174401)
Acrylic resin (Acrylate-like) (e.g., Stratasys, model: Vero ClearTM )
Silicone (e.g., Elkem Silicones, model: BLUESILTM RTV 3503)
Disposable autoclave bag (Sigma-Aldrich, catalog number: Z692212)
6-well plate, round (Thermo Fischer, catalog number: 140675)
Falcon 15 mL centrifuge tubes, PET, conical bottom w/plug seal cap (Sigma-Aldrich, catalog number: CLS430055)
Falcon 50 mL centrifuge tubes, PET, conical bottom w/plug seal cap (Sigma-Aldrich, catalog number: CLS4558)
Petri dish B60 (e.g., Corning, catalog number: BP53-03)
Tweezer (e.g., EMS 96, Sigma Aldrich, catalog number: 932922)
Disposable hemocytometer (e.g., Millicell®, Sigma-Aldrich, catalog number: MDH-2N1-50PK)
Positive displacement pipette (e.g., Microman 100–1,000 µL, Gilson, catalog number: FD10006)
Capillary pistons (e.g., CP1,000ST 2 × 91 TIPACK, Gilson, catalog number: F148180)
Microtube Eppendorf 2 mL (Dutscher, catalog number: 033297)
Equipment
Inkjet 3D printer (e.g., Stratasys, model: Objects 30 pro)
Autoclave (e.g., Sigma-Aldrich, model: BioCLAVETM mini digital autoclave, catalog number: Z680109)
Centrifuge (e.g., Sigma Aldrich, catalog number: C166500)
CO2 incubator (e.g., MEMMERT, catalog number: I227880)
Vacuum drying oven (e.g., Goldbrunn, model: 450)
Fluid aspiration systems (e.g., BVC control, Vaccubrand, catalog number: 20727200)
6 well-plate rotor (e.g., Eppendorf A-4-81 Rotor, Marshall Scientific, catalog number: EP-WPB)
Software and datasets
MATLAB (2022b, September 20, 2022)
Prism v9.0 (GraphPad, October 27, 2020)
Procedure
The aim of the procedure is to generate a biocompatible and autoclavable device, simplifying the induction of spheroids. As illustrated in Figure 1, the experimenter needs only to 3D-print the provided molds using the supplied 3D files (STL) to create an unlimited number of devices.
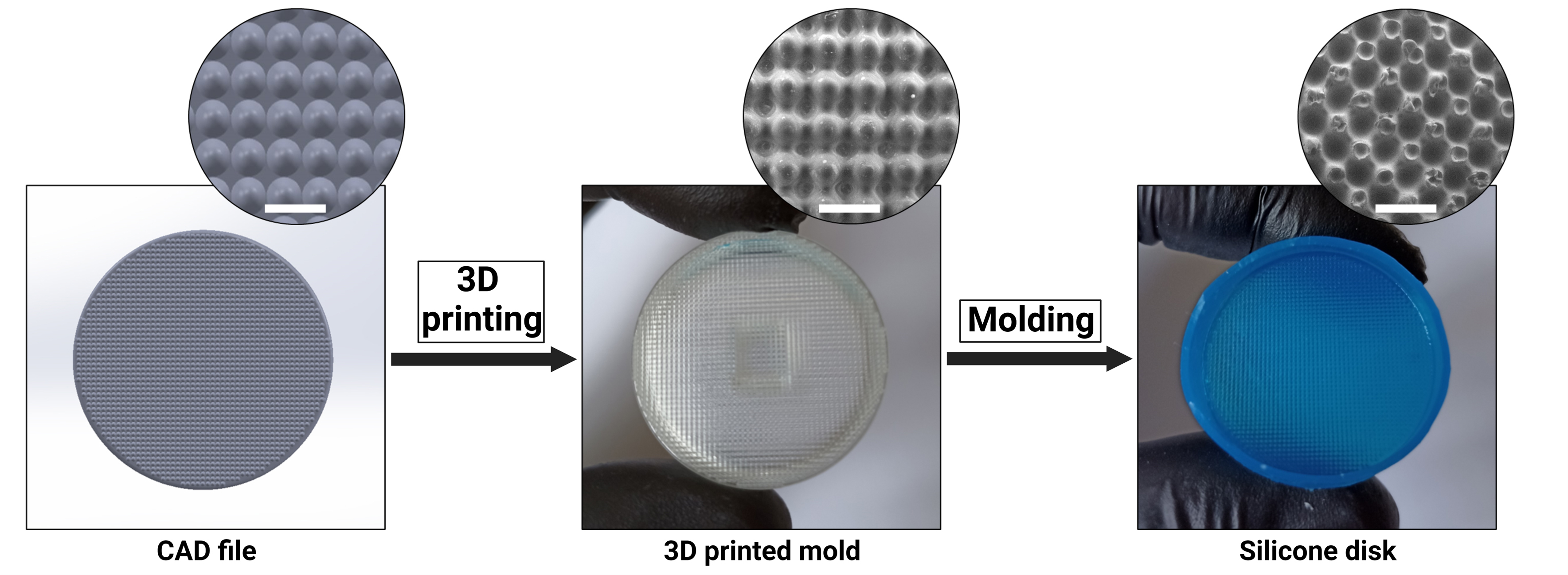
Figure 1. Silicone disc fabrication process. Scale bar = 1 mm. SEM: TM4000 (Itachi, Japan).
Mold 3D printing
Use an inkjet 3D printer to produce the acrylic resin molds from the “EBs-mold-base.STL,” “EBs-mold-intern.STL,” and “EBs-mold-extern.STL” STL files (available at https://github.com/Klux1rst/Spheroids-Inducer-Mold-Bioprotocol/tree/main). It is recommended to use the most resolution-enhancing default parameters to achieve a high-quality mold (See General Notes 1).
Perform post-printing cleaning on the 3D printed molds (usually by soaking in 50 mL of EtOH 70% in an open container for 12 h at room temperature but consult your material instructions because the post-printing process is resin dependent). Allow the acrylate mold to dry at room temperature for 30 min before use.
Silicone disc production
Note: The following steps are illustrated in Figure S1.
Handle the basal piece (EBs-mold-base).
Snap the central piece into the basal piece (EBs-mold-intern).
To complete the assembly, snap the external part into the basal piece (EBs-mold-extern).
Confirm proper assembly, ensuring all pieces fit securely. Place the mold in the upper lid of a B60 Petri dish.
Deposit 1 mL of silicone, starting with the peripheral groove between the central and external pieces. A positive displacement pipette is recommended to ensure an accurate volume.
Deposit another 1 mL a second time at the center of the mold.
Note: If only classic pipettes are available, approximately 2 mL of silicone can be deposited directly. Use a balance for accuracy (approximately 2 g).
Place the assembly in a sealed chamber and create a vacuum for 5 min. This step is crucial to eliminate bubbles resulting from the mixing of the two silicone phases (if RTV silicon) and to ensure silicone penetration into the peripheral groove.
Gently place the lower part of the B60 to smooth the silicone on the mold surface. Starting from one corner, gradually deposit to avoid trapping air. A weight can be placed on top to maintain pressure.
Incubate the assembly at 37–55 °C for 20 min to catalyze crosslinking. Alternatively, incubate for 1.5 h at room temperature (depending on the silicone used).
Retrieve the assembly after complete silicone crosslinking.
Remove the upper part of the B60 (no need to force).
Gently remove (lever from an angle) the lower part of the Petri dish while manually holding the mold.
Using a spatula, lever at the notches located at the base of the external part (EBs-mold-extern) of the mold. Gradually proceed from notch to notch until complete separation from the lower part (EBs-mold-base).
The base (EBs-mold-base) of the mold is completely removed.
Manually remove the external part (EBs-mold-extern) by applying gentle pressure.
The external part (EBs-mold-extern) of the mold is completely removed.
Finally, gently remove the silicone disk from the internal part ( EBs-mold-intern) with your fingers.
The internal part (EBs-mold-intern) of the mold is completely removed.
The molding process is complete. After a soap washing step (optional: use a toothbrush with soft bristle), the resulting disk can be autoclaved (liquid or dry) and used.
hiPSCs aggregation
Note: Perform the culture operation in a level 1 or 2 laboratory in a dedicated laminar airflow cabinet. Use autoclaved tweezers to ensure sterility. After use, store the forceps in a 15 mL Falcon filled with 70% EtOH until the next autoclaving.
Using tweezers, place the silicon discs at the bottom of 6-well plates.
Prepare the hiPSC spheroids induction medium (see Recipe 1) and TrypLETM inhibition medium (see Recipe 2).
Culture hiPSCs in mTeSRTM Plus containing medium on plates coated with Vitronectin XFTM until cells reach 80%–90% of confluency (Figure 2A).
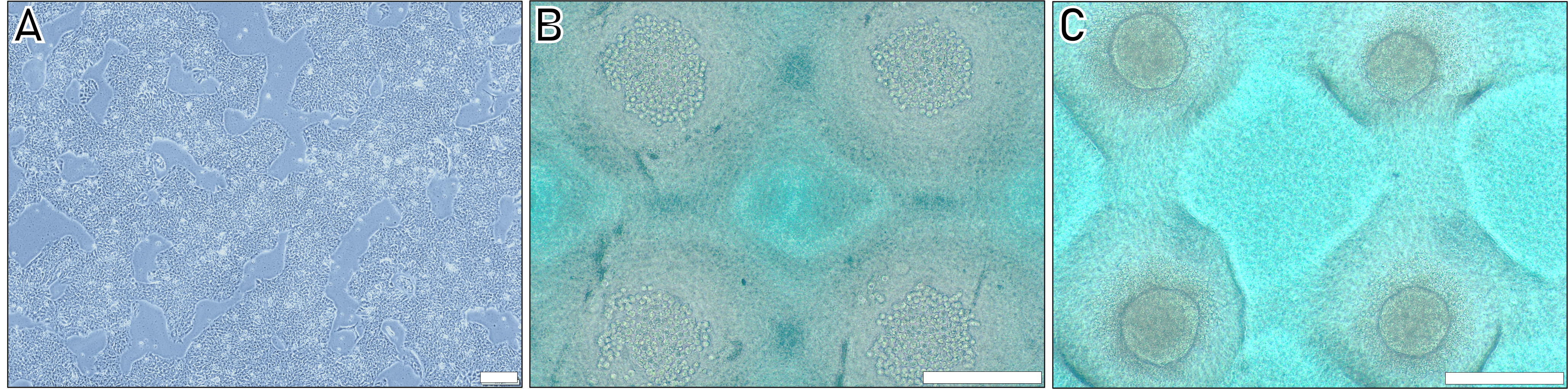
Figure 2. Different steps of the human induced pluripotent stem cells (hiPSC) spheroids induction protocol. A. Colonies of hIPSCs before trypsination. B. Appearance of cells in wells after plating and centrifugation. C. Aspect of spheroids after 24 h aggregation. Scale bar = 200 µm.Aspirate the medium from the cells and wash with 2.5 mL of room temperature DPBS. For aspiration, use a vacuum pump with a pipette and appropriate tubing.
Add 2 mL of TrypLETM and incubate for 5 min at 37 °C.
After cell detachment, add 2.5 mL of TrypLETM inhibition medium. Transfer the total volume to a 15 mL Falcon tube and centrifuge at 200× g for 4 min at room temperature.
Resuspend the cell pellet in 1 mL of hiPSC spheroids induction medium and count the cells (using a hemocytometer).
Transfer the desired cell quantity to a 2 mL Eppendorf tube. Gently mix.
Note: The diameter of the resulting hiPSC spheroids is directly related to the number of cells deposited on the disc, following linear Equation 1 (example: with 1 × 106 cells, D = 79.4 + 42.6 = 122 μm).
Equation 1: Spheroid diameter (D) as a function of the number of hIPSCs deposited on the disc.
D = 79.4x + 42.6
with D in µm and x the number of cells per disc in 10 5
Distribute the cell solution evenly on the molded disc by depositing drops in a circular motion. To ensure homogeneity, deposit the cell solution across the entire disc rather than locally, preventing strong size variations of the obtained spheroids.
Wait for 1 h in a 37 °C, 5% CO2 incubator for sedimentation. This step is crucial for ensuring good cell distribution and, consequently, uniformity of the spheroids (Figure 2B ).
Centrifuge at 500× g for 2 min at 20 °C to enhance cell aggregation.
Place the 6-well plate in a 37 °C, 5% CO2 incubator for 24 h (Figure 2C).
Spheroids extraction
Note: Perform the culture operation in a level 1 or 2 laboratory in a dedicated laminar airflow cabinet. Use autoclaved tweezers to ensure sterility. After use, store the forceps in a 15 mL Falcon filled with 70% EtOH until the next autoclaving.
After 24 h, use a P1000 pipette and 1 mL of DPBS warmed at 37 °C to pipette. Mix by aspiration, dislodging spheroids from the microwells.
Gently invert the silicone discs using fine forceps.
Centrifuge at 200× g for 4 min to extract the spheroids from the microwells.
Collect the culture medium containing spheroids in a 50 mL collection tube for each well.
Flush each disc with 5 mL of DPBS in order to recover any remaining spheroids at the well bottom and add this volume to the collection tube.
Rinse each silicone disc with 2 mL of DPBS. Submerge the discs in EtOH 70% for 30 min. Wash with water and soap, rinse in dezionized water, and autoclave in an autoclave bag for the next experiment.
Check spheroids integrity (refer to Figure 3A and Figure 3B) and gently centrifuge the collection tubes at 50× g for 4 min at 20 °C to obtain a pellet containing the spheroids.
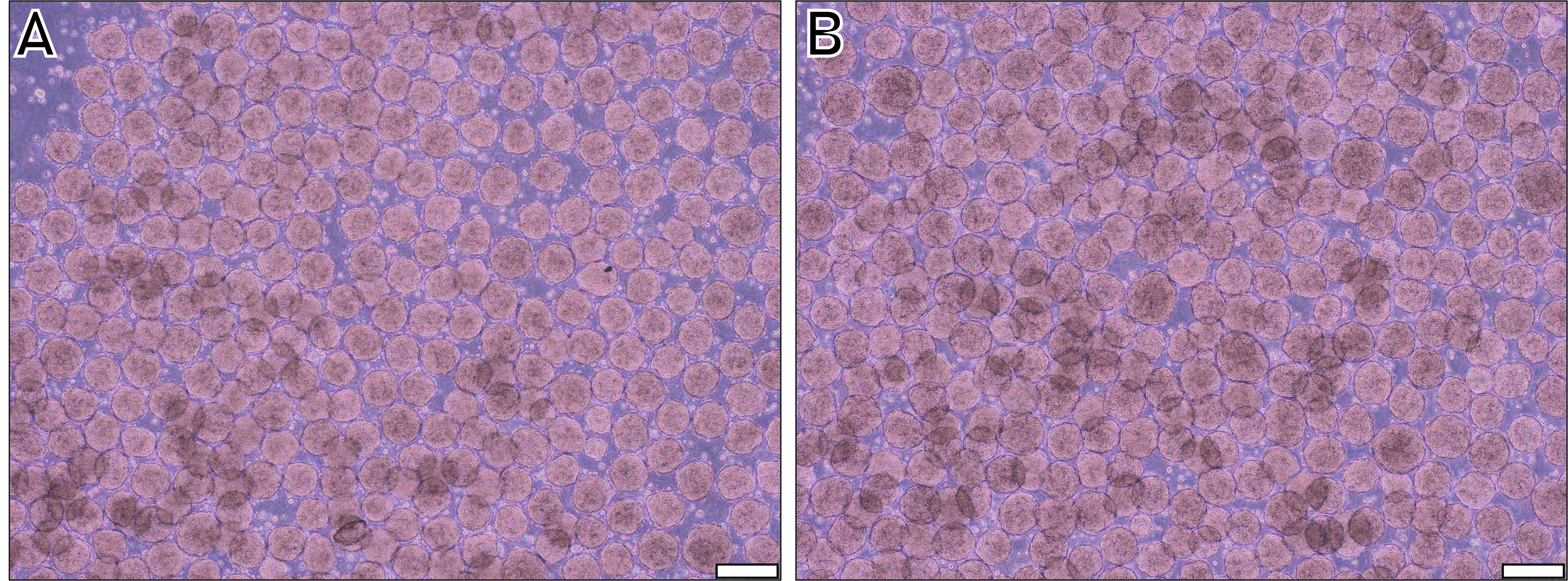
Figure 3. Spheroids of two different human induced pluripotent stem cells (hiPSC) cell lines 24 h after deposition of 1 × 106 cells per disc. A. Cell line AG08C5. B. Cell line SCTi003-A. Scale bar = 200 µm.Proceed with your experiment using the hiPSC spheroids.
Data analysis
To demonstrate the robustness of the methodology, varying quantities of hiPSCs were applied onto the discs following the protocol described above (Figure 4).
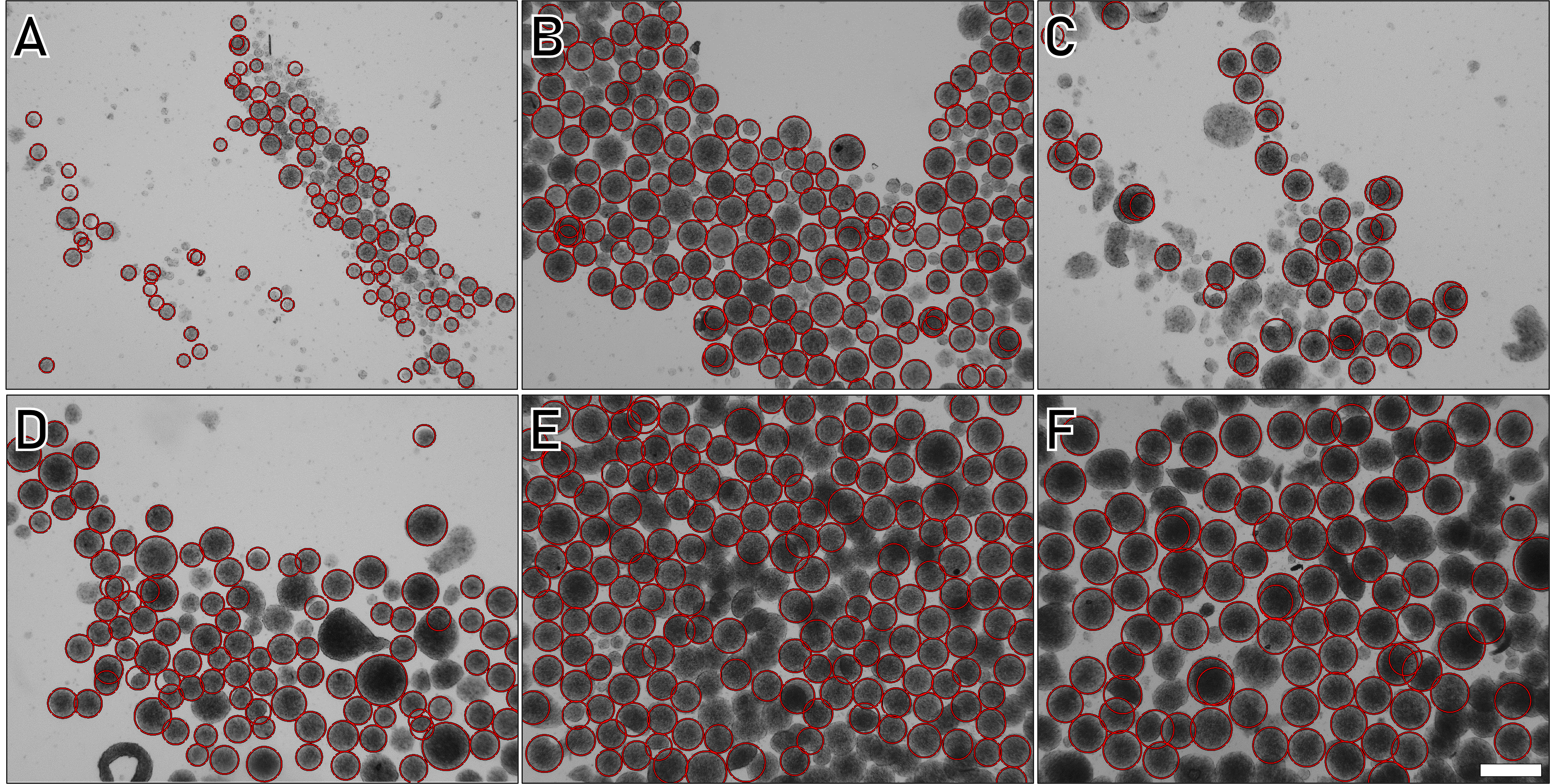
Figure 4. Human induced pluripotent stem cells (hIPSC) spheroids diameter depending on the number of cells deposited. A. 1 × 105. B. 2.5 × 105. C. 5 × 10 5. D. 7.5 × 105. E. 1 × 106. F. 1.5 × 106. Scale bar = 100 µm.
The obtained results were analyzed using a custom image analysis algorithm developed in MATLAB, leveraging the ImFindCircle function, which is relevant for the measurement of spheroid diameters (https://www.mathworks.com/help/images/ref/imfindcircles.html). Although not perfect due to occasional spheroid escape detection, the large number of events analyzed helps mitigate data variability. This analysis reveals a direct relationship between the quantity of cells deposited on a disc (each disc contains approximately 20,00 wells) and the resulting spheroid diameter. Spheroid polydispersity analysis based on each cell’s quantity is depicted in Figure 5.
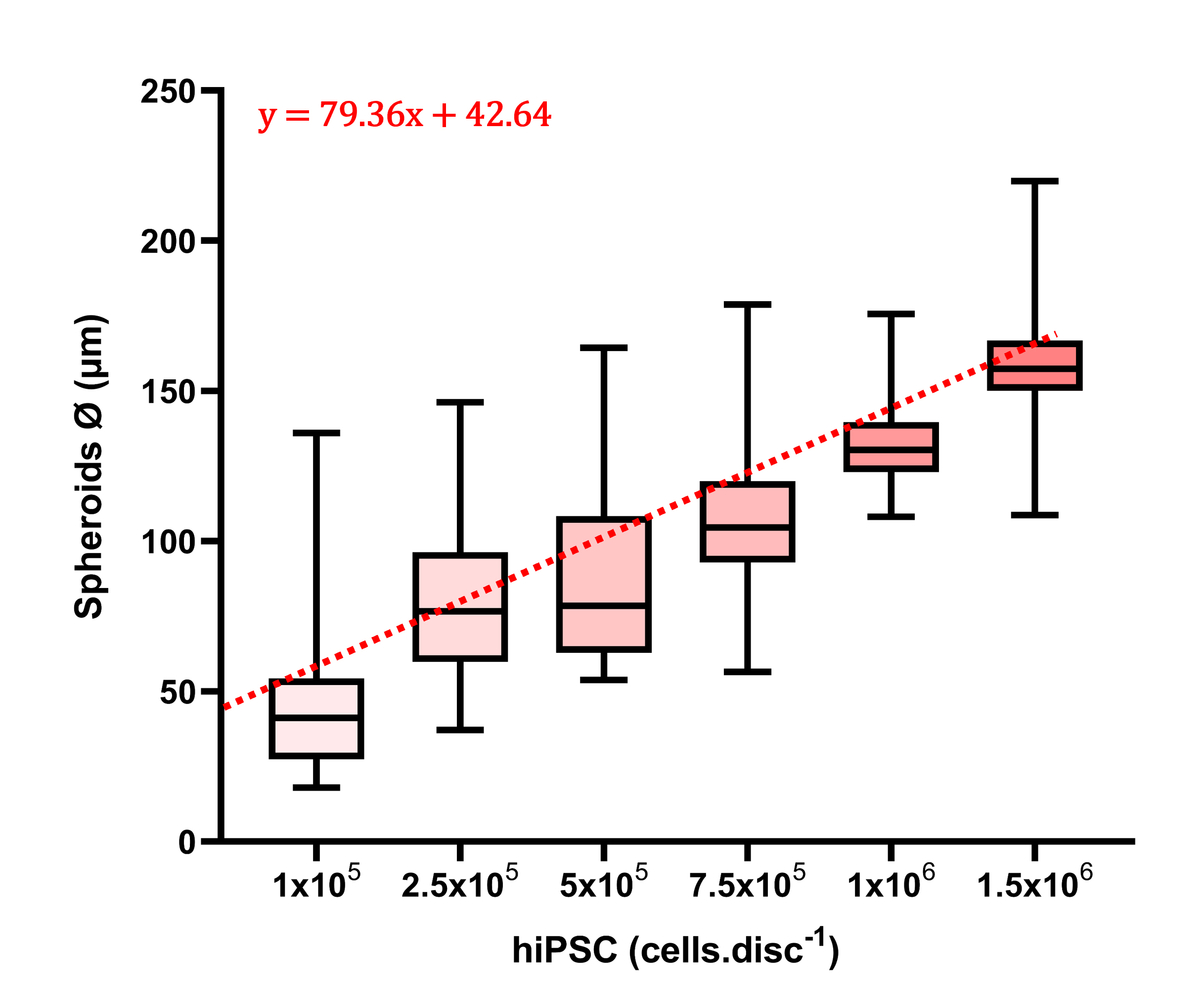
Figure 5. Human induced pluripotent stem cells (hIPSC) spheroids diameter distribution depending on the number of cells deposited (n = 300, D = trend equation, R = 0.87).
These box plots demonstrate the technique's versatility, generating spheroids ranging from 50 to 150 μm in diameter with the same mold by varying only the initial number of cells deposited. Notably, deviation is important for small cell numbers (<1 × 106), reaching a minimum value of 1 × 106 cells per disc. With the designed microwell diameter (approximately 400 µm), this condition appears most suitable for the production of homogeneous spheroids (Figure 4E). This observation can be attributed to a relation between microwell volume and the optimal volume occupied by cells (and hence cell quantity) for reproducible filling.
Moreover, the method demonstrates good reproducibility, with triplicate independent experiments using 1 × 106 cells per disc (approximately 5,000 cells per microwell) showing a deviation of spheroid median diameters of only 10.2 µm (run 1: median 130.2 ± 10.2 μm; run 2: 127.9 ± 9.9 μm; run 3: 128 ± 10.4 μm).
The integrity of hiPSC spheroids produced by the method was observed after extraction (Figure 6A). In all cases, a clear peripheral membrane was observed, indicating good aggregation. After 5 days in culture medium (mTeSR™ Plus), significant growth was observed (Figure 6B), validating the viability and quality of the produced spheroids.
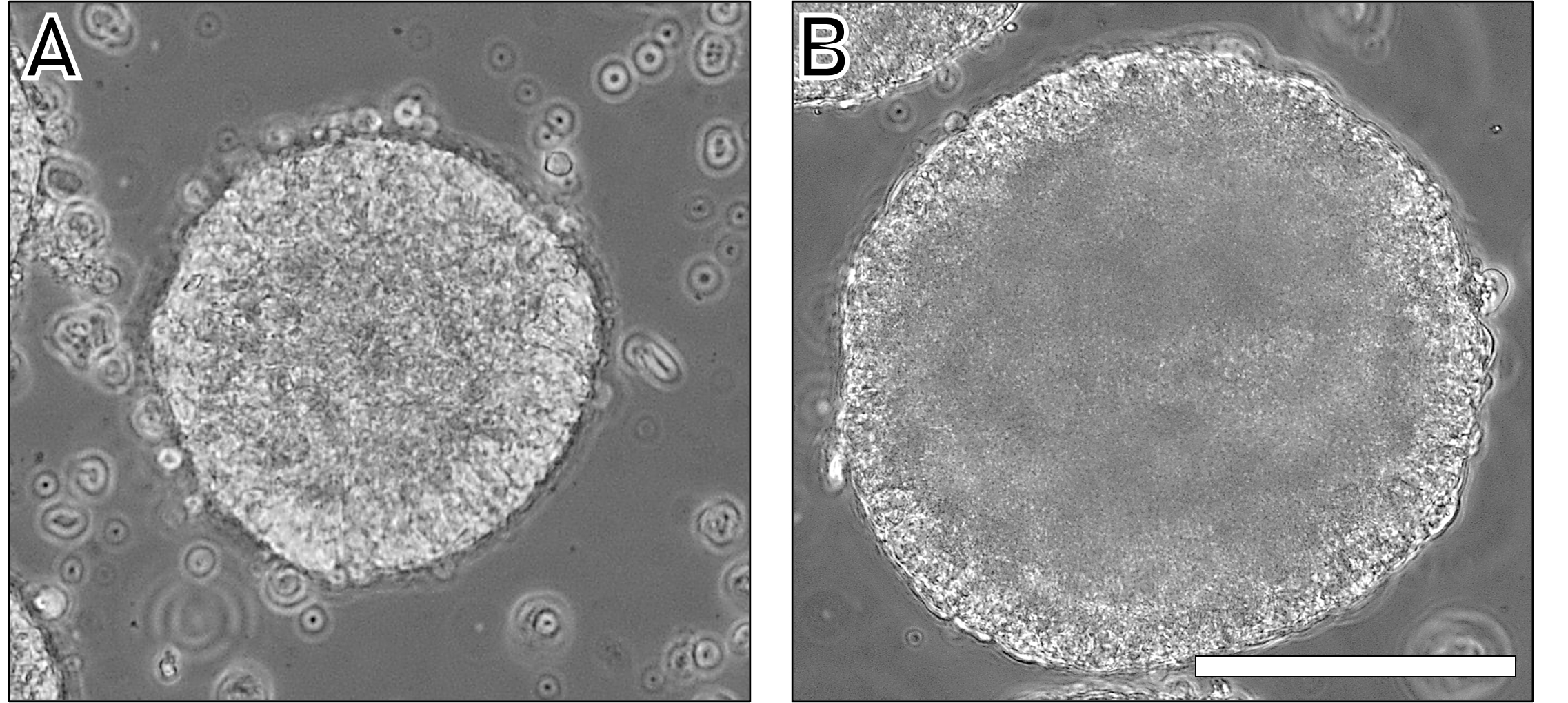
Figure 6. Spheroid diameter for the condition 1 × 106 cells per disc (≃5000 cells per well) at day 1 (A) and day 5 (B). Scale bar = 100 µm.
Validation of protocol
The protocol has been successfully employed in various studies in the lab, as well as in our most recent publication (Lemarié et al, DOI: 10.3390/bioengineering10121418). In this particular study, hiPSC-induced spheroids were incorporated into different alginate-gelatine hydrogels to investigate the impact of viscoelastic properties on the spheroid’s fate in the absence of growth factor.
General notes and troubleshooting
General notes
When 3D-printing molds, the nature of the resin is relatively unimportant as long as the post-process is executed correctly. Notably, non-polymerized resins can hinder silicone cross-linking [13]. Regarding printing parameters, it is strongly recommended to employ the maximum resolution (layer height < 50 μm) for the central part (EBs-mold-intern). Low resolution may result in a staircase effect in the microwells, negatively impacting cell aggregation and, consequently, spheroid quality.
The extraction of spheroids from the well is a critical step. Turning the disc over followed by centrifugation removes most spheroids, but some may remain in the microwells. The rinsing step with DPBS allows for their recovery. It is recommended to vigorously flush (employ an important backflow rate) the disc during this step. To standardize spheroid diameter more rigorously, a strainer with defined mesh size (usually < 200 μm) can be used to set aside spheroids of excessively large diameter, particularly at the disc’s edge, which can impact spheroid diameter distribution (see Figure S2a and Figure S2b). Silicone discs are, in principle, infinitely reusable if not damaged by handling with fine forceps or during washing steps.
The reduced dispersion observed in spheroids induced with a 106 cell deposit aligns with expectations. This deposition corresponds to approximately 500 cells per microwell, an optimal quantity considering the microwell's conical geometry with a depth of 400 μm and a base of 600 μm. The adaptability of this chosen format is elucidated in Figure 4. Note that the suitability of dimensions depends on cell type and quantity, highlighting the versatility of the selected configuration for this study.
After a 24 h incubation, hIPSC spheroids show optimal aggregation and structural integrity and do not disaggregate. It is recommended to use them within the first hour post-extraction to prevent fusion. They can be reintegrated into standard cell culture, adhering to the substrate coating and proliferating. Alternatively, they can be incorporated into viscous solutions like Matrigel® while maintaining integrity. 100 μm hIPSC spheroids were successfully embedded in alginate-gelatin hydrogels with preserved viability (live-dead staining, [10]). Note the increased risk of necrotic core formation with larger spheroid diameters due to diffusion constraints.
The 3D model presented here (designed using Autodesk Fusion 360) is the result of several iterations and is considered a very good compromise between the average resolution of inkjet printers currently on the market and the number of spheroids obtained in each experiment.
Troubleshooting
Problem 1: No aggregation.
Possible cause: Quantity of cells.
Solution: Deposit more cells (a minimum quantity is required to allow aggregation depending on cell type).
Problem 2: Spheroids’ diameter homogeneity.
Possible cause: Deposition of cells.
Solution: Achieving a uniform deposition across the entire disc structure, for example through circular motion, is crucial to prevent local cell concentration.
Problem 3: Several spheroids per well.
Possible cause: Bad centrifugation.
Solution: Double the centrifugation time.
Problem 4: Low spheroids yield.
Possible cause: Spheroids stuck in the silicone disc microwells.
Solution: Vigorously flush the disc before flipping and centrifuge the disc.
Acknowledgments
This research received support from SEGULA Technologies and the ANRT (National Association for Research and Technology). The authors express their gratitude to the IPS-PGNM core facility (https://pgnm.inmg.fr/plateformes-inmg/, Lyon, FRANCE) for providing the AG08C5 and SCTi003-A (Stem Cell Technologies) control IPS lines. The AG08C5 line is officially registered in the European hPSCreg as PGNMi001-A (https://hpscreg.eu/cell-line/PGNMi001-A) [12]. The SCTi003-A is a commercial cell line, female, and derived from blood cells (STEMCELL, Vancouver, Canada). The authors also acknowledge that all human cells utilized at the iPS-PGNM platform are duly declared to the French Ministry of Health (CODECOH DC-2022-5055).
The experiments were conducted with the invaluable assistance of the LBTI (Laboratory for Tissue Biology and Therapeutic Engineering, Lyon, France) and the academic platform 3d.FAB (Villeurbanne, France). Special thanks to Rania Hadji for her assistance in drafting the pictorial protocol. This protocol was applied in [10].
Competing interests
The authors declare no conflict of interest or competing interests.
References
- Laschke, M. W. and Menger, M. D. (2017). Life is 3D: Boosting Spheroid Function for Tissue Engineering. Trends Biotechnol. 35(2): 133–144. https://doi.org/10.1016/j.tibtech.2016.08.004
- Gilazieva, Z., Ponomarev, A., Rutland, C., Rizvanov, A. and Solovyeva, V. (2020). Promising Applications of Tumor Spheroids and Organoids for Personalized Medicine. Cancers 12(10): 2727. https://doi.org/10.3390/cancers12102727
- Gunti, S., Hoke, A. T., Vu, K. P. and London, N. R. (2021). Organoid and Spheroid Tumor Models: Techniques and Applications. Cancers 13(4): 874. https://doi.org/10.3390/cancers13040874
- Sakalem, M. E., De Sibio, M. T., da Costa, F. A. d. S. and de Oliveira, M. (2021). Historical evolution of spheroids and organoids, and possibilities of use in life sciences and medicine. Biotechnol. J. 16(5): e202000463. https://doi.org/10.1002/biot.202000463
- Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K. and Yamanaka, S. (2007). Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 131(5): 861–872. https://doi.org/10.1016/j.cell.2007.11.019
- Li, J., Hua, Y., Miyagawa, S., Zhang, J., Li, L., Liu, L. and Sawa, Y. (2020). hiPSC-Derived Cardiac Tissue for Disease Modeling and Drug Discovery. Int. J. Mol. Sci. 21(23): 8893. https://doi.org/10.3390/ijms21238893
- Nguyen, D., Hägg, D. A., Forsman, A., Ekholm, J., Nimkingratana, P., Brantsing, C., Kalogeropoulos, T., Zaunz, S., Concaro, S., Brittberg, M., et al. (2017). Cartilage Tissue Engineering by the 3D Bioprinting of iPS Cells in a Nanocellulose/Alginate Bioink. Sci. Rep. 7(1): e1038/s41598–017–00690–y. https://doi.org/10.1038/s41598-017-00690-y
- Baptista, L., Kronemberger, G., Côrtes, I., Charelli, L., Matsui, R., Palhares, T., Sohier, J., Rossi, A. and Granjeiro, J. (2018). Adult Stem Cells Spheroids to Optimize Cell Colonization in Scaffolds for Cartilage and Bone Tissue Engineering. Int. J. Mol. Sci. 19(5): 1285. https://doi.org/10.3390/ijms19051285
- Boot, R. C., Koenderink, G. H. and Boukany, P. E. (2021). Spheroid mechanics and implications for cell invasion. Adv. Phys.: X 6(1): e1978316. https://doi.org/10.1080/23746149.2021.1978316
- Lemarié, L., Dargar, T., Grosjean, I., Gache, V., Courtial, E. J. and Sohier, J. (2023). Human Induced Pluripotent Spheroids’ Growth Is Driven by Viscoelastic Properties and Macrostructure of 3D Hydrogel Environment. Bioengineering 10(12): 1418. https://doi.org/10.3390/bioengineering10121418
- Białkowska, K., Komorowski, P., Bryszewska, M. and Miłowska, K. (2020). Spheroids as a Type of Three-Dimensional Cell Cultures—Examples of Methods of Preparation and the Most Important Application. Int. J. Mol. Sci. 21(17): 6225. https://doi.org/10.3390/ijms21176225
- Badja, C., Maleeva, G., El-Yazidi, C., Barruet, E., Lasserre, M., Tropel, P., Binetruy, B., Bregestovski, P. and Magdinier, F. (2014). Efficient and Cost-Effective Generation of Mature Neurons From Human Induced Pluripotent Stem Cells. Stem Cells Transl. Med. 3(12): 1467–1472. https://doi.org/10.5966/sctm.2014-0024
- Venzac, B., Deng, S., Mahmoud, Z., Lenferink, A., Costa, A., Bray, F., Otto, C., Rolando, C. and Le Gac, S. (2021). PDMS Curing Inhibition on 3D-Printed Molds: Why? Also, How to Avoid It?. Anal. Chem. 93(19): 7180–7187. https://doi.org/10.1021/acs.analchem.0c04944
Supplementary information
The following supporting information can be downloaded here
- Figure S1: Illustrated protocol of the different steps for producing silicone discs from preprinted molds.
- Figure S2: Diameter distribution of AG08C5 spheroids for 1 × 106 cells per disc.
Article Information
Copyright
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Lemarié, L., Courtial, E. J. and Sohier, J. (2024). Method for Large-scale Production of hIPSC Spheroids. Bio-protocol 14(7): e4965. DOI: 10.21769/BioProtoc.4965.
Category
Biological Engineering > Biomedical engineering
Stem Cell > Organoid culture
Cell Biology > Cell isolation and culture > 3D cell culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








