- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Successful Transfection of MicroRNA Mimics or Inhibitors in a Regular Cell Line and in Primary Cells Derived from Patients with Rheumatoid Arthritis
Published: Vol 13, Iss 18, Sep 20, 2023 DOI: 10.21769/BioProtoc.4823 Views: 1892
Reviewed by: Navnita DuttaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

An in vitro Assay of mRNA 3’ end Using the E. coli Cell-free Expression System
Monford Paul Abishek N and Heon M. Lim
Feb 20, 2022 3683 Views

Profiling of Single-cell-type-specific MicroRNAs in Arabidopsis Roots by Immunoprecipitation of Root Cell-layer-specific GFP-AGO1
Lusheng Fan [...] Xuemei Chen
Dec 20, 2022 2231 Views

Preparing and Evaluating the Stability of Therapeutically Relevant Oligonucleotide Duplexes
Shreyas G. Iyer and Andrea L. Kasinski
Apr 20, 2024 4324 Views
Abstract
The transfection of microRNA (miRNA) mimics and inhibitors can lead to the gain and loss of intracellular miRNA function, helping us better understand the role of miRNA during gene expression regulation under specific physical conditions. Our previous research has confirmed the efficiency and convenience of using liposomes to transfect miRNA mimics or inhibitors. This work uses miR-424 as an example, to provide a detailed introduction for the transfection process of miRNA mimics and inhibitors in the regular SW982 cell line and primary rheumatoid arthritis synovial fibroblasts (RASF) cells from patients by using lipofection, which can also serve as a reference to miRNA transfection in other cell lines.
Key features
• MiRNA mimics and inhibitors transfection in regular SW982 cell line and primary RASF cells.
• Treatment and culture of RASF primary cells before transfection.
Using liposomes for transfection purposes.
Keywords: miRNABackground
Transfection is a specialized technique for introducing exogenous genes into cells. With the deepening of research on gene and protein functions, transfection has become a common basic method in laboratory work. Transfection can be roughly divided into three methods: physical, chemical, and biological mediation. Electroporation, microinjection, and gene gun are very promising physical methods. There are also many chemical methods, such as the classical calcium phosphate coprecipitation method and the lipofection method. Biological methods include primitive protoplast transfection and various virus-mediated transfection techniques (Kim and Eberwine, 2010).
Lipofection refers to the cationic liposome with a positive charge on the surface, which can interact with the phosphate group of nucleic acid through electrostatic interactions to encapsulate DNA or RNA molecules, forming lipid complexes. It can also be adsorbed by negatively charged cell membranes on the surface and then enter cells through fusion or endocytosis. Lipofection is suitable for transfect DNA or RNA into suspension or adherent cultured cells, and is currently one of the most convenient and efficient transfection methods in the laboratory (Felgner et al., 1987).
MicroRNAs (miRNAs) are a class of non-coding small RNAs composed of 18–25 nucleotides. Mostly, they regulate the expression of various protein cod genes at the post-transcriptional level, by inhibiting translation or inducing mRNA degradation, and have a significant impact on physiological processes such as cell differentiation, proliferation, apoptosis, and hematopoiesis. Hence, abnormal expression of miRNA is pathological and can result in the development and progression of multiple diseases (Bartel, 2004).
Some studies have found multiple miRNA expression disorders in rheumatoid arthritis (RA) patients, suggesting that an abnormal miRNA expression may be the molecular mechanism of the disease. These studies involve various cellular biological behaviors, as well as the regulation of signaling pathways and the study of target genes. With the expansion of research fields, the important role of miRNA in RA is gradually receiving increased attention. For example, miR-18a was found to be an inhibitor of nuclear factor kappa-B (NF-κB) in rheumatoid arthritis synovial fibroblasts (RASF) (Trenkmann et al., 2013); miR-19a is associated with the expression of Toll-like receptors in RASF (Philippe et al., 2012); and miR-23b is related to the expression of various inflammatory cytokines in RASF (Zhu et al., 2012).
As a key cell type in the research of pathogenesis of rheumatoid arthritis, the excessive proliferation and limited apoptosis of RASFs are considered to be the pathological basis of RA, which can directly promote joint destruction and cartilage damage by enhancing the production of matrix degrading enzymes (van der Helm-van Mil and Huizinga, 2008; Bartok and Firestein, 2010). Mimics and inhibitors of miRNAs were transfected into the regular SW982 cell line and the primary RASF cells from patients to study the molecular mechanism of these miRNAs during gain and loss of their function.
Materials and reagents
Biological materials
SW982 cell line (ATCC, catalog number: HTB-93)
RASF primary cell (origin)
Reagents
LipofectamineTM 3000 (Invitrogen, catalog number: L300001)
Mimics and inhibitors (GenePharma company, catalog number: B02001, B03001)
0.25% Trypsin-EDTA (HyClone, catalog number: SH30042.01B)
Fetal bovine serum (FBS) (Sangon biotechnology, catalog number: E51002)
DMEM high glucose culture medium (HyClone, catalog number: SH30022.01B)
Penicillin-Streptomycin 100× (P/S) (HyClone, catalog number: SV30010)
Saline (CR double-crane, catalog number: H20054037)
Phosphate buffered saline (PBS) (Biosharp, catalog number: BL601A)
DNase (Sigma-Aldrich, catalog number: AMPD1)
Hyaluronidase (Sigma-Aldrich, catalog number: 37259-53-3)
Type I collagenase (Sigma-Aldrich, catalog number: SCR103)
TRIzol reagent (Invitrogen, catalog number: 15596-026)
Pierce RIPA buffer (Thermo, catalog number: 89900)
NaOH (Xilong chemistry, catalog number: 7697-37-2)
HCI (Xilong chemistry, catalog number: 121-23-1)
Solutions
SW982 and RASF cell culture medium (see Recipes)
Recipes
SW982 and RASF cell culture medium
Reagent Final concentration Volume DMEM high glucose medium n/a 450 mL Fetal bovine serum 10% 50 mL P/S 0.1% 0.5 mL Total n/a 500.5 mL Adjust the pH with NaOH or HCl to 7.2–7.4.
Serum-free DMEM medium refers to the medium without the addition of FBS and antibiotics (the basal medium).
Laboratory supplies
100 mm sterile polystyrene culture dish (Nunc, catalog number: 150340)
6-well sterile polystyrene cell culture plate (Nunc, catalog number: 150239)
15 mL sterile centrifuge tube (KIRGEN, catalog number: KG2611)
1.5 mL centrifuge tube (KIRGEN, catalog number: KG2211S)
Ophthalmic scissors (Shinva, catalog number: ZC056R)
Hemacytometer (Bio-Rad, catalog number: 1450011)
Cell culture flask (Nunc, catalog number: 156340)
Equipment
Cell culture incubator: 37 and 5% CO2 (Thermo, catalog number: THM#3427)
Centrifuge (Eppendorf, catalog number: 5810R)
Biological safety cabinetry (Biobase, catalog number: BSC-1500IIB2-X)
Microscope (Nikon, catalog number: Eclipse E200)
Procedure
Cell preparation
SW982 cells are regularly cultured in DMEM high glucose medium supplemented with 10% FBS and 0.1% P/S and incubated at 37 °C in humid conditions with 5% CO2. RASF cells were isolated from synovial tissues of the knee joints from four RA patients; Table 1 displays the specific information of patients. The procedures for RASF isolation are as follows; a simple flowchart for cell extraction is shown in Figure 1.
Table 1. Patients’ information
Number Gender Age (years) RF (IU/mL) ESR (mm/h) CRP (mg/L) anti-CCP positive ratio 1 Female 37 18.62 90 8.7 18.5 2 Female 60 71.97 113 34.5 3.2 3 Female 45 195.6 87 42.96 110.4 4 Female 66 61.5 31 10.22 33.6 RF: rheumatoid factor
ESR: erythrocyte sedimentation rate
CRP: C-reactive protein
CCP: cyclic citrullinated peptide
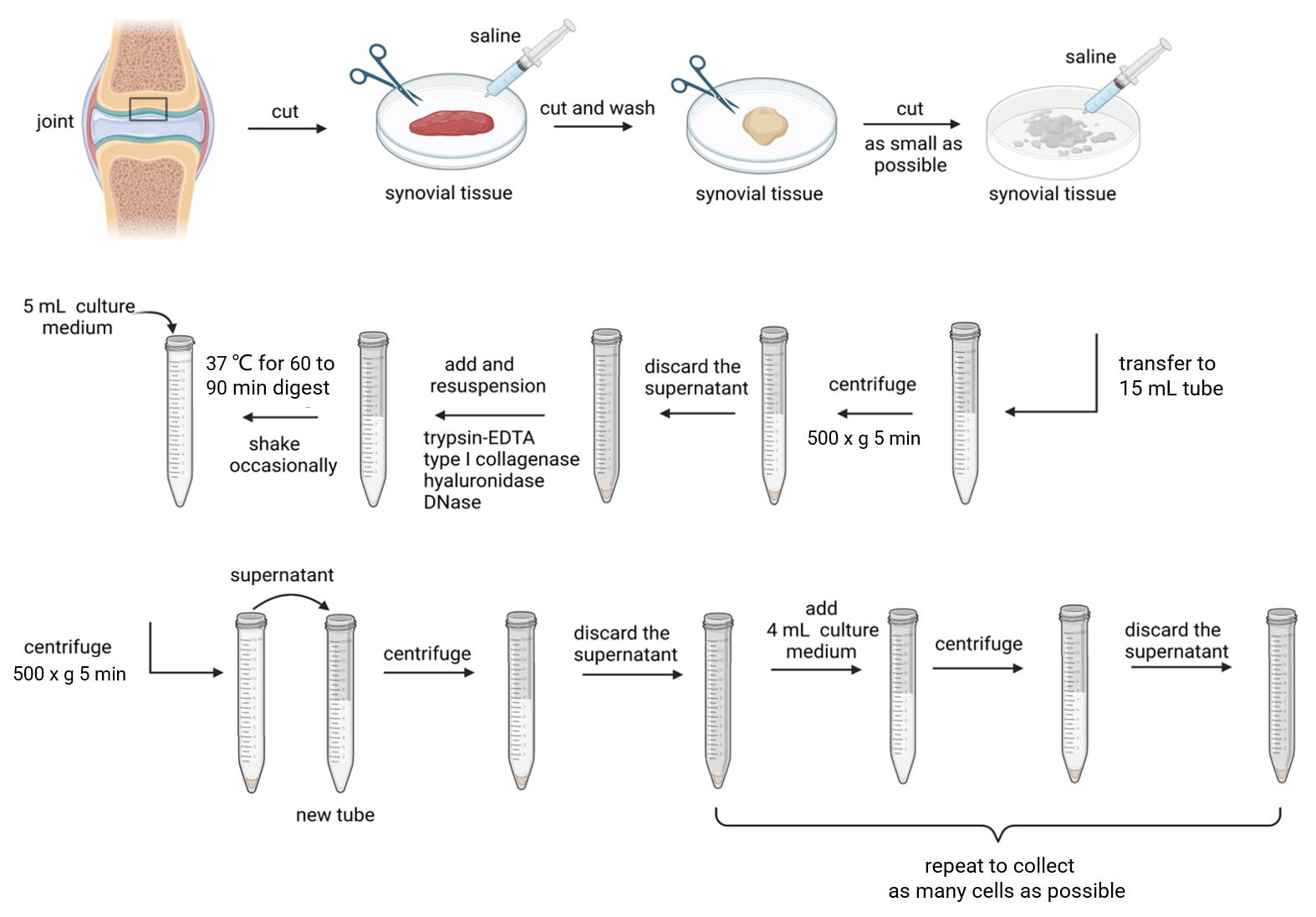
Figure 1. Schematic diagram of synovial fibroblasts extraction process. Created with BioRender.com.Cut off excess adipose tissue from the RA synovial tissue specimen, rinse the synovial tissue with sterile saline until there are no obvious blood stains, select suitable tissues, place it in a sterile culture dish, and use ophthalmic scissors to cut it as small as possible.
Add 5 mL of saline, soak the tissues, and then transfer it into a 15 mL centrifuge tube. Centrifuge at 500× g for 5 min and discard the supernatant.
Add 5 mL of 0.25% trypsin-EDTA for resuspension, type I collagenase (final concentration 1 mg/mL), hyaluronidase (final concentration 0.15 mg/mL), and DNase (final concentration 0.15 mg/mL). Mix them well, digest at 37 for 60–90 min, and shake the centrifuge tube occasionally.
After tissues’ digestion, add 5 mL of culture medium and resuspend. Let it stand until the remaining tissue block in the 15 mL centrifuge tube settles at the bottom and transfer the supernatant to another 15 mL centrifuge tube for centrifugation to obtain cell pellets.
Wash the pellets with 5 mL of culture medium, resuspend, centrifuge, and discard the supernatant. Add 4 mL of culture medium again and repeat the resuspension centrifugation step to collect as many cells as possible.
Count the collected cells with a hemacytometer. Seed 4 million cells per 25 cm2 of the culture bottle. The newly isolated cells are highly diverse. In addition to RASF, there are lymphocytes, osteoclasts, macrophages, and macrophage-like synovial cells. Therefore, the abundant cell density at the very beginning is crucial for the growth of synovial cells.
Place the culture flask inoculated with cells in a 5% CO2 cell incubator at 37 and observe the cell adhesion for any contamination on the next day. On the third day, change the fluid and discard the other types of cells or dead cells, which did not adhere to the bottom of the flask. Passage the cells when the confluence reaches 90%. Primary synovial fibroblasts in good condition are shown in Figure 2.
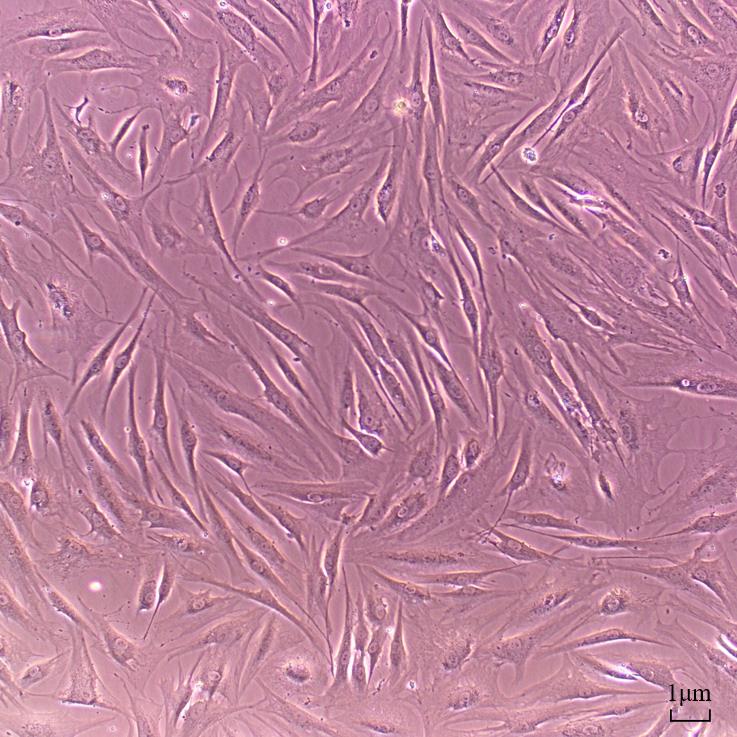
Figure 2. The expected cell morphology of rheumatoid arthritis synovial fibroblast primary cells. Scale bar: 1 μm.
Cell seeding
Wash thoroughly with PBS buffer three times. After discarding all the liquid, add 1 mL of trypsin, gently move the culture flask to make it even, and incubate it at 37 for approximately 2 min. Observe the cell condition and avoid excessive digestion, which may affect the cell morphology.
Add 4 mL of complete culture medium to terminate the reaction, gently detach the cells from the bottom with a pipette, transfer the cells to a 15 mL centrifuge tube, centrifuge at 1,000× g for 5 min to collect cell pellets, discard the supernatant, add 1 mL of the culture medium, and fully resuspend the cells.
According to the required number of cells in the experiment, inoculate SW982 cells and RASF cell in a 6-well plate overnight and make sure they grow to 80% confluence.
Preparation of the transfection reagents
For each well in the 6-well plate, add 5 μL of LipofectamineTM 3000 reagent and mix well using a pipette in a 1.5 mL centrifuge tube containing 250 μL of serum-free DMEM medium. Incubate at room temperature for 5 min.
Add miRNA mimics or inhibitors and mix them in another centrifuge tube containing 250 μL of serum-free DMEM medium. The sequence information of mimics and inhibitors is shown in Table 2.
Table 2. MiRNA mimics and inhibitors sequence information
Name Product Sequence (from 5′ to 3′) miRNA NC Mimic Sense UUCUCCGAACGUGUCACGUTT Antisense ACGUGACACGUUCGGAGAATT
miR-424-5p Mimic Sense CAGCAGCAAUUCAUGUUUUGAA
Antisense CAAAACAUGAAUUGCUGCUGUU
miRNA NC Inhibitor CAGUACUUUUGUGUAGUACAA miR-424-5p Inhibitor UUCAAAACAUGAAUUGCUGCUG NC: negative control
Combine the two mixtures from steps 1 and 2, gently mix 15 times, and incubate at room temperature for 20 min.
Prepare the cells during the incubation period of the previous step. Wash the cells in the 6-well plate with PBS and add 1.5 mL of serum-free culture medium. After the incubation of the transfection reagent, add 500 μL of the mixture from the previous step to each well, mix thoroughly, and incubate in a CO2 incubator. Forty-eight hours later, replace the medium with the complete SW982 and RASF cell culture medium.
Troubleshooting
The temperature of LipofectamineTM 3000 may affect transfection efficiency; therefore, when mixed with LipofectamineTM 3000, the culture medium should be kept at room temperature.
The number and status of cells have an impact on transfection. Resuscitated cells and primary cells should be transmitted to at least the third generation, and the number of cells should be stable before use.
After adding LipofectamineTM 3000 in the experimental steps, the mixing should be gentle to avoid damaging the liposome particles and affecting the transfection efficiency.
The transfection effect depends on the cell state, concentration of transfectants, transfection reagents, operation of transfection processes, etc. Therefore, a pilot study for subsequent transfection efficiency verification is always needed to determine whether they are effective for each individual study. We are usually confident to validate the gain or loss of function for miRNAs and their target genes following transfection with the miRNA mimics or inhibitors for 48 h.
Data analysis
Data were analyzed using SPSS 21. Experimental data were expressed as mean ± standard error of the mean (SEM). Differences between the groups were analyzed using the Mann-Whitney test. p < 0.05 was considered statistically significant (* p < 0.05).
Validation of protocol
The validation of transfection efficiency can be achieved by detecting the intracellular miRNA level following the transfection of exogenous miRNA mimics or inhibitors. Use TRIzol reagent to collect the cell lysate and perform RT-qPCR to verify transfection efficiency. In our published results (Wang et al., 2021), miRNA expression can increase up to a thousand times from its previous intracellular level after transfection of the mimics, and expression of some miRNAs can also be significantly reduced after transfection with inhibitors (Figure 3). This indicates the success of the lipofection method, which is simple and highly reproducible. Validation of target genes is achieved by western blotting, using RIPA buffer to collect protein lysate. After protein quantification, gel electrophoresis, and antibody incubation, the results of target gene expression changes were obtained; this part of the results can be referenced in our published article (Wang et al., 2021) and in Figure 4.
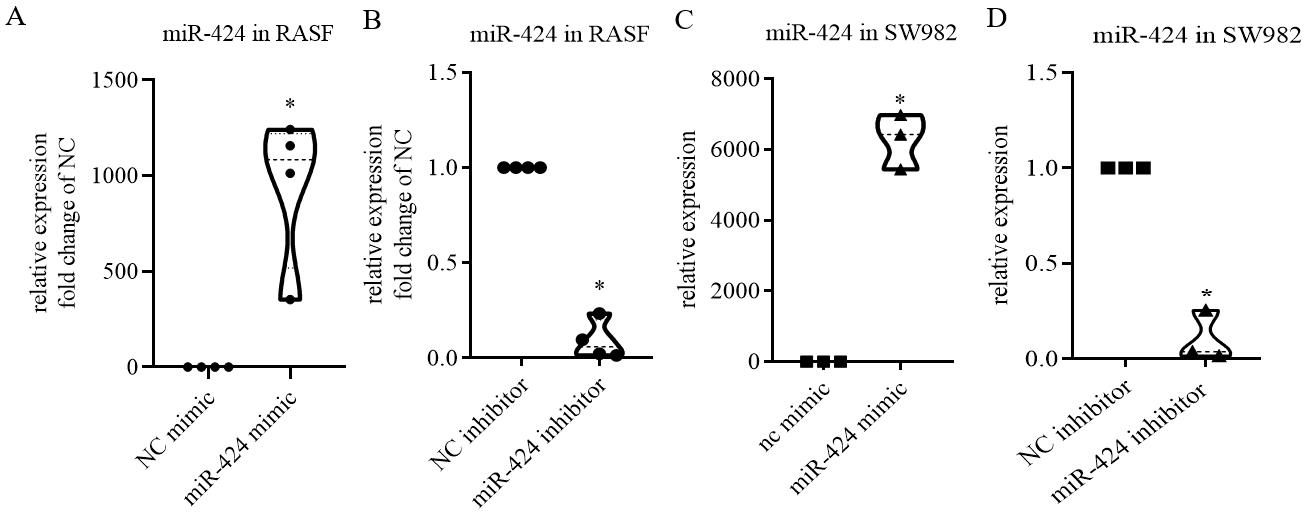
Figure 3. RT-qPCR results showing the RNA expression level of miR-424 in rheumatoid arthritis synovial fibroblasts (RASF) and SW982 cell line following the transfection of its mimics or inhibitors for 48 h. A, B. Expression of miR-424 in 10 nM mimic (A) or 100 nM inhibitor (B)-treated RASF. C, D. Expression of miR-424 in 10 nM mimic (C) or 100 nM inhibitor (D)-treated SW982 cells. Scale bar: mean ± SEM of samples from four patients (A and B) or from three independent cell experiments (C and D) p < 0.05, *: vs. negative control (NC), using Mann-Whitney test.
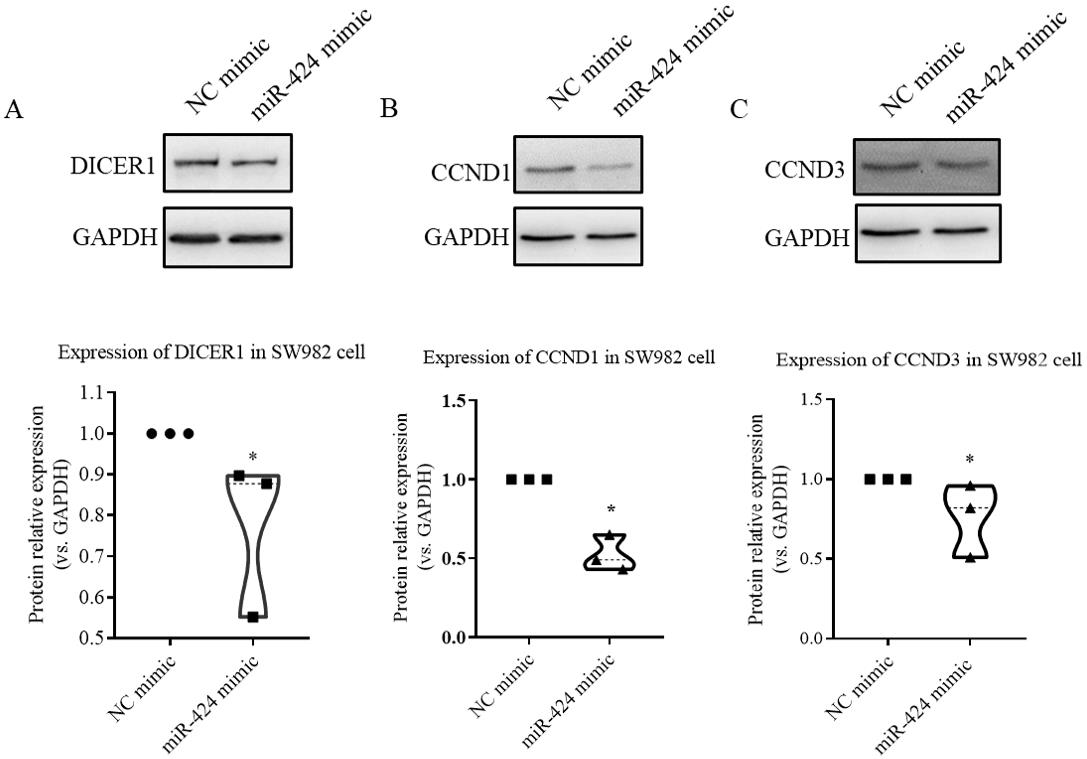
Figure 4. Western blotting results showing the protein expression of validated miR-424 target gene in SW982 cell line following miR-424 mimic transfection for 48 h. The protein expression of DICER1 (A), CCND1 (B), and CCND3 (C) in SW982 cells following miR-424 mimic transfection. Scale bar: mean ± SEM from three independent cell experiments. p < 0.05, *: vs. negative control (NC), using Mann-Whitney test.
General notes and troubleshooting
General notes
MiRNA mimics and inhibitors were synthesized by the company. Stock solutions at 20 μM were prepared before use and stored at -20 .
NC group with miRNA should be included. All experiments should be carried out in triplicates as a minimum.
According to our previous experience, for miRNA transfection, the final concentration of the 10 nM mimic substance is enough for most miRNA experiments; for inhibitors, a 100 nM should be ok. The optimal concentration could be obtained through pilot experiments.
Acknowledgments
We are grateful for the National Science Foundation of China (grant No. 81671629, 81701619, 81970029, and 81902249) and Shaanxi Province Natural Science Foundation (Project No. 2021JQ-024 and 2018JM7057).
Competing interests
These experimental procedures were adapted from our previous work published in Wang, S. et al., 2021. The authors declare that there are no known competing financial interests or personal relationships that could have appeared to influence this work.
References
- Bartel, D. P. (2004). MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2): 281–297.
- Bartok, B. and Firestein, G. S. (2010). Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol. Rev. 233(1): 233–255.
- Felgner, P. L., Gadek, T. R., Holm, M., Roman, R., Chan, H. W., Wenz, M., Northrop, J. P., Ringold, G. M. and Danielsen, M. (1987). Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. U. S. A. 84(21): 7413–7417.
- Kim, T. K. and Eberwine, J. H. (2010). Mammalian cell transfection: the present and the future. Anal. Bioanal. Chem. 397(8): 3173–3178.
- Philippe, L., Alsaleh, G., Suffert, G., Meyer, A., Georgel, P., Sibilia, J., Wachsmann, D. and Pfeffer, S. (2012). TLR2 Expression Is Regulated by MicroRNA miR-19 in Rheumatoid Fibroblast-like Synoviocytes. J. Immunol. 188(1): 454–461.
- Trenkmann, M., Brock, M., Gay, R. E., Michel, B. A., Gay, S. and Huber, L. C. (2013). Tumor Necrosis Factor α-Induced MicroRNA-18a Activates Rheumatoid Arthritis Synovial Fibroblasts Through a Feedback Loop in NF-κB Signaling. Arthritis Rheumatol. 65(4): 916–927.
- van der Helm-van Mil, A. H. and Huizinga, T. W. (2008). Advances in the genetics of rheumatoid arthritis point to subclassification into distinct disease subsets. Arthritis Res. Ther. 10(2): 205.
- Wang, S., Xu, J., Guo, Y., Cai, Y., Ren, X., Zhu, W., Geng, M., Meng, L., Jiang, C., Lu, S., et al. (2021). MicroRNA-497 Reduction and Increase of Its Family Member MicroRNA-424 Lead to Dysregulation of Multiple Inflammation Related Genes in Synovial Fibroblasts With Rheumatoid Arthritis. Front. Immunol. 12: e619392.
- Zhu, S., Pan, W., Song, X., Liu, Y., Shao, X., Tang, Y., Liang, D., He, D., Wang, H., Liu, W., et al. (2012).
- The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-α. Nat. Med. 18(7): 1077–1086.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Wang, S., Xu, J., Guo, Y., Cai, Y., Zhu, W., Meng, L., Jiang, C. and Lu, S. (2023). Successful Transfection of MicroRNA Mimics or Inhibitors in a Regular Cell Line and in Primary Cells Derived from Patients with Rheumatoid Arthritis. Bio-protocol 13(18): e4823. DOI: 10.21769/BioProtoc.4823.
Category
Molecular Biology > RNA > miRNA interference
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








