- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Streamlined Methods for Processing and Cryopreservation of Cell Therapy Products Using Automated Systems
(*contributed equally to this work) Published: Vol 13, Iss 24, Dec 20, 2023 DOI: 10.21769/BioProtoc.4900 Views: 3144
Reviewed by: Jan HuebingerKrishna NakuluriAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Identification and Sorting of Adipose Inflammatory and Metabolically Activated Macrophages in Diet-Induced Obesity
Dan Wu [...] Weidong Wang
Oct 20, 2025 2247 Views

Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1346 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1481 Views
Abstract
Streamlined procedures for processing and cryopreservation of cell therapies using good laboratory practices are integral to biomanufacturing process development and clinical applications. The protocol herein begins with the preparation of human cell types cultured as adherent (i.e., mesenchymal stromal cells, MSCs) or suspension cells (i.e., peripheral blood mononuclear cells, PBMCs) to comprehensively demonstrate procedures that are applicable to commonly used primary cell cultures. Cell processing steps consist of preparing high yields of cells for cryopreservation using instruments routinely used in cell manufacturing, including the Finia® Fill and Finish System and a controlled-rate freezer. The final steps comprise the storage of cells at subzero temperatures in liquid nitrogen vapor phase followed by the analysis of cell phenotypes before and after processing and cryopreservation, along with cell quality metrics for validation. Additionally, the protocol includes important considerations for the implementation of quality control measures for equipment operation and cell handling, as well as Good Laboratory Practices for cell manufacturing, which are essential for the translational use of cell therapies.
Key features
• The protocol applies to small- or large-scale manufacturing of cell therapy products.
• It includes streamlined procedures for processing and cryopreservation of cells cultured as adherent cells (MSCs) and suspension cells (PBMCs).
• Provides temperature control and rapid partitioning of sample in cryopreservation solution to maintain high viability of a range of cell types throughout the procedures.
• This protocol employs the Finia® Fill and Finish System and a controlled-rate freezer.
Graphical overview
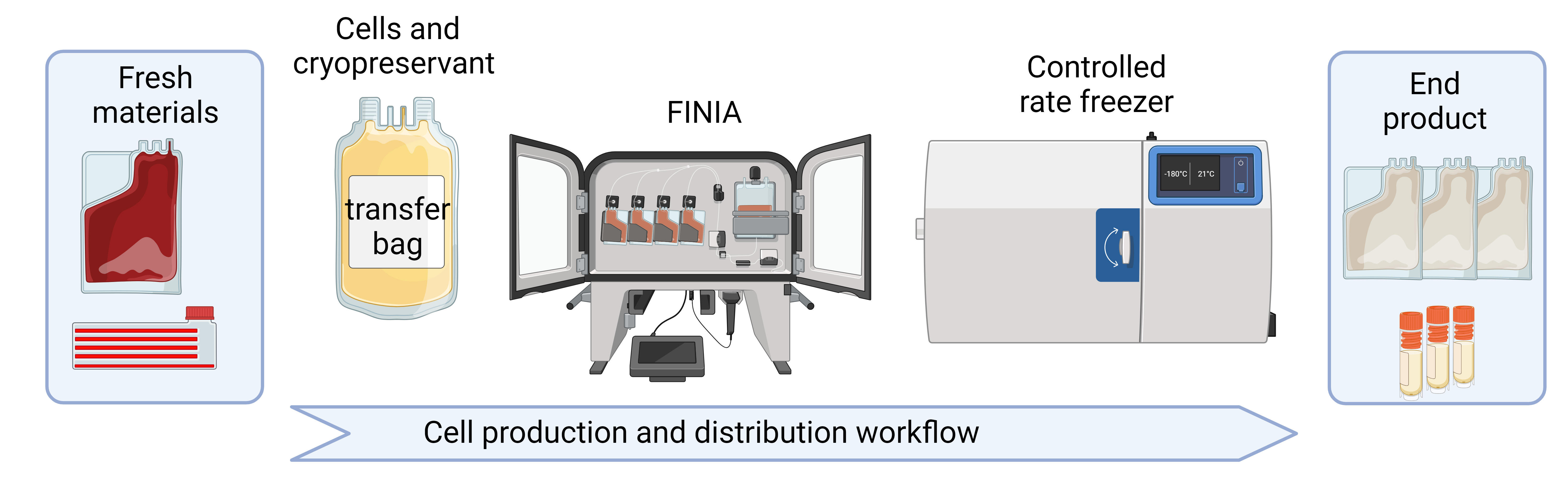
Background
Cell therapy investigations rely on ex vivo operations to adequately preserve the viability and functionality of the cell products prior to in vivo administration (Li et al., 2019). Process development for cell products requires standard operations with controlled systems, which should be monitored throughout the process of end-to-end manufacturing. Clinical laboratories routinely cryopreserve cells using cryopreservation solutions and operating conditions that need to be tightly controlled and amenable to the cell type for optimal cell viability and yield. The use of automated equipment that combines multiple manufacturing processes, such as fill-finish systems and controlled-rate freezers, can offer several advantages over manual operations including automation, better control of processing, and efficiency, thereby avoiding potential compromises to the viability and yield of cell products (van der Walle et al., 2021). Together, these systems can be employed and programmed to optimize the operations for cell cryopreservation at end-stage manufacturing as well as starting materials upstream of manufacturing.
Multiple cell types have been evaluated as cell therapies for clinical use to treat a broad spectrum of clinical indications. Depending on the cell type, specific reagents and operating conditions need to be considered for ex vivo manufacturing expansions to generate high yields for therapeutic dosages (Galipeau and Sensébé, 2018; Pigeau et al., 2018). For example, anchorage-dependent cells require adherence to substrates (e.g., plastic cultureware, microcarriers) to proliferate, whereas other cells proliferate in suspension without substrates (Merten, 2015; Cuesta-Gomez et al., 2023). Typically, cell therapy products are initially isolated from tissues or collected by apheresis from individuals and subsequently undergo various process development procedures to isolate, expand (i.e., proliferate), and harvest the cells (Bunnell et al., 2008; Palen et al., 2021). Herein, procedures that are applicable for both adherent (i.e., mesenchymal stromal cells, MSCs) and apheresis-derived suspension cells (i.e., peripheral blood mononuclear cells, PBMCs) were described to demonstrate how to prepare the cells (e.g., culture and harvest MSCs, isolated PBMCs) and subsequently execute automated and controlled process development procedures that are amenable to cell therapy product manufacturing.
The Finia® Fill and Finish System (Terumo Blood and Cell Technologies) is an automated, closed system that offers temperature-controlled processing for formulation and aliquoting of liquids, including cell suspensions, in preparation for cryopreservation (Sethi and Cunningham, 2021; Algorri et al., 2022). The system mixes and cools up to three materials (e.g., cells, buffers, cryopreservation solutions) to a specified temperature. The system offers flexible procedure programming to allow the user to define workstream needs, from simple aliquoting of a cell source to the stepwise cooling of cell solution, a relevant buffer, and a cryopreservation solution of choice. The final cell product is then aliquoted into individual product bags and automatically sealed. The single-use disposable set is composed of product bags appropriate for freezing, thawing, and administration in the clinic, and a quality control bag for testing (Sethi and Cunningham, 2020; Cunningham et al., 2022). The disposable set is offered in two volume configurations, allowing for the freezing of volumes from 10 to 70 mL per bag. The Finia system is governed by the cell processing application (CPA), which is a secure server-based software (i.e., protected from access by unauthorized individuals) for procedure management and record keeping, capable of tracking performance and protocol development for current Good Manufacturing Practices (cGMP) compliance.
The Finia system and controlled-rate freezer are two separate automated systems that together provide a closed system workflow, which has several advantages over common cell culture practices, including a reproducible cryopreservation procedure with >90% post-thaw cell viability and the prevention of risks related to contamination and operator error. In addition, product bags can store considerably more cells than cryovials (Sethi and Cunningham, 2021). Sethi and Cunningham performed studies to compare a robust manual process to the Finia system (automated process) using T cells and found that the targeted product volumes were more accurate using the automated processing, and cell viability (comparing pre-formulation, post-formulation, and post-thaw) was comparable between the two processes (Sethi and Cunningham, 2021). Cell therapy manufacturing can be further automated by the use of programmable, multi-step-controlled rate freezers, which can standardize and record freezing procedures (Meneghel et al., 2020). Based on these previous studies, our group has implemented streamlined methods for using the Finia system and a controlled-rate freezer for processing and cryopreservation of both adherent and suspension cell types commonly used in cell manufacturing as cell therapy products or as starting materials, respectively. Terumo Blood and Cell Technologies has performed internal testing on the Finia system to demonstrate it can be used for both adherent and suspension cells (data on file). Furthermore, the use of the Finia system in a workstream for a homogenous population of T cells was recently reported (Cunningham et al., 2022). This is the first published report on the use of the Finia system with adherent cells (i.e., MSCs) as well as heterogeneous suspension cells (i.e., PBMCs). Furthermore, we provide quality control results (based on cell count, viability, and phenotyping), troubleshooting guidance, and considerations for GMP and Good Laboratory Practice (GLP) compliance with application to automated and controlled process development procedures amenable to cell therapy product manufacturing.
Materials and reagents
Biological materials
Human MSCs derived from umbilical cord tissue cryovial, stored LN2 vapor phase (supplied by Duke University MC3)
Human peripheral blood one-tenth leukopak fresh (STEMCELL Technologies, 200-0092)
Reagents
Prime-XV MSC Expansion (XSFM) (Irvine Scientific, catalog number: 91149)
Penicillin/streptomycin (P/S) (Millipore Sigma, catalog number: P4333)
Phosphate buffered saline (PBS) Ca2+, Mg2+ free (Millipore Sigma, catalog number: TMS-012-A)
PLTGold® human platelet lysate (hPL) (Millipore Sigma, catalog number: SCM151;)
TrypLE Express 1× (Millipore Sigma, catalog number: 12605028)
Cryostor® CS-10 (Fisher Scientific, catalog number: NC9930384)
LymphoprepTM (STEMCELL Technologies, catalog number: 07801)
Fetal bovine serum (FBS) (any vendor)
Zombie UV Fixable Viability kit (BioLegend®, catalog number: 423107)
Human TruStain FcXTM (BioLegend®, catalog number: 422302)
BD CytofixTM fixation buffer (BD Biosciences, catalog number: 554655)
Solutions
Dilution buffer (see Recipes)
FC buffer (see Recipes)
Viability staining solution (see Recipes)
Fc block solution (see Recipes)
Recipes
Dilution buffer
Reagent Final concentration Quantity PBS Ca2+/Mg2+ free (1×) 98% 98 mL hPL 2% 2 mL Total 100% 100 mL FC buffer
Reagent Final concentration Quantity PBS Ca2+/Mg2+ free (1×) 98% 98 mL FBS 2% 2 mL Total 100% 100 mL Viability staining solution
Reagent Final concentration Quantity PBS Ca2+/Mg2+ free (1×) 99.999% 9.99 mL Zombie UV dye (reconstituted) 0.001% 10 μL Total 100% 10 mL Fc block solution
Reagent Final concentration Quantity FC buffer 90% 9 mL Human TruStain FcX 10% 1 mL Total 100% 10 mL
Laboratory supplies
CellBIND® surface HYPERFlask® cell culture vessel (Corning®, catalog number: 10020)
50 mL conical tubes (any vendor)
Storage bottle, sterile (any vendor)
Serological pipettes and tips, assorted volumes (any vendor)
Micropipettes and tips, P10–P1000 μL (any vendor)
T-150 transfer bag (Fisher Scientific, catalog number: NC0470433)
FINIA 50 tubing set (Terumo Blood and Cell Technologies, catalog number: 22050)
Note: The tubing set comprises a mixing bag, a QC bag (10–40 mL), and three storage bags (10–84 mL total).
FINIA 250 tubing set (Terumo Blood and Cell Technologies; catalog number: 22250)
Note: The tubing set comprises a mixing bag, a QC bag (10–40 mL), and three storage bags (29–210 mL total).
Vapor phase storage cryovials for cell samples including QC (any vendor)
Microcentrifuge tubes low binding (any vendor)
Via-1-CassetteTM cartridges (Chemometec, catalog number: 941-0012)
Syringe 10 mL with luer lok (any vendor)
Blunt fill needle 18-gauge length 1.5 inch (any vendor)
Controlled rate freezer canister rack adjustable (Thermo Fisher Scientific, catalog number: 11679084)
Stainless steel freezing canister with bag capacity 250 mL (Thermo Fisher Scientific, catalog number: 4000335)
Frame to hold canisters in LN2 storage (Thermo Fisher Scientific, catalog number: 4R5461)
Tank of liquid nitrogen (any vendor)
96-well plates (any vendor)
Equipment
Biosafety cabinet (BSC) level A2 (e.g., Thermo Fisher Scientific, catalog number: 1377)
Incubator set to 5% CO2 and 37 °C (e.g., HeraCell VIOS; Thermo Fisher Scientific)
Cell counter (e.g., Chemometec, model: NucleoCounter NC-200TM)
Note: Cell counting methods can vary. It is important to use a reliable and reproducible method for acquiring cell counts and diameter.
Refrigerated centrifuge capable of reaching 800 rcf/g and using 50 mL conical tubes (any vendor)
Micropipette P10–1000 μL (any vendor)
Pipette controller (any vendor)
Inverted microscope (e.g., Thermo Fisher Scientific, model: EVOS M5000)
Note: Microscope needs to be used for phase contrast imaging of cells only.
Finia Fill and Finish System (Terumo Blood and Cell Technologies)
Sterile tubing welder (Terumo Blood and Cell Technologies, model: TSCD-II)
Tubing sealer (Terumo Blood and Cell Technologies, model: T-SEAL Mobile)
Flow cytometer, e.g., CytoFlex (Beckman Coulter), LSRFortessa (BD)
Liquid nitrogen (LN2) dewar or cryo unit (any vendor)
Controlled rate freezer (e.g., Thermo Fisher Scientific, CryoMed, catalog number: 7454)
Note: Freezer needs to be programmable to -90 °C.
Software and datasets
Cell Processing Application (CPA) software version 2.1 with Finia Fill and Finish System
Flow cytometry software for data analysis, e.g., FlowJoTM software (BD Biosciences), CytoBank (Beckman Coulter)
Procedure
All cell handling procedures should be performed in a BSC. The Finia system does not require the use of a BSC. The following procedures outline cell preparation practices for MSCs and PBMCs as the starting material for subsequent processing and cryopreservation using automated systems. More specifically, the procedures include thawing, cell culture, and harvest of cryopreserved MSCs as well as isolation of PBMCs from a fresh (i.e., not cryopreserved) one-tenth leukopak.
Note: It is not advisable to follow the procedures for processing and cryopreservation of PBMCs directly thawed and isolated from a cryopreserved leukopak. If a cryopreserved leukopak is used, allow for a 24–48 h culture rescue period prior to processing and cryopreservation.
Cell thawing (cryopreserved cells, MSCs)
Thaw XSFM media at 4 °C overnight prior to the day of use.
Prepare fresh media by adding 1% P/S to XSFM.
Add 8 mL of fresh media to a 50 mL conical tube.
Retrieve a vial of frozen cells from the LN2 storage.
Thaw the vial in a water bath set at 37 °C for 2 min or until 90% of the ice has melted.
Note: This may depend on the size of the vial and content volume.
Record the thawing time.
Gently transfer the cells from the cryovial to a 50 mL conical tube using a 1,000 μL micropipette with tip.
Wash the vial with 1 mL of fresh media and add this to the conical tube. The final volume of the thawing media and cells should be approximately 10 mL.
Gently resuspend the cells with a 10 mL serological pipette.
Perform a cell count #1 (“Thawed Pre-Spin Count”) by adding 100 μL of cell suspension to a microcentrifuge tube.
Insert the Via-1-Cassette tip into the microcentrifuge tube to uptake approximately 60 μL of cell suspension.
Load the cassette into the NucleoCounter and record the cell count and diameter.
Note: Please complete reading within 1 min after loading cells into cassette.
Centrifuge the remaining cell suspension at 500× g for 5 min at 4 °C.
Aspirate the supernatant using a 10 mL serological pipette and add enough media to give an approximate cell concentration between 1 and 2.5 × 106 cells/mL (optimal concentration for accurate determination of cell count).
Thoroughly resuspend the cells.
Perform and record cell count #2 (“Thawed Post-Spin Count”) by adding 100 μL of cell suspension to a microcentrifuge tube.
Note: Measurement of cells post spin can give an adjusted measure of viability due to dead cells being eliminated in the spin and can also be more accurate as the cells are usually less dilute.
Insert the Via-1-Cassette tip to uptake approximately 60 μL of cell suspension.
Load into the NucleoCounter and record the cell count and diameter.
Calculate the volume needed of cell suspension to give 1.72 × 106 cells (number of cells needed for each HYPERFlask).
Add 560 mL of media (XSFM + 1% P/S) into a storage bottle. Add 1.72 × 106 cells (1,000 cells/cm2) to the storage bottle and rock to ensure that the cells are evenly distributed in media.
Gently pour the cell suspension into the HYPERFlask tilted at a 30° angle to the horizontal.
Place the cap and distribute the cell suspension among all layers of the flask by placing on its long side and then its short side; then, place it lying flat and repeat these movements for even distribution of cells on all layers.
Ensure that there are no air bubbles in the body of the flask by adding more media if needed. Small bubbles are acceptable near the neck of the flask.
Incubate for five to seven days in an incubator set at 5% CO2 and 37 °C.
Cell expansion and harvest
Take daily microscopy images of the lowest layer of the flask to gauge confluence. Do not allow the MSCs to become overcrowded. Harvest MSCs when 80% confluence is achieved.
Carefully pour 50 mL of media from the flask into a 50 mL conical tube. Label and set aside. This is used later to wash the flask.
Remove all remaining media from the flask by pouring gently into a waste container.
Add 50 mL of PBS 1× Ca2+, Mg2+ free to the HYPERFlask and tilt the flask on its long end, short end, and flat side multiple times for an even distribution of the reagent.
Remove the PBS by pouring it gently into a waste container.
Add 50 mL of TrypLE 1× to the flask and distribute evenly by rotating the flask along each end to detach cells.
Incubate the flask for 5 min at 37 °C.
Check the cells under a microscope. Tap the flask lightly against the microscope base to dislodge cells. If incomplete detachment of the cells from the flask is observed, re-incubate for at least another 5 min.
Once the cells are completely detached, add 50 mL of XSFM to the flask and distribute evenly by rotating the flask along each end to subdue the activity of TrypLE.
Note: Unlike TrypLE, other dissociation reagents may require neutralization with media containing serum.
Gently pour the contents of the flask (i.e., cells in solution) into two 50 mL tubes.
Centrifuge the tubes at 500× g for 10 min at 4 °C.
Carefully remove the supernatant from the tubes and combine the pellets into a single tube by adding 5 mL of fresh media to one of the tubes, resuspending the cells, and transferring the cells in suspension to the second tube with the cell pellet.
Thoroughly resuspend the cells.
Perform and record cell count #3 (“Harvested Cell Count”) by adding 10 μL of cell suspension and 90 μL of XSFM to a microcentrifuge tube.
Note: Given an expected high number of cells, it is recommended to perform cell counting with a dilution of the cell suspension to obtain an accurate cell count that is within the range of the instrument’s capabilities. Make sure to factor the dilution into the final cell count.
Uptake the diluted sample into a Via-1-Cassette and load it into the NucleoCounter.
Record the cell count and diameter.
Keep the cells in media at room temperature (RT) until the Finia system is ready.
Note: It is advisable to prepare the cells once the Finia system has been prepared for processing to minimize the time cells are kept at RT.
Cell preparation (fresh cells, PBMCs)
Bring PBS Ca2+, Mg2+ free, hPL, and Lymphoprep to RT.
Bring the centrifuge to RT by switching off the cooling function and remove the brake by setting the deceleration to 0.
Note: Removing the brake is necessary for density separation.
Prepare 100 mL of dilution buffer (see Recipes).
Transfer the contents of the one-tenth leukopak into a 50 mL conical tube.
Note: The volume is between 10 and 30 mL for one-tenth leukopak.
Record the total volume and add 2× volume of dilution buffer (i.e., 7 mL leukopak + 14 mL dilution buffer = 21 mL total volume).
Perform and record cell count #1 (“Pre-Separation Cell Count”) by adding 10 μL of cell suspension and 90 μL of thawing buffer to a microcentrifuge tube.
Note: Given an expected high number of cells, it is recommended to perform cell counting with a dilution of the cell suspension to obtain an accurate cell count that is within the range of the instrument’s capabilities. Make sure to factor the dilution into the final cell count.
Prepare a 50 mL tube with an equal volume of Lymphoprep to the total volume of diluted blood preparation.
Carefully layer the diluted blood preparation over the Lymphoprep by holding the tube at a 45° angle and slowly adding the blood approximately an inch above the Lymphoprep using a 1,000 μL micropipette with tip (see Figure 1A).
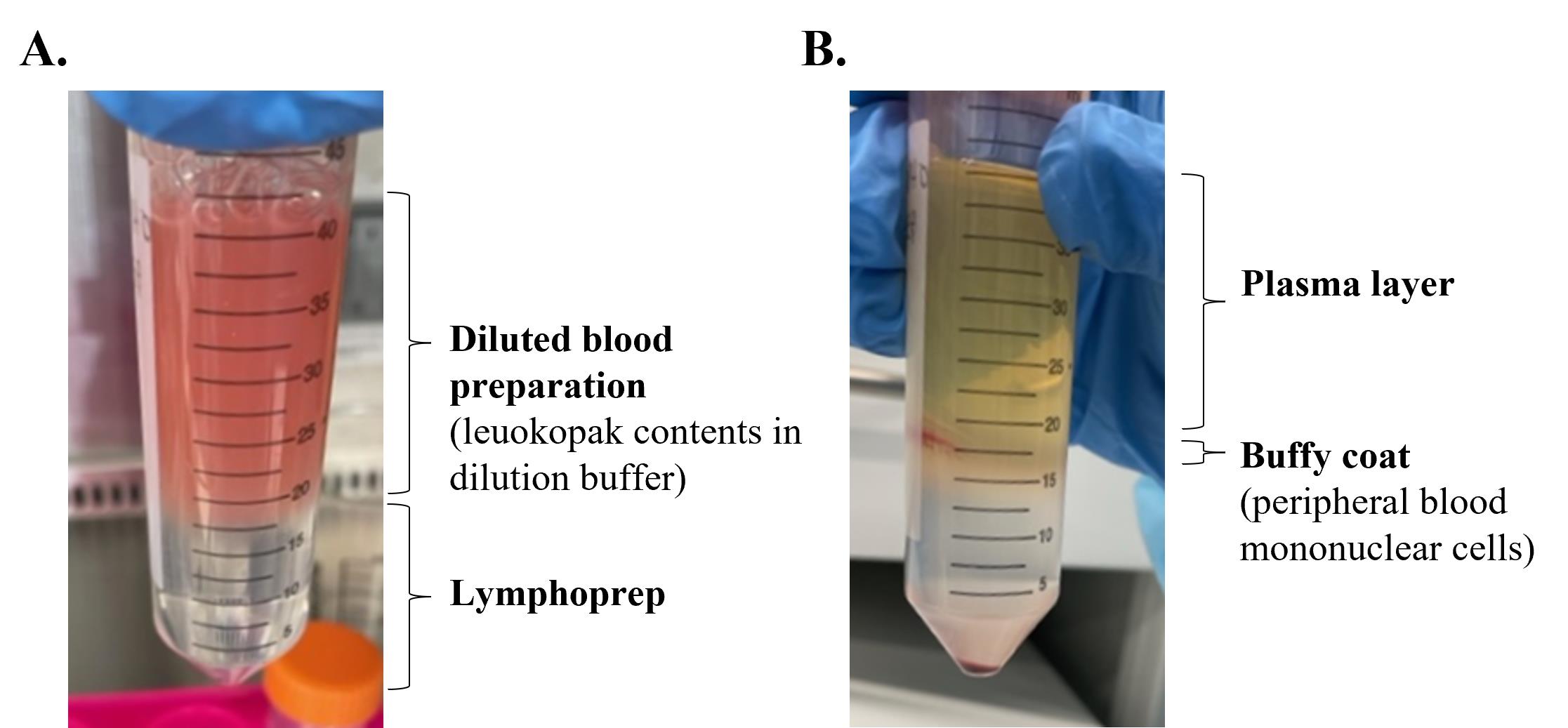
Figure 1. Images of leukopak processing before (A) and after separation (B) using Lymphoprep by density centrifugation to isolate peripheral blood mononuclear cells (PBMCs)Centrifuge the tube at 800× g for 20 min with the brake off and temperature set to RT.
Carefully remove and discard the upper plasma layer.
Collect the buffy coat containing the PBMCs in a 50 mL tube (see Figure 1B).
Add 10 mL of dilution buffer to the tube to wash the PBMCs.
Centrifuge at 300× g for 5 min at 4 °C with brake on by setting deceleration to 9.
Transfer the supernatant to a new tube and add 10 mL of dilution buffer to wash.
Retain and set aside the cell pellet.
Centrifuge the tube with supernatant at 300× g for 5 min at 4 °C to retrieve the remaining cells.
Remove the supernatant and combine the cell pellets from both centrifugations into a single one adding 5 mL to 10 mL of dilution buffer to one of the tubes, resuspending the cells, and transferring the cells in suspension to the second tube with cell pellet.
Perform count #2 (“Post-Separation Cell Count”). Take a 10 μL sample of the cell suspension and dilute 1:10 in a microcentrifuge tube.
Uptake the diluted sample into Via-1-Cassette and load it into the NucleoCounter.
Record the cell count and diameter.
Keep the cells in dilution buffer at RT until the Finia system is ready.
Preparation of the Finia system
Log into the Cell Processing Application (CPA) software, and under Configure Devices (see Figure 2), create a custom protocol to set up the material, product bag, and QC bag volume targets and temperature set point. Save the protocol.
Notes:
The temperature selected for MSCs and PBMCs was 8 °C. Temperature settings will affect the total process time. For example, setting the temperature of the protocol to 8 °C will reduce the time compared to setting to 4 °C.
For the FINIA 50 tubing set, the product bags hold 10–28 mL and the QC bag holds 10–40 mL, so the maximum product volume is 124 mL + 6 mL (retained in tubing). For the FINIA 250 tubing set, the product bags hold 29–70 mL, the QC bag holds 10–40 mL, and the maximum product volume is 250 mL + 6 mL (retained in tubing), including the QC volume.
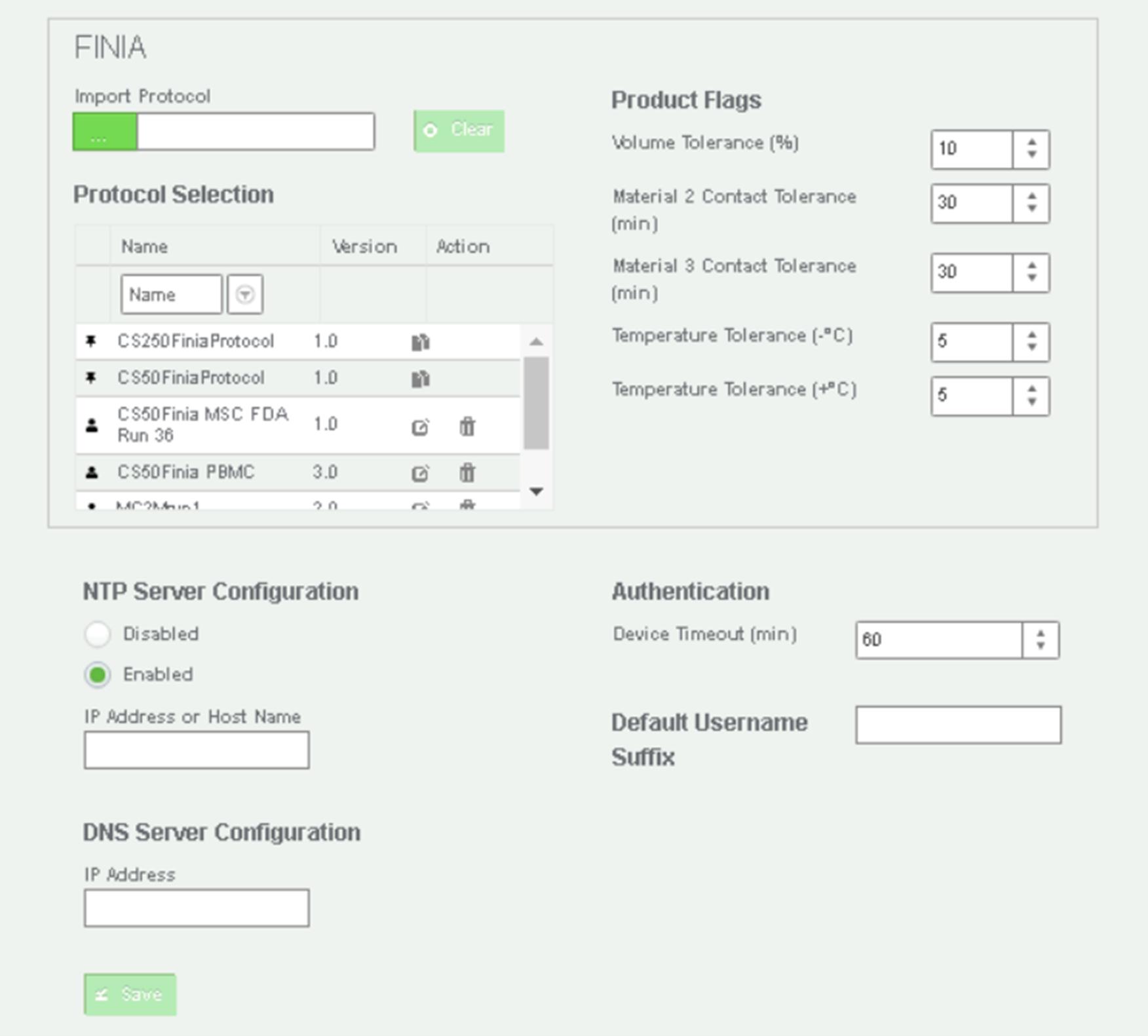
Figure 2. Image of the Cell Processing Application (CPA) in the Finia systemClick Configure Materials and define material IDs and specific gravity of each material. Touch Save.
Create barcodes for each material.
Note: It is important to ensure the material ID on the barcode matches the material ID in the CPA. Barcodes are created using the following schema: for Material 1 (cells): [Material ID]|[Material Batch ID]|Batch ID, e.g., MSCs|20230207|BatchID. For Material 2 (solution): [Material Type]|[Lot_ID], e.g., CS10|Lot 123. Remember to create a unique batch ID for each experiment to be able to recover the data stored in the CPA. “BatchID” is used by the system to identify that Material batch ID as the batch ID for the procedure report in the CPA.
Optionally, mark the sterile PVC section of the transfer bag every 3 inches from its opening with a marker to aid in welding of the transfer bag to the tubing set.
Note: To ensure you have sufficient length of tubing, tube welding at intervals of 3 inches is recommended.
Turn on the welder.
Note: It will take 5 min for the welder to warm up.
Turn on the Finia device and log in to the system. Touch Start Procedure to advance to the protocol selection screen.
Note: If the device was previously on, turn the device off for 60 s and then power it back on. The device receives configuration and material updates from the CPA each time it is powered on and the user logs in.
The list of custom protocols is displayed. Touch the protocol that was created to use for the procedure and then touch Confirm.
Check the function of the load sensor by first checking the 0-gram setting. With no weight on the load sensor, touch Check 0 g.
Place the 500 g test weight on the load sensor. Once the test weight is at rest, touch Check 500 g.
Remove the test weight from the load sensor and close the doors.
The custom protocol values for material, volume, and QC bag targets and temperature set point appear on the screen. Visually confirm the values and touch Confirm.
Notes:
This step refers to a protocol generated by following step D1.
To change a target or the temperature set point, touch any field on the screen and enter the new values. Touch Next to advance to the next screen. Continue until the procedure targets are correct and touch Confirm.
Scan the barcode of the tubing set and place the mixing bag on the load sensor.
Note: Wipe the plate behind the mixing bag to remove any condensation that may cause the mixing bag to stick to the plate.
Connect all tubing via the pumps, tubing holders, and fluid sensor, and close the pump covers.
Slide the product bags and QC bag into the bag holders and load the bag lines into the valves. Pull the bag lines up through the valve until the port touches the bottom of the slide holder (see Figure 3).

Figure 3. The Finia Fill and Finish System. A. The Finia system with general user interface and barcode scanner underneath the equipment manifold. B. Finia system setup with FINIA 50 tubing set.
Separation of the cells into bags and starting the Finia procedure
Use the Harvested Cell Count (MSCs) or Post-Separation Cell Count (PBMCs) to calculate the amount of CryoStor CS-10 required.
Note: For MSCs, the total volume placed into the transfer bag was 30 mL (10 mL product bag + 10 mL QC bag + 6 mL tubing + 15% extra). For PBMCs, the total volume was 41 mL (20 mL product bag + 10 mL QC bag + 6 mL tubing + 15% extra.) Confirm that the transfer bag (materials) contains enough volume for the Finia system to prepare in the mixing bag.
Print cryolabels with donor information, cell concentration, operator initials, date, and any additional information desired (e.g., PDL/passage #).
Retrieve the tube containing the cells from step B17 or C21.
Centrifuge the cells to obtain the cell pellet.
Note: Centrifugation for MSCs was 500× g for 5 min and for PBMCs was 300× g for 5 min.
Remove the supernatant without disturbing the cell pellet.
Add an appropriate amount of Cryostor CS-10 to the cell pellet at a desired cell concentration.
Notes:
Record the duration for which the cells were exposed to the cryopreservation solution. Perform the following procedures quickly to minimize the duration for which cells are in cryopreservation solution, because cryopreservation solutions contain chemicals that may compromise cell viability.
The Finia system is designed to add cryoprotectant solutions to a cell suspension.
Prepare the transfer bag by puncturing the sample port with the spike (on the transfer bag) to open it and release pressure.
Thoroughly resuspend the cells and transfer them into a syringe with an 18-gauge needle.
Situate the needle in the sample port and fill the bag with the cell suspension.
Plug the sample port with the spike and twist it until tight to secure closure of sample port.
Use the T-SEAL Mobile to seal the tubing shut by the sample ports. This leaves the tubing available for welding to the tubing set.
Place the transfer bag on the hook outside the Finia system.
Connect the transfer bag to the Finia disposable set by placing the ends of the tubing into the welding device.
Note: The transfer bag line can be welded only onto the supply line/PVC portion of the Finia disposable set.
Close the welder and start the welding process.
Open the welder and open the weld.
Scan the barcode for the material and touch Confirm.
Confirm that the weld from the transfer bag to the disposable set is open and touch Continue to start the Finia procedure.
Note: The Finia system mixes and cools the cell suspension to the temperature set point. The system pumps three boluses of cell suspension into the mixing bag, after each bolus weighing the bag and reporting the measurement. Then, the system primes each product bag with cell suspension before extracting all air from the product bags. Once the product bags are filled, the bags are automatically sealed and the Finia process is complete.
Carefully remove the product bags and QC bag separately by pulling down the tubing at the top of each bag. Remove and process all product bags before removing any other material from the system. After the bags have been removed, touch Confirm on the screen to store all details of the process on the CPA.
In a BSC, open the QC bag and aliquot the contents into cryovials for downstream analysis of the cells.
Note: Air is not removed from the QC bag. The contents of the QC bag can be analyzed before or after cryopreservation.
On the Finia device, select View Procedure Summary to view the procedure details on the screen.
Remove the rest of the disposable set from the system.
Use the batch ID to retrieve the batch report from the CPA.
Cryopreservation of cells
Label and place the product bags into the stainless-steel canisters.
Place the canisters into the adjustable freezing rack.
Label and place QC cryovials in tube racks.
Perform freezing operations using the controlled-rate freezer set to a desired freezing program.
Note: The CryoMed program cools at a freezing rate of 1 °C/min from 4 to -40 °C, then at 10 °C per minute cooling rate, until reaching -90 °C end temperature.
Transfer the canisters to the accompanying frame.
Transfer the QC cryovials into storage boxes and place them in the storage rack.
Store the frame and rack into the LN2 vapor phase maintained at -140 °C to -180 °C.
Cell phenotype analysis by flow cytometry
Follow steps A1–A18 to thaw and record pre-and post-spin cell counts on MSCs and PBMCs.
Note: XSFM + 1% P/S was used to thaw MSCs or PBMCs.
Add 1 × 105 cells per well into a 96-well V-bottom plate [four technical replicates per sample and fluorescence minus one (FMO) control samples].
Centrifuge the plate at 500× g for 5 min and decant the supernatant.
Resuspend the cell pellets in 100 μL of viability stain solution (see Recipes) or PBS for controls (Zombie FMO and unstained control samples).
Incubate for 15 min at RT in the dark.
Perform a wash by resuspending the cells in 150 μL of FC buffer (see Recipes).
Centrifuge the plate at 500× g for 5 min and decant the supernatant.
For PBMC staining only, add 50 μL of Fc block solution (see Recipes) per well to all wells and incubate for 5 min at RT in the dark.
Resuspend cell pellets in surface marker stain mixture (see Table 1) or FMO mixture for controls and incubate for 20 min at 4 °C in the dark.
Table 1. Antibody information for cell phenotype panels
Centrifuge the plate at 500× g for 5 min and decant the supernatant.
Resuspend all cell pellets in BD CytofixTM fixation buffer.
Incubate for 15 min at 4 °C in the dark.
Centrifuge the plate at 500× g for 5 min and decant the supernatant.
Resuspend all cell pellets in the FC buffer and then perform data acquisition on the flow cytometer with appropriate settings and compensation for the designed panel.
Surface marker Dilution (in FC buffer) Conjugate Vendor Catalog # Clone Excitation (nm) Emission (nm) Human PBMCs
panel antibodiesCD3 1:100 AF700 BioLegend 317340 OKT3 638 719 CD14 1:100 PE BioLegend 325606 HCD14 561 576 CD16 1:100 PerCP-Cy5.5 BioLegend 302028 3G8 488 679 CD19 1:100 PE-Cy7 BioLegend 302216 HIB19 561 780 CD56 1:100 APC BioLegend 362504 5.1H11 638 660 HLA-DR 1:100 FITC BioLegend 327006 LN3 488 517 Human MSCs panel antibodies CD14 1:100 PE-Cy7 BioLegend 367112 63D3 561 780 CD19 1:100 APC-Cy7 BioLegend 302218 HIB19 650 785 CD34 1:100 FITC BioLegend 343504 581 488 517 CD45 1:100 APC BioLegend 304012 H130 638 660 CD73 1:100 PE BioLegend 344004 AD2 561 576 CD90 1:100 PerCPCy5.5 BioLegend 328118 5E10 488 679 CD105 1:100 BV605 BD Biosciences 562664 266 405 421 HLA-DR 1:100 BV421 BioLegend 307636 L243 405 605
Data analysis
Phenotypic analysis of cells “pre-cryopreservation” (post-Finia procedure, i.e., MSCs and isolated PBMCs prior to cryopreservation) and “post-cryopreservation” (post-Finia procedure, i.e., following thaw of cryopreserved MSCs and PBMCs) was performed by flow cytometry. The percentage of cells expressing surface markers outlined in Table 1 were analyzed following an initial gating strategy of forward and side scatter > singlets > live cells (Zombie UV live/dead discrimination) on >10K acquired events. Phenotypic analysis of MSCs showed comparable surface marker expressions for pre- and post-cryopreservation cells. Results showed positive expression (>95%) of markers CD73, CD90, and CD105 and negative expression (<5%) of markers CD14, CD19, CD34, CD45, and HLA-DR. For PBMCs, surface marker expressions of CD3 (34.0% for pre- and 33.0% for post-cryopreservation) and CD56 (16.3% for pre- and 16.9% for post-cryopreservation) were comparable (Figure 4A and 4C). In contrast, results showed marked differences between pre- and post-cryopreserved PBMCs related to the surface marker expressions of CD14 (24.2% for pre- and 44.2% for post-cryopreservation; P < 0.01), CD16 (25.7% for pre- and 30.8% for post-cryopreservation; P < 0.05), CD19 (13.6% for pre- and 17.1% for post-cryopreservation; P < 0.05), and HLA-DR (46.9% for pre- and 56.7% for post-cryopreservation; P < 0.05) (Figure 4B and 4D). The cell population that expressed CD14, CD16, CD19, and HLA-DR was enriched after cryopreservation, which suggests changes to surface marker expressions directly related to cryopreservation or incomplete recovery of all cell types within PBMCs following cryopreservation in which the loss of cells was not captured by the employed phenotyping panel. Overall, post-cryopreserved cells maintained surface marker expression as did fresh cells (pre-cryopreservation). Using the Finia system along with a controlled rate freezer can maintain surface marker expression for cells cultured as both adherent cells and suspension cells. The process can generate high-quality cells to ensure successful cell production.
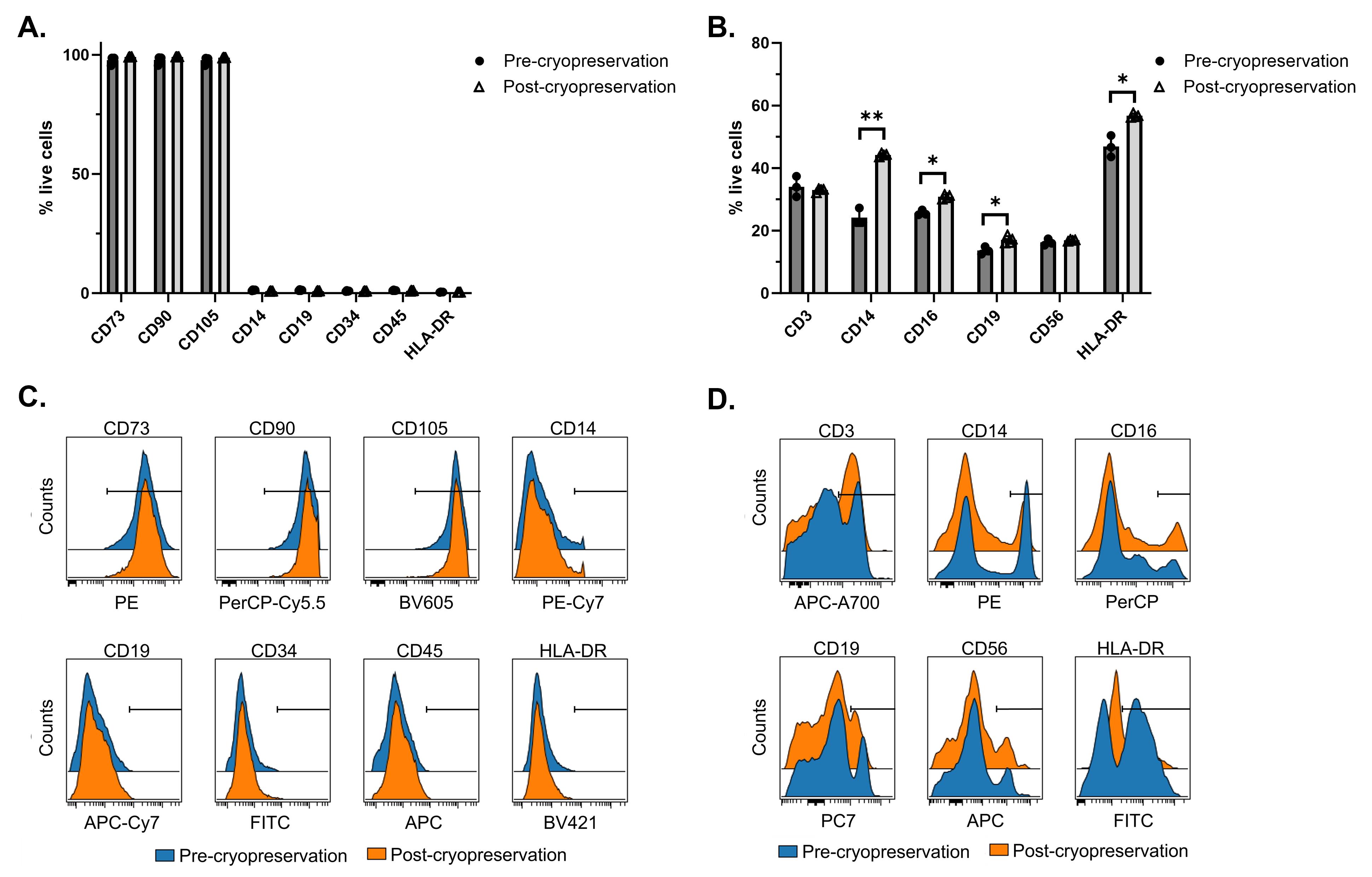
Figure 4. Results of flow cytometry analysis of phenotypes pre- and post-cryopreservation. Quantitative comparison of surface marker expressions of mesenchymal stromal cells (MSCs) (A) and peripheral blood mononuclear cells (PBMCs) (B). Representative histograms of MSCs (C) and PBMCs (D) showing positive gate applied to each surface marker based on FMO controls. Statistical comparison of pre- and post-cryopreserved samples was performed using a paired t test. *, P < 0.05; **, P < 0.01; ***P < 0.001.
Validation of protocol
The protocol offers a robust and reproducible workflow for the processing and cryopreservation of adherent and suspension cells, which was demonstrated with MSCs and PBMCs. The following results are provided as validation of the protocol performed on the different cell types to compare measurable parameters representing the quality of the cell products before and after cryopreservation (Tables 2 and 3). Additionally, examples of the expected results generated from the CPA of the Finia system for each cell type are shown (Tables 4 and 5).
Table 2. Validation results for MSCs
| Sample | Cells/mL | Viability (%) | Diameter (μm) | Total cells (× 106) |
| Sample 1 | ||||
| Fresh cell at harvest (pre-cryopreservation) | 6.39 × 105 | 98.3 | 14.7 | 19.1 (in 30 mL) |
| Thawed cells pre-spin (QC vial; post-cryopreservation) | 6.91 × 105 | 96.0 | 15.4 | 13.8 (in 20 mL) |
| Thawed cell post-spin (QC vial; post-cryopreservation) | 6.67 × 105 | 93.6 | 15.4 | 13.3 (in 20 mL) |
| Thawed cell post-spin (product bag; post- cryopreservation) | 5.65 × 105 | 90.4 | 15.8 | 5.65 (in 10 mL) |
| Sample 2 | ||||
| Fresh cell at harvest (pre-cryopreservation) | 1.02 × 106 | 93.9 | 15.7 | 30.6 (in 30 mL) |
| Thawed cell pre-spin (QC vial; post-cryopreservation) | 1.16 × 106 | 92.4 | 16.3 | 24.4 (21 mL) |
| Thawed cell post-spin (QC vial; post-cryopreservation) | 1.14 × 106 | 90.8 | 16.1 | 23.9 (in 21 mL) |
Table 3. Validation results for PBMCs
| Sample: PBMCs | Cells/mL | Viability (%) | Diameter (μm) | Total cells (× 106) |
| Fresh cell at harvest (pre-cryopreservation) | 3.36 × 106 | 99.0 | 9.9 | 137.8 (in 41 mL) |
| Thawed cell pre-spin (QC vial; post-cryopreservation) | 2.99 × 106 | 97.1 | 10.0 | 101.7 (in 34 mL) |
| Thawed cell post-spin (QC vial; post-cryopreservation) | 2.6 × 106 | 96.7 | 9.9 | 88.4 (in 34 mL) |
In summary:
Both MSC runs resulted in >90% viabilities of the thawed cells post-cryopreservation with similar cell counts.
The Finia system allows for overage in the QC bag. Prior to sealing, the system pumps in all residual volume from the mixing bag to the QC bag.
In both cases, the Finia system run was 10 min from connecting the transfer bag to the end of the filling procedure.
Table 4. Example of results from the FINIA device run report for MSCs
Sample Planned (CPA protocol) Actual (CPA report) Total volume for cell suspension in Cryostor CS10 26 mL 26 mL Bag 1 MSC product 10 mL 9.9 QC bag 10 mL 11.8 Residual volume in tubing 6 mL - Total end product (include QC vials) 20 mL 21.7 Note: Confirm that the transfer bag (materials) contains enough volume for the Finia system to prepare the mixing bag.
Table 5. Example of results from the FINIA device run report for PBMCs
Sample: PBMCs Planned (CPA protocol) Actual (CPA report) Total volume for cell suspension in Cryostor CS10 36 mL 35.1 mL Bag 1 product 20 mL 19.8 QC bag 10 mL 14.3 Residual volume in tubing 6 mL - Total end product (include QC vials) 30 mL 34.1 mL Note: Confirm that the transfer bag (materials) contains enough volume for the Finia system to prepare the mixing bag.
In summary:
The viability of the thawed cells post-cryopreservation was >90% with some cell loss from centrifugation.
The Finia system run was 10 min from connecting the transfer bag to the end of the filling procedure.
General notes and troubleshooting
General notes
Optimal thawing solutions (e.g., media), time, and centrifugation speeds need to be considered and may be cell type–specific for thawing cryopreserved cells.
Automated cell counters and manual counting methods may result in discrepancies to cell counts and viability assessments. Selecting the appropriate cell counting method, instruments, and associated ranges of detection needs to be considered and may be cell type–specific when obtaining cell counts and viability measurements.
Selection of culture media, enzyme-based digestion solutions for detachment of cells, proliferation rates, and culture conditions and duration are cell type–specific.
When estimating confluence of adherent cells cultured in HYPERFlask culture vessels, observing the bottom-most layer assumes an even distribution among the ten layers.
A density gradient centrifugation procedure is typically followed for the isolation of PBMCs from a leukopak or apheresis-derived products. Consideration of density gradient solutions, temperature, and centrifugation time and conditions are necessary to obtain a sufficient buffy coat comprised of PBMCs.
Retaining both the pellet and the supernatant from the first wash after Lymphoprep separation is important, as some PBMCs will remain in the supernatant.
The CPA can be adjusted for the number of materials used (i.e., cell suspension only or cell suspension plus cryopreservation solution), the volume of each material, the temperature, and the desired end product volume in each bag. The specific gravity information is important, as that is used by the Finia system to determine the volume in the bags. For PBMCs (1.077) and MSCs (1.056–1.075) and Cryostor CS10 (1.065–1.069), a specific gravity of 1.07 g/mL at RT was used.
The QC bag does not have air removal, and the allowable volume range on the QC bag is 10–40 mL.
An amount equaling 15% of the volume of cells plus cryopreservation solution was added to the transfer bag to account for dead volume in the transfer bag. Material left in the transfer bag (not pumped into the Finia system) is not recorded in the Finia run report. If an alarm is triggered due to insufficient volume being drawn from the transfer bag, it is usually necessary to transfer the bag contents to a new transfer bag and start the process again.
Start the welding process only when directed by the Finia system. Welding prior to loading the disposable set could cause unintended flow of material out of the transfer bag and trigger alarms.
Measure the height of the first canister insert in the frame to determine if the bag will sit in the liquid or vapor phase of LN2. If needed, add a platform riser to raise up the frame.
As described above, Finia has a user-configurable flexible protocol option. In this procedure, the authors utilized a manual method of cryoprotectant addition prior to loading into the mixing bag. The Finia system is designed to mix up to three materials and can automatically add a cryoprotectant solution to a cell suspension if desired. The system allows the user to establish notification flags to monitor the amount of time the cell suspension has been in contact with the cryoprotectant solution.
Troubleshooting
The Finia system must be restarted to receive a new protocol configured from the CPA.
Unloading prior to the end of the run should be avoided and only be used as a last resort, as this requires the whole process to start over, i.e., remove, recalculate based on the lost volume in the lines, and start again.
Finia procedures can be performed in an offline mode if the system loses connection to the network. The Finia system will send a run report once it re-establishes connection. The device may need to be restarted to send the run report. Updates from the CPA (e.g., protocol, users) cannot be added to the Finia device when the system is offline.
Always perform an action before reporting that an action has been taken, as the confirmation will trigger the next step in the process, and you cannot go back to the previous screen.
Considerations for current good laboratory practices (cGLP)
cGLP is a set of standards and guidelines that ensure the quality and reliability of non-clinical laboratory studies (Tarlengco, 2023). The FDA’s Code of Federal Regulations (21 CFR 58) outlines GLPs, which include considerations for Organization and Personnel, Testing Facilities Operation, and Test and Control Articles. The methods performed within this manuscript adhere to these cGLP guidelines. Within our facility, personnel are trained in donning appropriate PPE (hairnet, safety glasses, face mask, gown, shoe covers, and gloves) and aseptic technique is practiced, as with all cell culture studies, to ensure the correct preparation of cells in a sterile environment (Siddiquee, 2017). Along with the creation and adherence to standard operating procedures, reagents and solutions are properly labeled to be in accordance with cGLP guidelines. All fill-finish work was carried out in clean room space capable of supporting open and closed manufacturing processes for phase I good manufacturing practices evaluation. Environmental monitoring of non-viable and viable particle counts ensured that these counts remained under specified limits. For cryopreservation of samples, each container was labeled with initials, code/batch number, and expiration date and was properly handled and stored. All samples within this study were barcoded to distinguish each batch filled with the Finia system. Once the system had completed a run, the bags were collected and run through a controlled-rate freezing program for proper handling and storage of the samples. By following the GLP considerations, our laboratory ensured the reliability, reproducibility, and quality of the data generated.
Considerations for current good manufacturing practice (cGMP)
cGMP is a set of quality management principles and guidelines that govern the production, distribution, and supply of a health product (Gouveia et al., 2015). The World Health Organization (WHO) defines quality metrics for production and quality control (World Health Organization, 2023). Considerations and key aspects of GMP include Quality Management, Facility and Equipment, Personnel, Raw Materials, Process Control, Validation and Qualification, and Documentation. The FDA outlines the GMP guidelines within the Code of Federal Regulations (21 CFR 211). There are five Ps of GMP: people, products, processes, procedures, and premises (Tarlengco, 2023). Personnel are fully trained in their roles and duties and all products have clear specifications at every phase of production. All products must undergo testing and quality assurance before being distributed to customer s. The Finia system and the controlled rate freezer can be operated in GLP space such that potential cGMP applications can be executed.
Acknowledgments
This study was supported by the Billi and Bernie Marcus Foundation and Terumo Blood and Cell Technologies.
Competing interests
Authors A.R.H. and S.L.G. are affiliated with Terumo Blood Cell and Technologies.
Ethical considerations
No human subjects were used for any experiments included in this study. All procedures using MSCs derived from umbilical cord tissue supplied by collaborators at Duke University were in accordance with the ethical standards and approved by the ethics committee of the institutional review boards at Georgia Institute of Technology and Duke University (IRB Protocol No. H17348).
References
Algorri, M., Abernathy, M. J., Cauchon, N. S., Christian, T. R., Lamm, C. F. and Moore, C. M. (2022). Re-Envisioning Pharmaceutical Manufacturing: Increasing Agility for Global Patient Access. J. Pharm. Sci. 111(3): 593–607.
- Bunnell, B., Flaat, M., Gagliardi, C., Patel, B. and Ripoll, C. (2008). Adipose-derived stem cells: Isolation, expansion and differentiation. Methods 45(2): 115–120.
- Cuesta-Gomez, N., Verhoeff, K., Dadheech, N., Dang, T., Jasra, I. T., de Leon, M. B., Pawlick, R., Marfil-Garza, B., Anwar, P., Razavy, H., et al. (2023). Suspension culture improves iPSC expansion and pluripotency phenotype. Stem Cell Res. Ther. 14(1): e1186/s13287-023-03382-9.
- Cunningham, A. W., Jones, M., Frank, N., Sethi, D. and Miller, M. M. (2022). Stem-like memory T cells are generated during hollow fiber perfusion-based expansion and enriched after cryopreservation in an automated modular cell therapy manufacturing process. Cytotherapy 24(11): 1148–1157.
- Sethi, D. and Cunningham, A. (2020). De-risking the final formulation, fill and finish step in cell therapy manufacturing: considerations for an automated solution. Cell and Gene Therapy Insights 6(10): 1513–1519.
- Sethi, D. and Cunningham, A. (2021). Are you finishing strong in cell therapy manufacturing? Tackling your final fill and finish challenges with automation. Cell and Gene Therapy Insights 7(9): 1163–1171.
- Galipeau, J. and Sensébé, L. (2018). Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 22(6): 824–833.
- Gouveia, B. G., Rijo, P., Gonçalo, T. S.and Reis, C. P. (2015). Good manufacturing practices for medicinal products for human use. J. Pharm. Bioallied Sci. 7(2): 87–96.
- Li, R., Johnson, R., Yu, G., Mckenna, D. H. and Hubel, A. (2019). Preservation of cell-based immunotherapies for clinical trials. Cytotherapy 21(9): 943–957.
- Meneghel, J., Kilbride, P. and Morris, G. J. (2020). Cryopreservation as a Key Element in the Successful Delivery of Cell-Based Therapies—A Review. Front. Med. 7: e592242.
- Merten, O. W. (2015). Advances in cell culture: anchorage dependence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 370(1661): 20140040.
- Oyama, K., Hori, Y., Nagai, Y., Miyakawa, N., Mimura, K., Hirabayashi, T., Inoue, K. i., Suhara, T., Takada, M., Higuchi, M., et al. (2021). Chemogenetic dissection of the primate prefronto-subcortical pathways for working memory and decision-making. Sci. Adv. 7(26): eabg4246.
- Palen, K., Zurko, J., Johnson, B. D., Hari, P. and Shah, N. N. (2021). Manufacturing chimeric antigen receptor T cells from cryopreserved peripheral blood cells: time for a collect-and-freeze model?. Cytotherapy 23(11): 985–990.
- Pigeau, G. M., Csaszar, E. and Dulgar-Tulloch, A. (2018). Commercial Scale Manufacturing of Allogeneic Cell Therapy. Front. Med. 5: e00233.
- Siddiquee, S. (2017). The Basic Concept of Microbiology. Fungal Biol.: 1–15.
- Tarlengco, J. (2023). Good Manufacturing Practices: What You Need to Know. Available: https://safetyculture.com/topics/gmp/
- van der Walle, C., Godbert, S., Saito, G. and Azhari, Z. (2021). Formulation Considerations for Autologous T Cell Drug Products. Pharmaceutics 13(8): 1317.
- World Health Organization. (2023). Considerations in developing a regulatory framework for human cells and tissues and for advanced therapy medicinal products, Annex 3, TRS 1048. Available: https://www.who.int/publications/m/item/considerations-in-developing-a-regulatory-framework-for-human-cells-and-tissues-and-for-advance-therapy-medicinal-products--annex-3--trs-1048
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Li, Y., Stevens, H. Y., Sivaraman, S., Porter, L. N., Hoffman, A. R., Gibb, S. L., Selvam, S. and Bowles-Welch, A. C. (2023). Streamlined Methods for Processing and Cryopreservation of Cell Therapy Products Using Automated Systems. Bio-protocol 13(24): e4900. DOI: 10.21769/BioProtoc.4900.
Category
Cell Biology > Cell isolation and culture
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









