- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Botrytis cinerea in vivo Inoculation Assays for Early-, Middle- and Late-stage Strawberries
Published: Vol 13, Iss 20, Oct 20, 2023 DOI: 10.21769/BioProtoc.4859 Views: 2776
Reviewed by: Samik BhattacharyaIgnacio Lescano

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

In Vitro Hyphal Branching Assay Using Rhizophagus irregularis
Takaya Tominaga and Hironori Kaminaka
Aug 20, 2024 2339 Views

Micrografting Technique of Hevea brasiliensis In Vitro Plantlets
Florence Dessailly [...] Julie Leclercq
Feb 20, 2025 1492 Views
Abstract
Strawberries are delicious and nutritious fruits that are widely cultivated and consumed around the world, either fresh or in various products such as jam, juice, and ice cream. Botrytis cinerea is a fungal pathogen that causes gray mold disease on many crops, including strawberries. Disease monitoring is an important aspect for growing commercial crops like strawberry because there is an urgent need to develop effective strategies to control this destructive gray mold disease. In this protocol, we provide an important tool to monitor the gray mold fungal infection progression in different developmental stages of strawberry. There are different types of inoculation assays for B. cinerea on strawberry plants, such as in vitro (in/on a culture medium) or in vivo (in a living plant). In vivo inoculation assays can be performed at early, middle, and late stages of strawberry development. Here, we describe three methods for in vivo inoculation assays of B. cinerea on strawberry plants. For early-stage strawberry plants, we modified the traditional fungal disc inoculation method to apply to fungal infection on strawberry leaves. For middle-stage strawberry plants, we developed the flower infection assay by dropping fungal conidia onto flowers. For late-stage strawberry plants, we tracked the survival rate of strawberry fruits after fungal conidia infection. This protocol has been successfully used in both lab and greenhouse conditions. It can be applied to other flowering plants or non-model species with appropriate modifications.
Key features
• Fungal disc inoculation on early-stage strawberry leaves.
• Fungal conidia inoculation on middle-stage strawberry flowers.
• Disease rating for late-stage strawberry fruits.
• This protocol is applicable to the other flowering plants with appropriate modifications.
Graphical overview
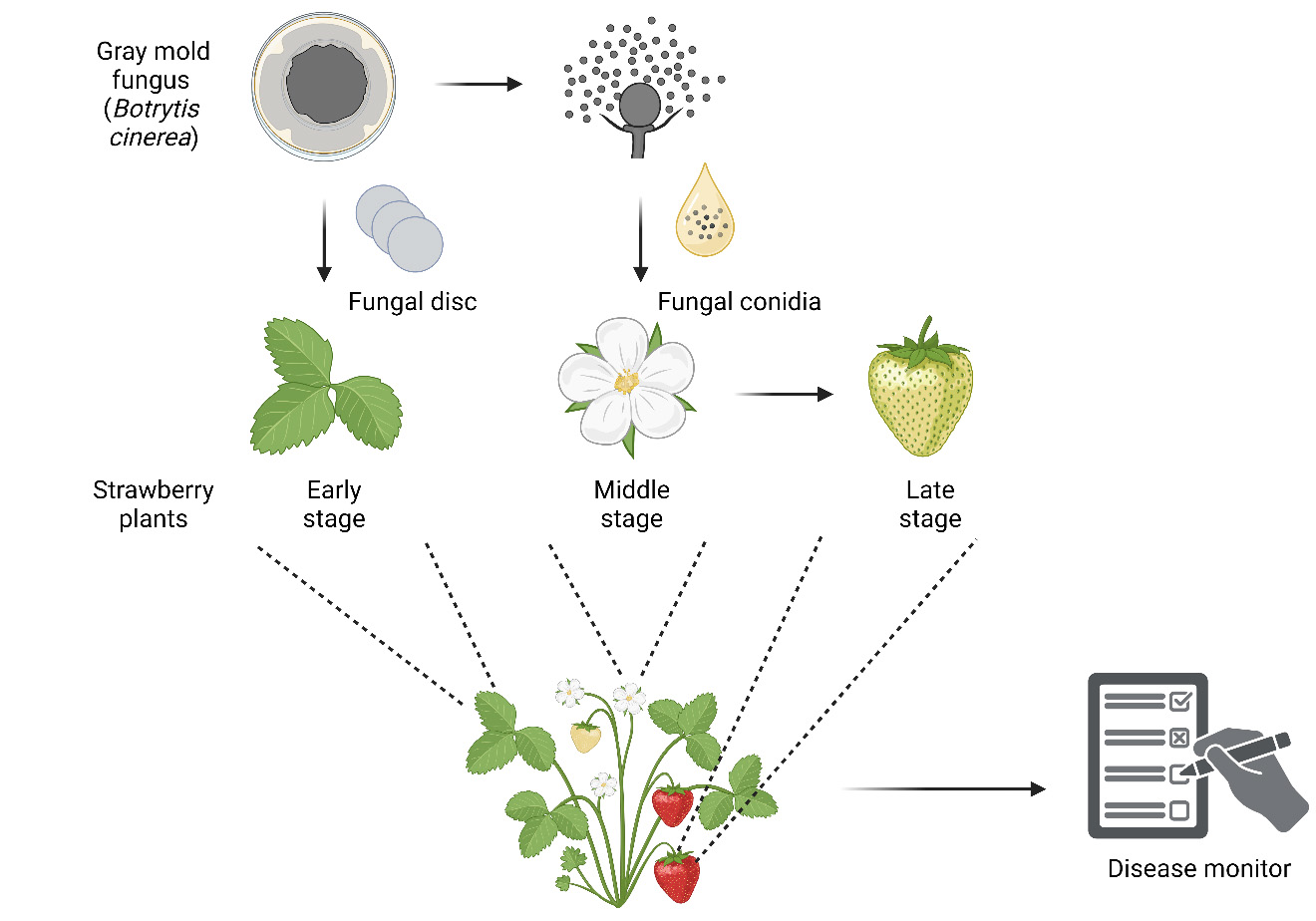
In vivo infection progression assays of gray mold fungus Botrytis cinerea at different developmental stages of strawberry. Created with BioRender.com.
Background
Strawberries are valuable commercial fruit crops that provide critical income and food security for many farmers and consumers around the world. Strawberry plants can be affected by bacterial and fungal infections like gray mold, anthracnose, and powdery mildew, which can damage the plant, reduce fruit quality, and even lead to plant death (Yang et al., 2023a). Botrytis cinerea is the causing agent of the gray mold disease in almost all vegetable and fruit crops, including strawberry plants and post-harvest fruits (Dean et al., 2012; Yang et al., 2023b). This necrotrophic fungal phytopathogen causes an estimated $10–100 billion yearly loss (Fillinger and Elad, 2016). The airborne conidia are produced by B. cinerea in diseased plant tissues, posing a long-lasting threat as part of the secondary infection cycle. An outbreak of the gray mold disease will likely occur under low temperature and high humidity in greenhouse and field conditions. Importantly, B. cinerea is also widely accepted as the second most important fungal pathogen in the research field of molecular plant pathology (Dean et al., 2012), ranked after Magnaporthe oryzae (Chen et al., 2023) but before Fusarium graminearum (Yang et al., 2018). Although plant species such as Arabidopsis and tomato have been used as classic models to study plant response against B. cinerea (Sun et al., 2011), other economically valuable crop species, like strawberries, are not well studied (Petrasch et al., 2019), especially from the flower and fruit perspectives. This protocol provides a detailed method for assessing the response of strawberry plants to the infection by the gray mold fungus B. cinerea at different stages of plant development. It can be adapted for other research purposes (e.g., in vitro detached leaf/fruit inoculation) and other flowering plants with appropriate modifications. The main factors to consider are the inoculation method (fungal disc vs. fungal conidia depending on the plant tissues) and the inoculation dosage (different plant species have varying levels of resistance to the fungus). For example, leaves and fruits of strawberry, tomato, and tobacco can be detached from plants and kept in optimal in vitro conditions (e.g., low temperature and high humidity) for subsequent fungal disc/conidia inoculation. This protocol can also be combined with other methods in related research fields, such as plant–microbe interactions. For instance, some beneficial bacteria can induce plant defense mechanisms that make the plants more resilient to future attacks (Sahib et al., 2019; Zhao et al., 2021; Yang et al., 2022). These defense mechanisms are called induced systemic resistance (ISR) and systemic acquired resistance (SAR) (Chanda et al., 2011; Yang et al., 2023b). This protocol can be used to study ISR/SAR of flowering plants against the gray mold fungus and help develop strategies for plant disease management from the plant’s perspective. However, this protocol has not been tested yet in field conditions and should also be modified according to plant protection principles such as the plant disease triangle, the plant fitness tetrahedron, and the plant disease management hexagon (Yang et al., 2023a), which should take into account multiple factors rather than a single one.
Materials and reagents
B. cinerea strain SS2Ap1 (isolated from diseased strawberry field by IFF)
Petri dishes (90 mm × 14 mm) (VWR, catalog number: 391-0439)
2 mL microcentrifuge tubes (Fisher Scientific, catalog number: 02-681-320)
Sterile water
Lens paper (VWR, catalog number: 52846-000)
Strawberry (day-neutral variety Monterey-UC)
2-gallon pots (greenhouse megastore)
Commercial potting mix (PRO-MIX Company, PRO-MIX BX)
Ziploc sandwich bags (Ziploc, model: 664546)
Spray bottle
Micropore tape (MicroporeTM, catalog number: 1530S-1)
BactoTM agar (BD, catalog number: 214010)
Potato dextrose agar (PDA) (Sigma-Aldrich, catalog number: 70139)
Commercial V8 100% vegetable juice (V8) (Campbell, catalog number: 0067-17KN)
Half-strength PDA medium (see Recipes)
Half-strength V8 medium (see Recipes)
Recipes
Half-strength PDA medium: for culturing B. cinerea
Half-strength of PDA diluted with sterile water.
Half-strength V8 medium: for preparing conidia suspension
Half-strength of supernatant of V8, diluted with sterile water.
Equipment
Autoclave machine (Panasonic Healthcare, model: MLS-3781L)
Sterile pipettes (VWR, catalog number: VHPA23004)
Microcentrifuge (Eppendorf, model: 5415D)
Hemocytometer (Hausser Scientific, catalog number: 497559)
Digital Calipers (Fisher Scientific, catalog number: 1464817)
Light microscope (Nikon, model: E100, required objective: 10×)
Laminar flow hood (SterilGARD 3 Advance)
Freezer (-80 °C) (Panasonic VIP Plus, model: MDF-V76VC-PA)
Vortex mixer (VWR, catalog number: 58815-232)
Laboratory gloves (Fisher Scientific, catalog number: 19-130-1597B)
Forceps (Grainger, catalog number: 4CR15)
Software and datasets
Microsoft Excel Spreadsheet Software (Microsoft 365)
GraphPad Prism (v9.0.2)
All raw example datasets are included in this protocol.
Procedure
Preparation of plant materials
Purchase fresh commercial strawberry seedlings (e.g., day-neutral variety Monterey-UC) from Lassen Canyon Nursery INC (Lassen Canyon | Strawberry Plants | Lassen Canyon Nursery). We conducted a greenhouse trial to test the susceptibility of the Monterey-UC seedlings to the fungal pathogen Botrytis cinerea, which causes gray mold disease. We inoculated the seedlings with the fungus and observed infection symptoms.
Select the uniform seedlings and transplant them in a commercial potting mix in the greenhouse (temperature: 20–32 ; humidity: 40%–60%) with a 16:8 h light/darkness cycle. We selected uniform seedlings based on the following criteria: similar root length, root biomass, and crown (stem) diameter.
Fungal inoculum preparation and inoculation
Fungal inoculum preparation
Withdraw B. cinerea SS2Ap1 strain from -80 °C glycerol stock (25%) and grow for four days on half-strength PDA at room temperature (RT).
Keep the fungal agar plate for another ~10 days at RT for conidiation.
Strawberry leaf inoculation
Punch new fungal discs from the edge of the PDA agar plates using the reverse side of sterile 1 mL tips (Figure 1A).
Inoculate the fully expanded strawberry leaves with fungal discs (Figure 1B).
Secure them with sterile tape (Figure 1C).
Punch a hole on each leaf through the attached fungal disc with a sterile needle (Figure 1D).
Spray sterile water mist on the leaf surface (Figure 1E).
Place the inoculated leaves in plastic bags to maintain high humidity (Figure 1F).
For early-stage (~2-month-old) strawberry leaf inoculation, the lesion diameter was recorded at 4 days post inoculation (dpi) (Figure 1G).
Analyze the data (Figure 1H and Table 1). “Mock” stands for the inoculation with no fungal conidia on strawberry leaves. “Infected” stands for the inoculation with fungal conidia on strawberry leaves (two-tailed unpaired t-test).
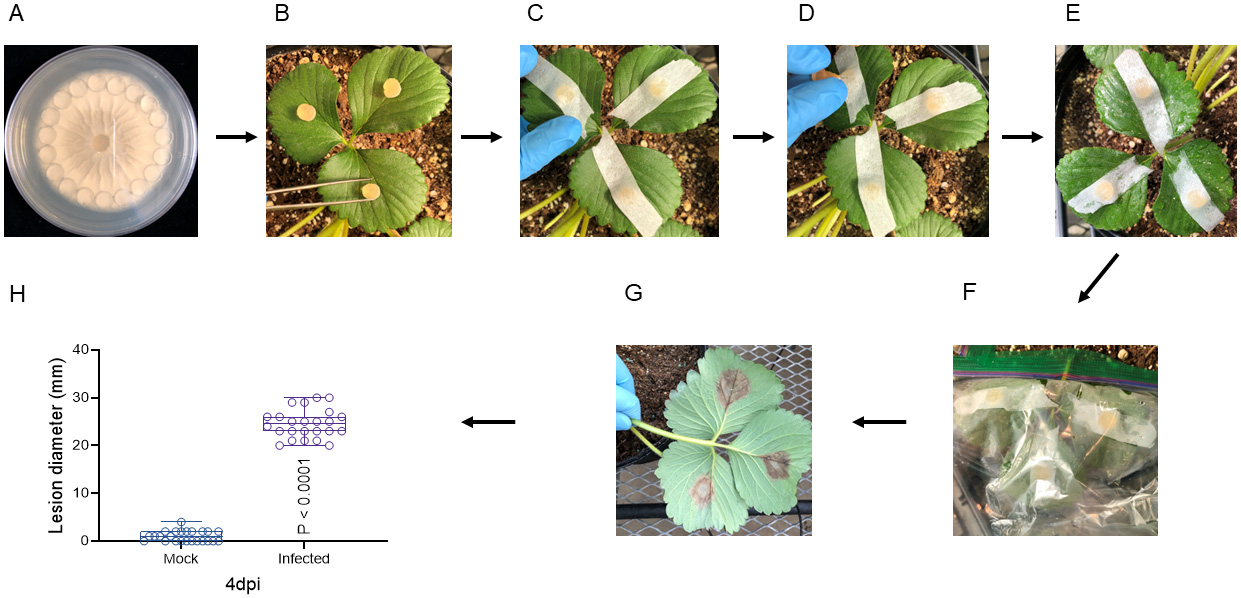
Figure 1. Procedure for inoculating strawberry leaves with Botrytis cinerea at early stage. After the seedling transplant, fresh four-day-old fungal discs (A) were taken from the agar plate edge and inoculated to early-stage (~2-month-old) strawberry leaves (B). The fungal discs' mycelia side was firmly attached to the upside of strawberry leaves with micropore tapes (C). A sterilized needle (D) was used to wound the leaf and facilitate fungal penetration. Sterile water was sprayed on infected leaves (E) and enclosed in a sandwich bag to maintain relative high humidity (F). A dark-brown necrosis lesion (G) was detected on the inoculated leaves at 4 dpi, and the lesion diameter was measured for data analysis (H). “Mock” stands for the inoculation with no fungal conidia on strawberry leaves. “Infected” stands for the inoculation with fungal conidia on strawberry leaves (two-tailed unpaired t-test).Strawberry flower inoculation
Wash fungal conidia from the agar plates with sterile water (Figure 2A).
Filter washed conidia with sterile lens paper to a 2 mL tube and centrifuge to enrich the conidia (Figure 2B).
Dilute the conidia with half-strength V8 medium to appropriate concentration by counting conidia under a light microscope (10× objective) using a hemocytometer (Figure 2C). We used the following formula to calculate the conidial concentration using the hemocytometer: conidia concentration = N × 104 conidia/mL, where N is the number of conidia counted under the 10× objective (when 10 μL of conidial solution was applied to the hemocytometer).
Adjust the conidial suspension to the final inoculum concentration as needed. For example, we used 100 conidia/mL suspension (20 μL drop) to inoculate middle-stage (~3-month-old) strawberry flowers (Figure 2D).
Spray mint water to the inside of a plastic bag (e.g., sandwich bag) and use it to cover the inoculated flower carefully (Figure 2E).
Record the disease symptom of inoculated flowers with a 0–4 scoring system (Figure 2F).
Analyze the data (Figure 2G and Table 2). “Mock” stands for the inoculation with no fungal conidia on strawberry flowers. “Infected” stands for the inoculation with fungal conidia on strawberry flowers (two-tailed unpaired t-test).
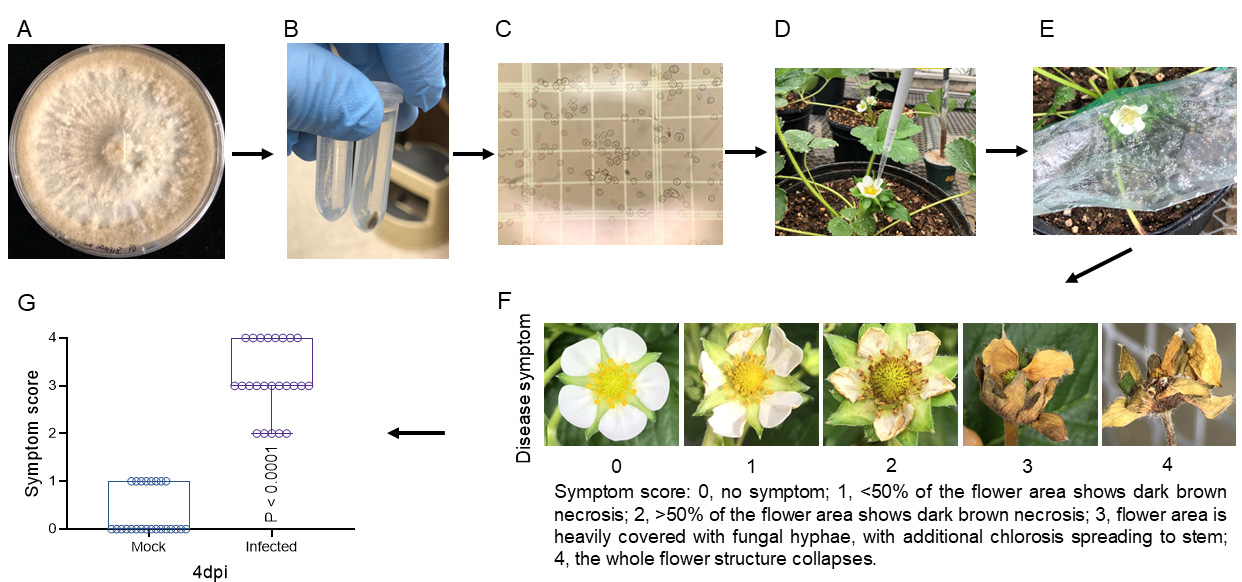
Figure 2. Procedure for inoculating strawberry flowers with Botrytis cinerea at middle stage. Fungal conidia were washed from potato dextrose agar (PDA) plates (A), enriched by centrifuge (B), and calculated using a hemocytometer (C). A 20 μL droplet of conidia suspension was inoculated in the middle-stage (~3-month-old) newly opened strawberry flower (D) and enclosed into a sandwich bag pre-sprayed with sterile water to maintain high relative humidity (E). Four days later, disease symptoms of inoculated flowers were scored on a 0–4 scale (F) and further analyzed (G). “Mock” stands for the inoculation with no fungal conidia on strawberry flowers. “Infected” stands for the inoculation with fungal conidia on strawberry flowers (two-tailed unpaired t-test).Strawberry fruit inoculation
Prepare the conidia inoculum as above in steps B3a–B3e, with the exception that newly emerging fruits (~3.5-month-old) are inoculated instead of flowers.
Record the disease development at 4, 8, and 12 dpi (Figure 3A). We counted the number of healthy and diseased fruits at 12 dpi.
Analyze the data (Figure 3B and Table 3). We calculated the survival rate using this formula: survival rate (%) = the number of healthy fruits/the number of total fruits × 100. “LD” stands for inoculation with low dosage of fungal conidia (100 conidia/mL). “HD” stands for inoculation with fungal high dosage of fungal conidia (5,000 conidia/mL) (two-tailed unpaired t-test).
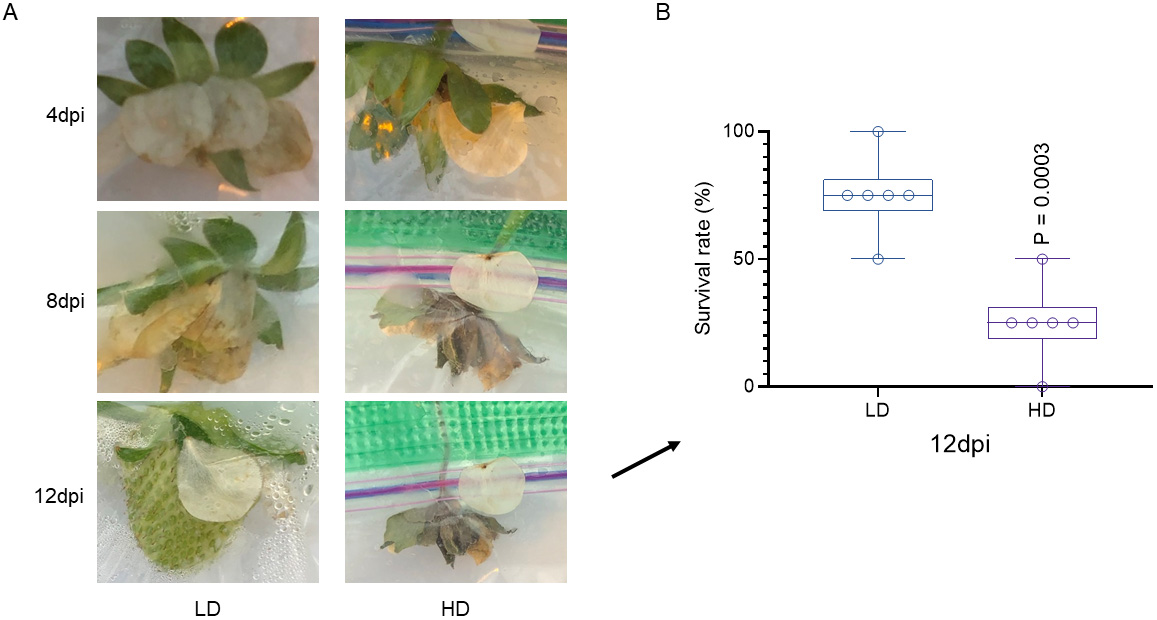
Figure 3. Procedure of evaluating fruit survival rate to Botrytis cinerea infection at the late-flowering to early-fruiting stage. Inoculated newly emerging fruits (~3.5-month-old) were monitored until 12 dpi to evaluate fruit development (A) and its survival rate to overcome the gray mold disease (B). “LD” stands for inoculation with low dosage of fungal conidia. “HD” stands for inoculation with fungal high dosage of fungal conidia (two-tailed unpaired t-test).
Data analysis
The strawberry lesion diameter was measured using a vernier caliper/ruler.
Strawberry flower symptoms were scored on a 0–4 scale. Symptom score: 0, no symptom; 1, < 50% of the flower area shows dark brown necrosis; 2, > 50% of the flower area shows dark brown necrosis; 3, flower area is heavily covered with fungal hyphae, with additional chlorosis spreading to stem; 4, the whole flower structure collapses (see Figure 2F).
Strawberry fruit disease symptoms were assayed regarding fruit survival rate (%), overcoming the fungal infection.
All statistical analyses were performed using the GraphPad Prism v9.0.2 software. Data were presented as box & whiskers (min to max, show all points). Exact p value (two-tailed unpaired t-test) was shown in each graph.
All raw example datasets were provided in this protocol.
Validation of protocol
B. cinerea, a fungal pathogen, is known to cause gray mold disease in strawberries and many other crops (Dean et al., 2012; Yang et al., 2023b). In vivo inoculation assays have been developed in this study to assess the resistance or susceptibility of in planta strawberry leaf and flower samples to B. cinerea. The idea of infecting strawberry flowers with fungal conidia was inspired by the research of Yang et al. (2018). This protocol outlines the procedure for conducting in vivo inoculation assays at different stages of strawberry development, utilizing either fungal discs or fungal conidia suspension under realistic conditions.
The protocol has demonstrated successful implementation in both strictly controlled laboratory settings and less-controlled greenhouse environments, yielding highly effective results (see Figures 1–3). Its robustness and reproducibility are ensured through consistent employment of standardized fungal disc sizes or conidia concentrations, uniform inoculation techniques, and the use of controlled sandwich bags that mimic optimal growth conditions for B. cinerea. Additionally, the protocol incorporates an ample number of replicates (at least 24 leaves/flowers/fruits per inoculation) and appropriate statistical analysis (two-tailed unpaired t-test) to validate the outcomes and account for potential sources of variation.
The outcome of the protocol is measured by assessing lesion diameter (for leaf infections), symptom score (for flower infections), or survival rate (%, for fruit infections) after 4–12 days of incubation. These parameters reflect the degree of resistance or susceptibility exhibited by each strawberry sample to B. cinerea infection.
Below is the raw dataset as Table 1 presented in Figure 1H.
Table 1. Raw dataset presented in Figure 1H
| Mock | Infected |
|---|---|
| 2 | 20 |
| 4 | 25 |
| 2 | 30 |
| 1 | 25 |
| 1 | 24 |
| 2 | 23 |
| 0 | 23 |
| 0 | 23 |
| 0 | 21 |
| 2 | 23 |
| 0 | 26 |
| 1 | 26 |
| 0 | 20 |
| 2 | 21 |
| 1 | 29 |
| 0 | 29 |
| 0 | 21 |
| 0 | 26 |
| 2 | 25 |
| 2 | 23 |
| 0 | 25 |
| 0 | 30 |
| 0 | 27 |
| 2 | 23 |
| Unpaired t-test | |
| p value | < 0.0001 |
| p value summary | **** |
| Significantly different (p < 0.05)? | Yes |
| One- or two-tailed p value? | Two-tailed |
| t, df | T = 36.09, df = 46 |
Below is the raw dataset presented in Figure 2G (Table 2).
Table 2. Raw dataset presented in Figure 2G
| Mock | Infected |
|---|---|
| 0 | 2 |
| 1 | 3 |
| 0 | 4 |
| 0 | 4 |
| 0 | 4 |
| 0 | 4 |
| 0 | 3 |
| 1 | 3 |
| 0 | 3 |
| 0 | 3 |
| 0 | 4 |
| 1 | 4 |
| 1 | 3 |
| 1 | 3 |
| 1 | 2 |
| 0 | 3 |
| 0 | 3 |
| 0 | 3 |
| 0 | 2 |
| 0 | 4 |
| 0 | 4 |
| 1 | 2 |
| 1 | 2 |
| 0 | 3 |
| Unpaired t-test | |
| p value | < 0.0001 |
| p value summary | **** |
| Significantly different (p < 0.05)? | Yes |
| One- or two-tailed p value? | Two-tailed |
| t, df | t = 15.48, df = 46 |
Below is the raw dataset presented in Figure 3B (Table 3).
Table 3. Raw dataset presented in Figure 3B
| LD | HD |
|---|---|
| 75 | 50 |
| 75 | 25 |
| 75 | 25 |
| 100 | 0 |
| 50 | 25 |
| 75 | 25 |
| Unpaired t-test | |
| p value | 0.0003 |
| p value summary | *** |
| Significantly different (p < 0.05)? | Yes |
| One- or two-tailed p value? | Two-tailed |
| t, df | t = 5.477, df = 10 |
General notes and troubleshooting
General notes
B. cinerea SS2Ap1 was isolated from a commercially grown and harvested strawberry in California and tends to produce mass conidia at the edge of the agar plate under darkness at RT.
Miracloth might be a better alternative to lens paper due to its fixed pore size. We used lens paper for its lower cost and higher availability.
Fungal conidia were diluted in half-strength V8 medium to facilitate reproducible pathogenesis.
The high relative humidity is critical for reproducible disease symptom development.
This protocol is also applicable to other research objectives (e.g., in vitro detached leaf/fruit inoculation) and flowering plants with appropriate modifications.
Troubleshooting
Problem 1: The fungal conidia production is low.
Possible cause: The culture condition is not optimal.
Solution: You can try to incubate the fungal plate under darker and cooler conditions, as this might affect its growth. Another option is to reduce the nutrient strength of the agar media, for instance, from half to 1/10.
Problem 2: The symptoms of the disease are uneven.
Possible cause: The optimal dose for inoculation has not been determined.
Solution: To find the optimal dose of inoculum, you should consider the differences in plant tissues, species, and size. It is important to note that high relative humidity is critical for the reproducibility of disease symptoms.
Acknowledgments
We want to thank the support from International Flavors & Fragrances Inc (IFF), USDA Hatch Project, and OSU-OARDC Start-Up Fund.
Competing interests
The authors declare no competing interests.
References
- Chanda, B., Xia, Y., Mandal, M. K., Yu, K., Sekine, K., Gao, Q. m., Selote, D., Hu, Y., Stromberg, A., Navarre, D., et al. (2011). Glycerol-3-phosphate is a critical mobile inducer of systemic immunity in plants. Nat. Genet. 43(5): 421–427.
- Chen, X., Selvaraj, P., Lin, L., Fang, W., Wu, C., Yang, P., Zhang, J., Abubakar, Y. S., Yang, F., Lu, G., et al. (2023). Rab7/Retromer‐based endolysosomal trafficking is essential for proper host invasion in rice blast. New Phytol. 239(4): 1384–1403.
- Dean, R., Kan, J. a. L. V., Pretorius, Z. A., Hammond‐Kosack, K. E., Pietro, A. D., Spanu, P. D., Rudd, J. J., Dickman, M., Kahmann, R., Ellis, J., et al. (2012). The Top 10 fungal pathogens in molecular plant pathology.Mol. Plant Pathol. 13(4): 414–430.
- Fillinger, S. and Elad, Y. (Eds.). (2016). Botrytis – the Fungus, the Pathogen and its Management in Agricultural Systems. Cham: Springer International Publishing.
- Petrasch, S., Knapp, S. J., van Kan, J. A. L. and Blanco-Ulate, B. (2019). Grey mould of strawberry, a devastating disease caused by the ubiquitous necrotrophic fungal pathogen Botrytis cinerea. Mol. Plant Pathol 20(6): 877–892.
- Sahib, M. R., Yang, P., Bokros, N., Shapiro, N., Woyke, T., Kyrpides, N. C., Xia, Y. and DeBolt, S. (2019). Improved Draft Genome Sequence of Microbacterium sp. Strain LKL04, a Bacterial Endophyte Associated with Switchgrass Plants. Microbiol. Resour. Announce. 8(45): e00927–19.
- Sun, J. Q., Jiang, H. L. and Li, C. Y. (2011). Systemin/Jasmonate-Mediated Systemic Defense Signaling in Tomato. Mol. Plant 4(4): 607–615.
- Yang, P., Bokros, N., Debolt, S., Zhao, Z. and Xia, Y. (2022). Genome Sequence Source of Bacillus amyloliquefaciens Strain GD4a, a Bacterial Endophyte Associated with Switchgrass Plants. Phytobiomes Journal 6(4): 354–357.
- Yang, P., Chen, Y., Wu, H., Fang, W., Liang, Q., Zheng, Y., Olsson, S., Zhang, D., Zhou, J., Wang, Z., et al. (2018). The 5-oxoprolinase is required for conidiation, sexual reproduction, virulence and deoxynivalenol production of Fusarium graminearum. Curr. Genet 64(1): 285–301.
- Yang, P., Zhao, L., Gao, Y. G. and Xia, Y. (2023a). Detection, Diagnosis, and Preventive Management of the Bacterial Plant Pathogen Pseudomonas syringae. Plants 12(9): 1765.
- Yang, P., Zhao, Z., Fan, J., Liang, Y., Bernier, M. C., Gao, Y., Zhao, L., Opiyo, S. O. and Xia, Y. (2023b). Bacillus proteolyticus OSUB18 triggers induced systemic resistance against bacterial and fungal pathogens in Arabidopsis. Front. Plant Sci. 14: e1078100.
- Zhao, Z., Bokros, N., DeBolt, S., Yang, P. and Xia, Y. (2021). Genome Sequence Resource of Bacillus sp. RRD69, a Beneficial Bacterial Endophyte Isolated from Switchgrass Plants. Mol. Plant Microbe Interact. 34(11): 1320–1323.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Yang, P., Zhao, Z., Virag, A., Becker, T., Zhao, L., Liu, W. and Xia, Y. (2023). Botrytis cinerea in vivo Inoculation Assays for Early-, Middle- and Late-stage Strawberries. Bio-protocol 13(20): e4859. DOI: 10.21769/BioProtoc.4859.
Category
Plant Science > Plant immunity > Disease bioassay
Plant Science > Plant physiology > Biotic stress
Biological Sciences > Biological techniques > Microbiology techniques
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









