- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation, Purification, and Culture of Embryonic Melanoblasts from Green Fluorescent Protein–expressing Reporter Mice
Published: Vol 13, Iss 17, Sep 5, 2023 DOI: 10.21769/BioProtoc.4805 Views: 1992
Reviewed by: Olga KopachPhilipp WörsdörferKrishna NakuluriSreejith Perinthottathil

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Isolation and ex vivo Expansion of Limbal Mesenchymal Stromal Cells
Naresh Polisetti [...] Günther Schlunck
Jul 20, 2022 2968 Views

Thrombopoietin-independent Megakaryocyte Differentiation of Hematopoietic Progenitor Cells from Patients with Myeloproliferative Neoplasms
Chloe A. L. Thompson-Peach [...] Daniel Thomas
Jan 20, 2023 2363 Views

Identification and Sorting of Adipose Inflammatory and Metabolically Activated Macrophages in Diet-Induced Obesity
Dan Wu [...] Weidong Wang
Oct 20, 2025 2243 Views
Abstract
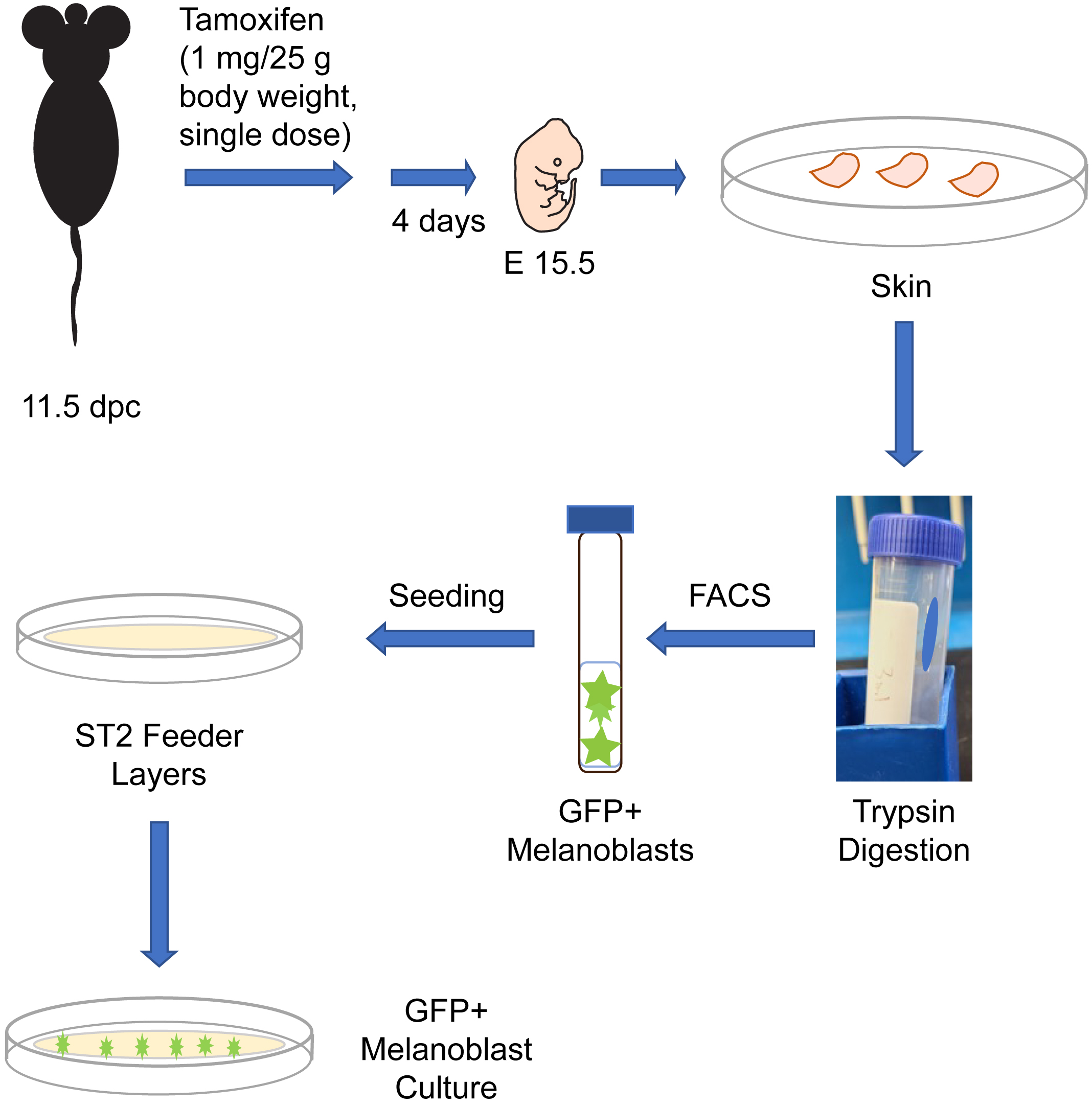
In this article, we provide a method to isolate embryonic melanoblasts from reporter mouse strains. The mice from which these cells are isolated are bred into the ROSA26mT/mG reporter background, which results in green fluorescent protein (GFP) expression in the targeted melanoblast population. These cells are isolated and purified by fluorescence-activated cell sorting using GFP fluorescence. We also provide a method to culture the purified melanoblasts for further analysis. This method yields > 99% purity melanoblasts specifically targeted, and can be used for a variety of studies, including gene expression, clonogenic experiments, and biological assays, such as viability, capacity for directional migration, or differentiation into melanin-producing melanocytic cells.
Graphical overview
Background
Melanocytes are melanin pigment–producing cells that are found in the skin, inner ear, nervous system, and in the heart (Li, 2014). Melanocytes and their precursor cells, melanoblasts, originate from neural crest cells. In mice, a subset of neural crest cells becomes specified as melanoblasts and begin their migration around embryonic day (E) 10.5 from the neural crest, to ultimately colonize the entire body (Mort et al., 2015). A second wave of melanoblast formation occurs around E14.5, when neural crest cells that migrated along a ventral path detach from nerve endings and differentiate into melanoblasts (Delmas et al., 2007; Adameyko et al., 2009). Melanoblasts proliferate, migrate, and interact with their surrounding environment to localize to specific sites in the body. By E16.5, melanoblasts from both waves will have homed to the basal layer of the epidermis, as well as the developing hair follicles (Mort et al., 2015). Understanding the ontogeny of melanocytic cells is a key area in developmental biology with significant implications for human health, as evidenced by the plethora of human diseases linked to defects in melanocytic lineage cells. In this protocol, we describe the isolation from reporter mice of embryonic melanoblasts which express green fluorescent protein (GFP). These mice express in a Cre recombinase enzyme fused to a modified estrogen receptor (CreET2). In these animals, CreET2 expression is restricted to melanocytic lineage cells, as its transcription is under the control of the Tyrosinase promoter. Tamoxifen administration to these mice induces CreET2 nuclear translocation in melanoblasts, allowing activation of GFP expression. As a result, targeted melanoblasts can be identified through GFP fluorescence. This protocol can also be used to purify and characterize embryonic melanoblasts in which a gene of interest flanked by loxP sites can be inactivated by Cre recombinase (Crawford et al., 2017). In this protocol, we also describe methods to culture the embryonic melanoblasts using ST2 feeder layers. Specifically, seeding the purified melanoblasts onto a confluent monolayer of ST2 cells maintains their viability and supports their maturation into melanin-producing cells (Crawford et al., 2020). This system allows studies including single-cell analyses and whole-genome screens of melanoblasts. Under the conditions used in our cultures, melanoblasts remain viable for at least seven days and transition into melanin-producing cells.
Materials and reagents
Biological materials
Mice. The experiments we describe are conducted with mice generated by breeding the reporter strain Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J (hereafter termed ROSAmT/mG, The Jackson Laboratory, strain number 007676) (Muzumdar et al., 2007) with B6.Cg-Tg (Tyr-cre/ERT2)13Bos/J (hereafter termed Tyr::CreERT2, The Jackson Laboratory, strain number 012328) (Bosenberg et al., 2006). Our protocol uses melanoblasts isolated from timed-pregnant dams that are homozygous for the ROSAmT/mG allele and homozygous for Tyr::CreERT2. These reporter mice can be further bred into other backgrounds in which a gene of interest is flanked by loxP sites and can therefore be used to generate GFP-tagged melanocytic cells in which a gene of interest has also been inactivated upon administration of tamoxifen (Crawford et al., 2020). Non-targeted cells can be identified by their expression of mTomato fluorescent protein. These experiments require time-pregnant mice, which are first treated with tamoxifen at 11.5 days post-coitus (dpc; midday of the vaginal plug is considered 0.5 dpc), as described below in the procedure.
ST2 cells. Bone marrow stroma–derived cell line (Riken BioResource Research Center, catalog number: RCB0224). ST2 cells express collagens and fibroblast growth factors and support melanoblasts in culture (Abdallah et al., 2019).
Reagents
Tamoxifen (Millipore Sigma, catalog number: T-5648)
Absolute ethanol (Millipore Sigma, catalog number: 493511)
70% ethanol (Millipore Sigma, catalog number: 63350-M)
Bovine serum albumin fraction V (Wisent, catalog number: 800-095-EG)
Cremophore/Kolliphor EL (Millipore Sigma, catalog number: C5135)
MGM-4 basal medium (Lonza, catalog number: CC-3250)
MGM-4 SingleQuot kit with supplements & growth factors (Lonza, catalog number: CC-4435)
Endothelin 3, lyophilized (ET3) (Lonza, catalog number: CC-4510)
2.5% trypsin (Invitrogen, catalog number: 15090046)
Trypsin neutralizing solution (Life Technologies, Thermo Fisher Scientific, catalog number: R-002-100)
Fetal bovine serum (FBS) (Thermo Fisher Scientific, Gibco, catalog number: 26140079)
0.25% trypsin/EDTA (Invitrogen, Thermo Fisher Scientific, catalog number: 25200072)
7-Amino-actinomycin D (7-AAD) (Millipore Sigma, catalog number: A9400)
Paraformaldehyde (PFA) (Millipore Sigma, catalog number: 158127)
Sterile RPMI medium (Millipore Sigma, catalog number: R8758)
NaCl (Millipore Sigma, catalog number: S5150)
KCl (Millipore Sigma, catalog number: P5405)
Na2HPO4 (Millipore Sigma, catalog number: S7907)
KH2PO4 (Millipore Sigma, catalog number: P5655)
NaOH (Millipore Sigma, catalog number: S8045)
Triton X-100 (Millipore Sigma, catalog number: X100-100ML)
Mouse Anti-TRP1 antibody (Abcam, catalog number: 3312)
Goat anti-mouse IgG Alexa Fluor® 594 Conjugate (Thermo Fisher Scientific, catalog number: A-11032)
Mounting medium with DAPI (ibidi, catalog number: 50011)
Solutions
Tamoxifen stock solution (50 mg/mL) (see Recipes)
Melanoblast culture medium (see Recipes)
ST2 culture medium (see Recipes)
Sterile Ca2+- and Mg2+-free phosphate-buffered saline (PBS), pH 7.4 (see Recipes)
FACS buffer: sterile Ca2+- and Mg2+-free PBS containing 2% FBS (see Recipes)
7-Amino-actinomycin D (7-AAD) stock solution (see Recipes)
7-Amino-actinomycin D (7-AAD) working staining solution (see Recipes)
1 N NaOH solution (see Recipes)
4% paraformaldehyde (PFA) (see Recipes)
Recipes
Tamoxifen stock solution (store at -20 °C)
Reagent Final concentration Quantity Tamoxifen 50 mg/mL 25 mg Absolute ethanol 50% 250 μL Cremophore/Kolliphor 50% 250 μL Total n/a 500 μL Melanoblast culture medium (store at 4 °C)
Reagent Final concentration Quantity MGM-4 basal medium n/a 500 mL MGM-4 SingleQuot Kit n/a 1 kit (9 mL) ET3 255 ng/mL 130 μg Total n/a 509 mL ST2 cell culture medium (store at 4 °C)
Reagent Final concentration Quantity RPMI medium 90% 450 mL FBS 10% 50 mL Total n/a 500 mL Ca2+- and Mg2+-free phosphate-buffered saline (PBS, store at 4 °C)
Reagent Final concentration Quantity NaCl 137 mM 8 g KCl 2.7 mM 0.2 g Na2HPO4 10 mM 1.44 g KH2PO4 1.8 mM 0.24 g Total n/a to 1,000 mL Autoclave to sterilize.
FACS buffer (store at 4 °C)
Reagent Final concentration Quantity Sterile Ca2+- and Mg2+-free PBS 98% 98 mL FBS 2% 2 mL Total n/a 100 mL Keep sterile.
7-Amino-actinomycin D (7-AAD) stock solution (store at -20 °C, protected from light)
Reagent Final concentration Quantity 7-AAD 1 mg/mL 1 mg Methanol 5% 50 μL Sterile Ca2+- and Mg2+-free PBS 950 μL Total n/a 1 mL 7-Amino-actinomycin D (7-AAD) working staining solution (freshly prepared)
Reagent Final concentration Quantity 7-AAD stock solution 1% 100 μL FACS buffer 99% to 10 mL Total n/a 10 mL 1 N NaOH solution
Reagent Final concentration Quantity NaOH 1 N 40 g H2O n/a to 1,000 mL Total n/a 1,000 mL 4% Paraformaldehyde (PFA) solution
Reagent Final concentration Quantity PFA 4% 0.4 g Ca2+- and Mg2+-free PBS n/a to 10 mL Total n/a 10 mL Prepare just prior to use.
Laboratory supplies
Bacterial grade 100 mm Petri dishes (Fisher Scientific, catalog number: FB0875713A)
Surgical scissors (Fine Science Tools, catalog number: 14002-12)
Two sets of forceps (Fine Science Tools, catalog number: 11000-12)
Scalpel (Fine Science Tools, catalog number: 10003-12)
Scalpel blades (Fine Science Tools, catalog number: 10011-00)
Sterile 15 mL conical tubes (Sarstedt, catalog number: 62.554.502)
Sterile 50 mL conical tubes (Sarstedt, catalog number: 62.547.205)
2 mL microcentrifuge tubes (VWR, catalog number: 20170-098)
Sterile 10 mL disposable plastic syringes (BD Biosciences, catalog number: 302995)
Sterile 21 G needles for syringe (BD Biosciences, catalog number: 305165)
Sterile 5 mL round-bottom snap cap tubes (BD Falcon, catalog number: 52054)
Sterile 40 μm nylon cell strainers (Fisher Scientific, catalog number: 08-771-1)
Neubauer hemocytometer (Hausser Scientific, catalog number: 15170-173)
BioLite T25 flasks, cell culture treated (ThermoFisher Scientific, catalog number: 130189)
35 mm μ-dishes with a 4-well insert (ibidi, catalog number: 80466)
Pipet-aid (Thermo Fisher Scientific, S1 Pipet Filler, catalog number: 9521)
Micropipettes (Eppendorf Research plus, P20, P200, P2000)
12 mm glass coverslips (Mandel Scientific, catalog number: NEU-GG-12)
Equipment
Stereomicroscope (Leica Stereo Zoom GZ6E)
Phase contrast and fluorescence inverted microscope with camera and humidified CO2 37 °C chamber for time-lapse videomicroscopy (Leica DMIRBE or equivalent)
Becton Dickinson FACSAria III cell sorter (BD Biosciences)
Becton Dickinson FACSCanto flow cytometer (BD Biosciences)
Centrifuge (Eppendorf 5810R)
Biosafety cabinet (Microzone BK-2-4)
CO2 cell culture incubator (VWR, catalog number: 10710-944)
Water bath (PolyScience, catalog number: WBE05A11B)
Rocking platform (Bio-Rad, catalog number: 166709 or equivalent)
Software
FACSDiva Software (v. 8.0.1) or equivalent
Volocity software for cellular imaging and analysis (PerkinElmer), or equivalent
Procedure
Preparation of tamoxifen stock solution
Weigh 25 mg of tamoxifen placing it in a microfuge tube. Caution: Handle the tamoxifen with protective gloves and in a fume hood to avoid inhalation.
Add 250 μL of absolute ethanol.
Vortex and incubate this suspension at 55 °C with vigorous shaking for 5–10 min, ensuring complete dissolution.
Caution: Using protective gloves, add 250 μL of Cremophor using a 1 mL sterile serological pipette, as Cremophor is highly viscous.
Mix by vigorous vortexing.
Store at -20 °C in single-use aliquots of 100 μL.
In vivo activation of CreERT2 recombinase
Immediately prior to use, thaw rapidly a 100 μL aliquot of tamoxifen stock solution (50 mg/mL) by incubating at 37 °C for 1–2 min. Caution: Use protective gloves when handling the tamoxifen solution.
Following institutional biosafety regulations, add 400 μL of sterile Ca2+- and Mg2+-free PBS to 100 μL of tamoxifen stock solution (50 mg/mL), to yield a final concentration of 10 mg/mL of tamoxifen (i.e., 1 mg/100 μL). Vortex to mix well, protect from the light, and use promptly to avoid tamoxifen precipitation from the solution.
Note: The final volume of diluted tamoxifen administered to each mouse will depend on its body weight and will be approximately 100 μL (steps B3 and B4).
Weigh the time-pregnant 11.5 dpc mice to be treated with tamoxifen.
Briefly restraining the conscious mice with one hand (Machholz et al., 2012), administer intraperitoneally (IP) the PBS-diluted tamoxifen solution at a dose of 1 mg/25 g body weight.
Note: Administration of tamoxifen in Cremophor EL-containing solutions reduces variability in pharmacokinetic parameters, including plasma concentrations and half-life (Chevalier et al., 2013). A single IP injection is required.
Return the mice to their housing cage, allowing normal housing conditions for four days.
Culture of ST2 stromal cells
ST2 cells are maintained in ST2 culture medium. Procedures to process cell cultures from T25 tissue culture flasks are described below. All steps are conducted aseptically in a laminar flow biosafety cabinet.
Once ST2 cultures reach 80% confluence, remove the growth medium by aspiration.
Gently add 5 mL of warm (37 °C) sterile Ca2+- and Mg2+-free PBS to rinse the cell monolayer and remove by aspiration.
Trypsinize the cells, adding 1 mL of 0.25% trypsin/EDTA solution, and place the cells in the cell culture incubator.
Examine the extent of cell detachment by phase contrast microscopy every 2–4 min.
Once ~ 90% of the cells have detached, add 7 mL of warm (37 °C) growth medium to inactivate the trypsin and gently mix the cell suspension to generate a single-cell suspension.
Transfer 1 mL of the cell suspension into a new T25 cell culture flask containing 5 mL of warm (37 °C) growth medium. Mix gently and place the cells in the incubator.
To maintain exponential cell proliferation, aspirate the growth medium every third day and replace with 5 mL of fresh growth medium. Do not allow the cultures to reach over 80% confluence.
Preparation of ST2 feeder cell monolayers for melanoblast culture
Trypsinize ST2 cells cultured in a T25 flask, as described in steps C1–C5.
Transfer the cell suspension to a 15 mL conical tube.
Centrifuge the cells at 200× g for 5 min at 22 °C. Aspirate the supernatant and gently resuspend the cell pellet in 1 mL of warm (37 °C) RPMI growth medium.
Determine the cell density using a hemocytometer and adjust to 70,000 cells/mL by adding the appropriate volume of warm RPMI growth medium.
Add 100 μL of the cell suspension to each well of a 4-well insert placed in a 35 mm μ-dish (7,000 ST2 cells/well) and culture overnight.
Note: The use of μ-dishes with optical grade plastic allows high quality imaging and optimal cell attachment and spreading.
The day after plating, and just prior to isolation and purification of targeted melanoblasts (see below), remove ST2 growth medium by aspiration.
Add 100 μL of warm (37 °C) fresh culture medium consisting of a 1:1 mixture of melanocyte growth medium and ST2 RPMI growth medium to each well.
Place the ST2 feeder cells back in the culture incubator.
Isolation of targeted embryonic melanoblasts
Four days after single-dose IP administration of tamoxifen (step B4), euthanize time-pregnant dams at 15.5 dpc by CO2 inhalation or isoflurane overdose, following appropriate institutionally approved animal care ethics procedures.
Lay the animal in a supine position on an absorbent pad and soak the thorax and abdomen with 70% ethanol.
Using a pair of forceps, gently pinch outwards the abdominal skin below the center of the belly and, using surgical scissors, make a small incision across the midline, ensuring not to cut through the abdominal cavity.
Cut the skin along the ventral midline and gently pull the skin to each side to separate it from the underlying abdominal cavity, thus exposing the uterus (Figure 1).
Using forceps, gently remove the uterus containing the string of embryos out of the body cavity. Transfer the uterus to a Petri dish containing sterile PBS kept at room temperature (22 °C). Separate the embryos by carefully cutting between implantation sites along the uterine horn (Figure 1).
Embryo isolation is conducted under a stereomicroscope. To this end, transfer a single embryo and associated uterine tissues to another Petri dish containing PBS. Maintaining the embryo in PBS, use a pair of forceps to peel away the muscular uterine layers from the embryo, followed by removal of the decidua and the ectoplacental cone.
With a pair of forceps, gently tear the yolk sac and fully separate it from the embryo by gently tearing the umbilical cord.
Transfer embryos to a sterile tissue culture dish containing PBS and place on ice. At this stage, a small fragment of the embryo tail can be removed and processed for genomic DNA extraction and genotyping if necessary (Figure 1).
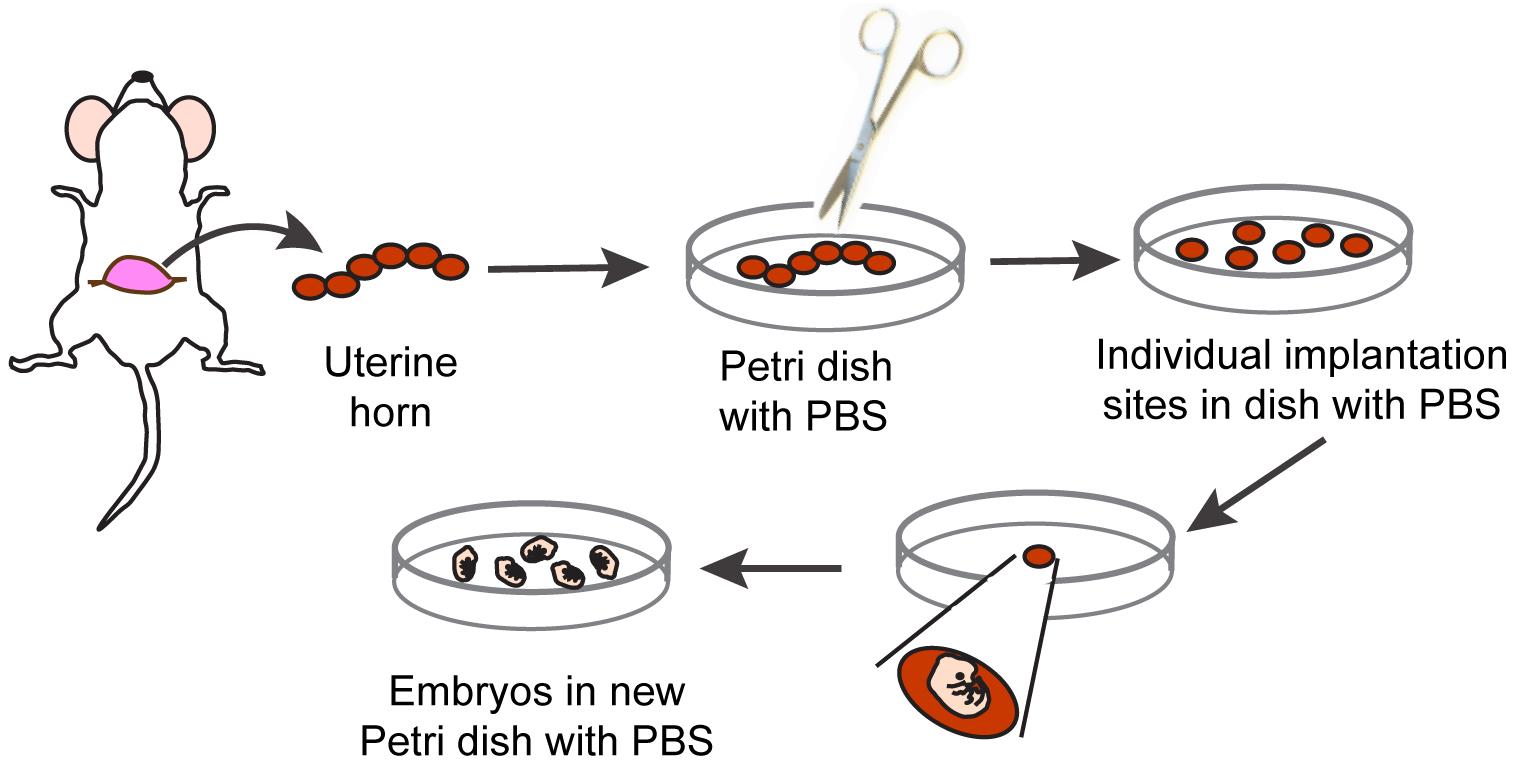
Figure 1. Schematic representation of procedure to isolate individual E15.5 mouse embryosTransfer an embryo to a Petri dish containing sterile PBS placed on the stage of the stereomicroscope.
Holding the embryo in a prone position with forceps, gently make a superficial longitudinal dorsal incision along the midline with a scalpel below the head and through the entire carcass, avoiding deep incisions that will result in exposure of the organs.
Using a pair of forceps, gently and gradually separate the skin from the carcass, starting at the incision point closest to the neck.
Note: Embryonic skin is very fragile. Use one set of forceps to hold the embryo through its head and the other set to gently separate the skin from the carcass.
Float each harvested skin in a sterile Petri dish containing ice-cold sterile PBS. Continue harvesting the skin of all remaining embryos.
Carefully transfer with forceps the embryonic skins into a sterile conical tube containing 500 μL of sterile 2.5% trypsin per skin.
Note: If analysis of individual embryos is necessary, each skin can be digested in individual 2 mL microfuge tubes containing 500 μL of 2.5% trypsin and then minced.
Mince skin using small scissors into fragments of approximately 2 mm × 2 mm and incubate at 37 °C for 10 min with gentle rocking.
Add 1 mL of trypsin neutralizing solution for every skin digested and mix gently.
Filter the cell suspension through a sterile 40 μm pore size cell strainer into a 15 mL sterile conical tube to remove tissue debris.
To obtain a single cell suspension, gently pass the filtered cell mixture 10 times through a 5 or 10 mL syringe fitted with a 21 G needle.
Centrifuge at 140× g for 5 min at 22 °C.
Carefully remove the supernatant by aspiration, avoiding disturbing the cell pellet.
Resuspend the embryonic cells in 300–500 μL of ice-cold FACS sorting buffer.
Note: Resuspending the cell suspension in larger volumes of FACS sorting buffer will result in decreased flow rates and increased sorting times, leading to decreased cell viability.
Transfer the cell suspension into 5 mL BD Falcon glass tubes and place on ice.
Immediately proceed to cell staining and FACS purification of melanoblasts.
FACS purification and culture of targeted, GFP-positive melanoblasts
Approximately 15 min prior to fluorescence-activated sorting of melanoblasts, add 7-AAD stock solution to the melanoblast suspension (5 μL of 7-AAD stock solution for every 450 μL of cell suspension), to obtain a final concentration of 10 μg/mL, and mix gently.
Note: 7-AAD will stain apoptotic and necrotic cells, allowing their separation from the viable melanoblasts to be cultured.
For FACS purification of melanoblasts, set up sorting at 4 °C, using a 100 μm nozzle at low pressure (20 psi), and at a maximum event rate of 2,000 events/s.
Note: Increasing the event rate will decrease the recovery yield of GFP-positive cells.
Configure the blue laser trigon to detect GFP from detector B (530/30 bandpass and 502 long pass filter). The yellow-green laser octagon is configured to detect mTomato from detector C (610/20 band pass and 600 long pass filter). 7-AAD is detected from detector B (670/14 bandpass and 630 long pass filter).
Exclude cell doublets using consecutive gates on side scatter (SSC)-height vs. SSC-width plots and forward scatter (FSC)-height vs. FSC-width (Figure 2A and 2B).
The FSC and SSC detectors are set in a linear scale, whereas the fluorescence channels are set to logarithmic scale. Use the appropriate voltage on each detector to generate a cell population with a wide distribution but ensure that all events remain visible in the FSC vs. SSC plots for the GFP fluorescence channel, as exemplified in Figure 2.
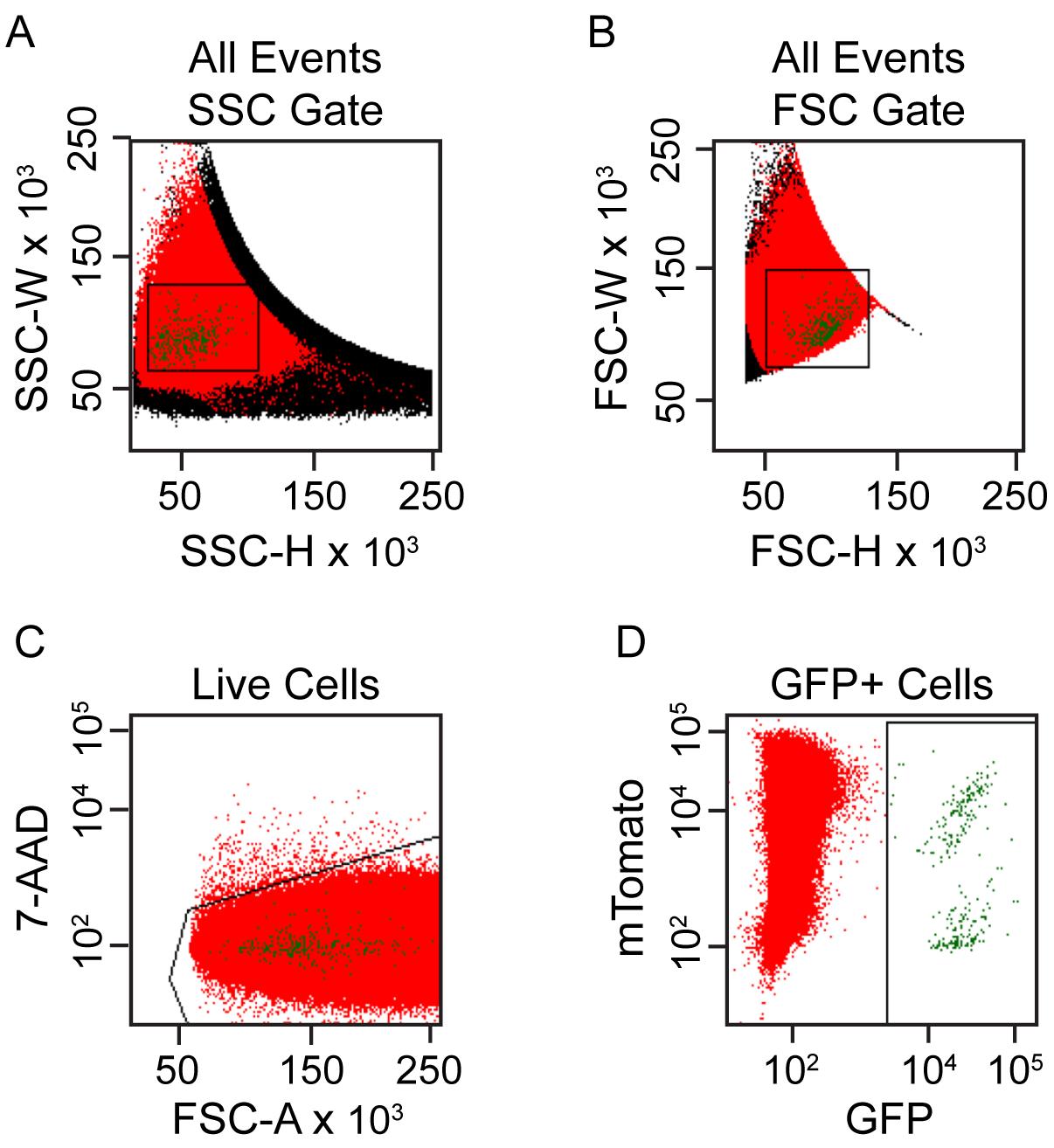
Figure 2. FACS gating strategy to purify targeted melanoblasts from single cell suspensions of embryonic skin cells. (A, B) Cell doublets are excluded using consecutive gates on side scatter (SSC)-height vs. SSC-width plots and forward scatter (FSC)-height vs. FSC-width. (C) Cells with low 7-AAD staining are selected to exclude non-viable cells. (D) Cells gated for GFP expression are selected as targeted melanoblasts for subsequent culture.Select viable cells based on the exclusion of 7-AAD (Figure 2C).
Run the GFP-negative control samples and set gates for GFP-positive and negative regions.
Create a region of GFP-positive, 7-AAD-negative cells and display on a plot of mTomato vs. GFP as shown in Figure 2D. These are the viable GFP-positive melanoblasts that have been targeted.
Sort cells into sterile tubes containing ice-cold melanoblast culture medium.
Run a small aliquot of the sorted cell suspension in the sorter to verify that > 95% of purified cells are GFP-positive.
Seal the tubes containing the remainder of the sorted melanoblasts using their caps and transport into the laminar flow hood for subsequent seeding.
Seed the sorted GFP-positive melanoblasts into the insert wells of the 35 mm μ-dishes containing the previously prepared confluent monolayers of ST2 feeder cells (section D) and culture at 37 °C.
Note: The average number of GFP-positive melanoblasts obtained per embryo is approximately 500 (ranging from 50 to 1,400). Melanoblasts obtained from 4–8 embryos can be pooled into one well.
Sixteen hours after sorting, gently remove the growth medium aspirating with a pipette to avoid disturbing the cell monolayer and replace with fresh growth medium.
Note: Sixteen hours after seeding, approximately 70% of melanoblasts will be fully spread and begin to exhibit dendricity (Figure 3).
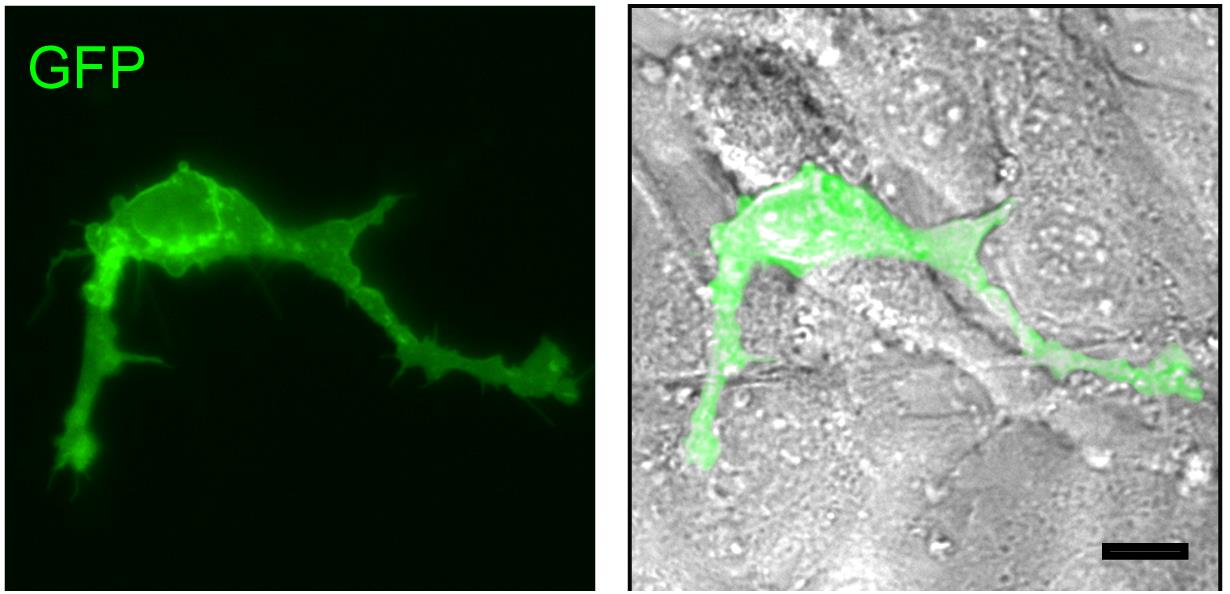
Figure 3. GFP-expressing melanoblasts. Targeted, GFP-expressing melanoblasts isolated from tamoxifen-treated reporter mice (15.5 dpc) were isolated, and FACS-sorted cells were seeded on ST2 cell feeder layers and cultured for 16 h. GFP fluorescence and GFP/phase contrast overlay micrographs show the GFP-positive melanoblasts. Note confluent monolayer of ST2 cells around and underneath the GFP-expressing melanocyte, visible in the phase contrast micrograph. Scale bar, 25 μm.Replace the growth medium every 72 h.
Note: Remove growth medium gently using a pipette to avoid disturbing the cell monolayer. Melanoblasts will mature into melanin-producing cells and become highly dendritic approximately within one week after isolation.
Basic characterization of melanocytic lineage identity
The melanocytic lineage cell-restricted expression of Cre-ERT2 recombinase under the regulation of the Tyr promoter and its tamoxifen-dependent activation to induce melanocytic cell-specific expression of fluorescent reporter proteins (e.g., GFP, tdTomato) has been documented in several independent reports (Bosenberg et al., 2006; Crawford et al., 2017 and 2020; Sun et al., 2023). In addition, cultured melanoblasts can be morphologically identified by their characteristic ability to develop highly dynamic dendritic extensions (Figure 3). Although E15.5 melanoblasts are amelanotic, they express the specific transcription factor MITF-M (Thomas and Erickson, 2008), which can be detected by immunofluorescence microscopy. Further, 4–5 days after isolation, cultured melanoblasts will mature into melanocytic cells with dendritic projections that contain melanin granules distributed throughout the cell body (Figure 4). At this stage, the cells also express enzymes involved in melanin biosynthesis, such as tyrosinase and tyrosinase-related protein 1 (TRP1; Figure 4). The protocol to process these cultures for TRP1 detection by indirect immunofluorescence microscopy is described below.
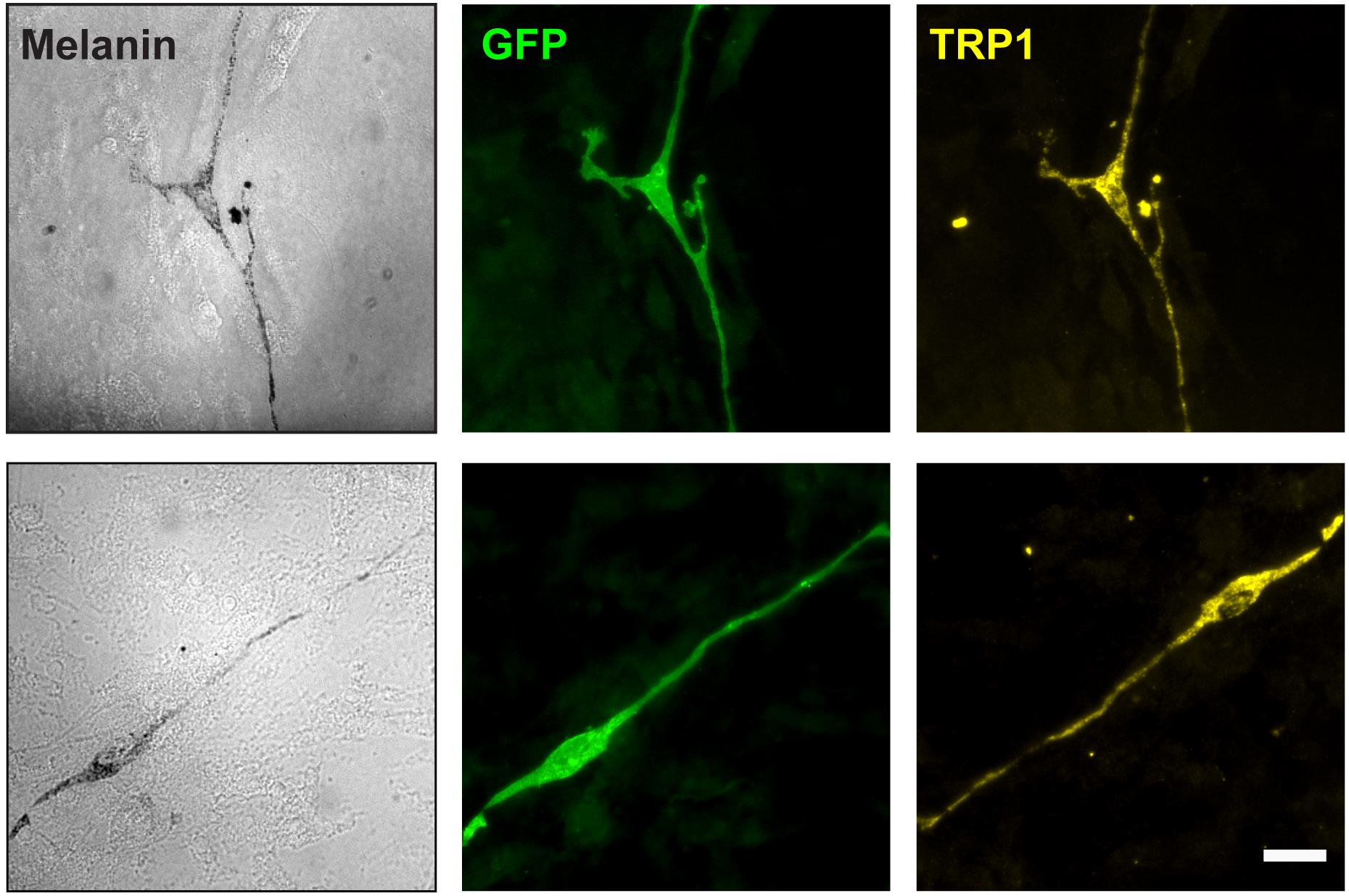
Figure 4. Expression of GFP and tyrosinase-related protein 1 (TRP1) in purified melanoblasts. Targeted, GFP-expressing melanoblasts isolated from tamoxifen-treated reporter mice (15.5 dpc) were isolated, FACS-sorted, and seeded on ST2 cell feeder layers. The cells were cultured for seven days and processed for immunofluorescence microscopy. Two different melanoblasts are shown. Brightfield micrograph panels (labeled “Melanin”) illustrate dendritic cell morphology and presence of dark melanin granules distributed throughout the cell. Scale bar, 25 μm.Place the culture dish with melanoblasts cultured for 3–7 days on ST2 feeder cells on ice and remove the insert with forceps, taking care to avoid disturbing the cell monolayer.
Remove the culture medium by aspiration.
If used, remove the insert from the culture dish.
Gently add 1 mL of ice-cold PBS to rinse the cells and remove by aspiration.
Fix the cells by adding 1 mL of freshly prepared 4% PFA in PBS and incubate with gentle rocking for 20 min at room temperature (22 °C).
Remove the PFA solution by aspiration.
Rinse once by adding 1 mL of PBS, as in step G4.
Note: Fixed cells can be processed immediately or stored in PBS at 4 °C for up to a week.
Permeabilize the cells by adding 1 mL of 0.1% Triton X-100 in PBS and incubate for 10 min at room temperature with gentle rocking.
Rinse thrice with PBS, as in step G4.
Mix 40 μL of goat serum with 756 μL of PBS and add 4 μL of the primary anti-TRP1 antibody. Mix well.
Note: The primary antibody can also be diluted in PBS containing 3% bovine serum albumin in place of goat serum.
Add the anti-TRP1 antibody solution from step G10 to the cells and incubate at 4 °C overnight.
Remove the anti-TRP1 antibody solution by aspiration.
Wash the cells by incubating with 2 mL of PBS for 10 min at room temperature (22 °C), with gentle rocking. Remove the PBS by aspiration.
Repeat step G13 two additional times.
Mix 40 μL of goat serum with 756 μL of PBS and add 1–2 μL of the secondary Anti-mouse IgG Alexa Fluor® 594 Conjugate. Protect sample from light from this step onwards.
Add the secondary antibody solution to the cells and incubate for 1 h at room temperature (22 °C) with gentle rocking and protected from the light.
Note: The secondary antibody can also be diluted in PBS containing 3% bovine serum albumin in place of goat serum.
Wash the cells by incubating with 2 mL of PBS for 10 min at room temperature, with gentle rocking. Remove the PBS by aspiration.
Repeat step G17 two additional times.
Immediately after aspirating the PBS from the last wash, gently add 50 μL of mounting medium to the center of the culture dish.
Gently place a 12 mm glass coverslip on the mounting medium, ensuring there are no bubbles.
Place samples flat and store at room temperature (22 °C) overnight, protected from light.
Samples are ready for fluorescence imaging.
Processed samples should be subsequently stored at 4 °C, protected from light.
Validation of protocol
This protocol was validated in: J. Invest. Dermatol. (2020), DOI: 10.1016/j.jid.2019.07.681
Acknowledgments
We thank K. Chadwick for expert help with FACS experiments. The authors acknowledge financial support for this work to LD from the Natural Sciences and Engineering Research Council and the Canadian Institutes of Health Research. Studies on melanoblasts isolated and cultured following this protocol have been published in Crawford et al. (2020).
Competing interests
The authors declare no competing interests or conflicts of interest.
Ethical considerations
All animal experiments were approved by the University of Western Ontario Animal Care Committee (Protocol No. 2018-169), according to regulations and guidelines of the Canadian Council on Animal Care.
References
- Abdallah, B. M., Alzahrani, A. M., Abdel-Moneim, A. M., Ditzel, N. and Kassem, M. (2019). A simple and reliable protocol for long-term culture of murine bone marrow stromal (mesenchymal) stem cells that retained their in vitro and in vivo stemness in long-term culture. Biol. Proced. Online 21(1): e1186/s12575-019-0091-3.
- Adameyko, I., Lallemend, F., Aquino, J. B., Pereira, J. A., Topilko, P., Müller, T., Fritz, N., Beljajeva, A., Mochii, M., Liste, I., et al. (2009). Schwann Cell Precursors from Nerve Innervation Are a Cellular Origin of Melanocytes in Skin. Cell 139(2): 366–379.
- Bosenberg, M., Muthusamy, V., Curley, D. P., Wang, Z., Hobbs, C., Nelson, B., Nogueira, C., Horner, J. W., DePinho, R., Chin, L., et al. (2006). Characterization of melanocyte-specific inducible Cre recombinase transgenic mice. genesis 44(5): 262–267.
- Chevalier, C., Nicolas, J. F. and Petit, A. C. (2013). Preparation and Delivery of 4-Hydroxy-Tamoxifen for Clonal and Polyclonal Labeling of Cells of the Surface Ectoderm, Skin, and Hair Follicle. In: Turksen, K. (Ed.). Epidermal Cells (pp. 239–245). Methods in Molecular Biology. Springer, New York.
- Crawford, M., Leclerc, V., Barr, K. and Dagnino, L. (2020). Essential Role for Integrin-Linked Kinase in Melanoblast Colonization of the Skin. J. Invest. Dermatol. 140(2): 425–434.e10.
- Crawford, M., Leclerc, V. and Dagnino, L. (2017). A reporter mouse model forin vivotracing andin vitromolecular studies of melanocytic lineage cells and their diseases. Biol. Open 6(8): 1219–1228.
- Delmas, V., Beermann, F., Martinozzi, S., Carreira, S., Ackermann, J., Kumasaka, M., Denat, L., Goodall, J., Luciani, F., Viros, A., et al. (2007). β-Catenin induces immortalization of melanocytes by suppressing p16INK4a expression and cooperates with N-Ras in melanoma development. Genes Dev. 21(22): 2923–2935.
- Li, A. (2014). The biology of melanocyte and melanocyte stem cell. Acta Biochim. Biophys. Sin. 46(4): 255–260.
- Machholz, E., Mulder, G., Ruiz, C., Corning, B. F. and Pritchett-Corning, K. R. (2012). Manual Restraint and Common Compound Administration Routes in Mice and Rats. J. Vis. Exp. (67): 2771.
- Mort, R. L., Jackson, I. J. and Patton, E. E. (2015). The melanocyte lineage in development and disease. Development 142(4): 620-632.
- Muzumdar, M. D., Tasic, B., Miyamichi, K., Li, L. and Luo, L. (2007). A global double-fluorescent Cre reporter mouse. genesis 45(9): 593–605.
- Sun, Q., Lee, W., Hu, H., Ogawa, T., De Leon, S., Katehis, I., Lim, C. H., Takeo, M., Cammer, M., Taketo, M. M., et al. (2023). Dedifferentiation maintains melanocyte stem cells in a dynamic niche. Nature 616(7958): 774–782.
- Thomas, A. J. and Erickson, C. A. (2008). The making of a melanocyte: the specification of melanoblasts from the neural crest. Pigment Cell & Melanoma Res. 21(6): 598–610.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Crawford, M., Barr, K. and Dagnino, L. (2023). Isolation, Purification, and Culture of Embryonic Melanoblasts from Green Fluorescent Protein–expressing Reporter Mice. Bio-protocol 13(17): e4805. DOI: 10.21769/BioProtoc.4805.
Category
Cell Biology > Cell isolation and culture > Cell isolation > Flow cytometry
Cell Biology > Cell imaging > Fluorescence
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link