- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Determination of Antibody Activity by Platelet Aggregation
Published: Vol 13, Iss 17, Sep 5, 2023 DOI: 10.21769/BioProtoc.4804 Views: 2246
Reviewed by: Meenal SinhaLuis Alberto Sánchez Vargas

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Human Auto-IgG Purification from High Volume Serum Sample by Protein G Affinity Purification
Serena Sensi and Andreas Goebel
Dec 5, 2022 2772 Views

Mesenchymal Stromal Cell (MSC) Functional Analysis—Macrophage Activation and Polarization Assays
Hazel Y. Stevens [...] Annie C. Bowles-Welch
Mar 20, 2024 4289 Views
Assessing Human Treg Suppression at Single-Cell Resolution Using Mass Cytometry
Jonas Nørskov Søndergaard [...] James B. Wing
Aug 20, 2025 2868 Views
Abstract
Platelets play an important role in hemostasis by forming clots and stopping bleeding. In immune thrombotic conditions, platelets and leukocytes are aberrantly activated by pathogenic antibodies resulting in platelet aggregates and NETosis, leading to thrombosis and thrombocytopenia. A simple assay that assesses platelet function and antibody activity is light transmission aggregometry. This assay can be used to determine antibody activity in patients with disorders such as heparin-induced thrombocytopenia (HIT) and vaccine-induced thrombotic thrombocytopenia (VITT). Briefly, for detection of pathogenic antibody, platelet-rich plasma (PRP) is treated with a specific agent (e.g., patient sera or purified patient antibodies) with constant stirring. Upon activation, platelets undergo a shape change and adhere to each other forming aggregates. This causes a reduction in opacity allowing more light to pass through PRP. Light transmission through the cuvette is proportional to the degree of platelet aggregation and is measured by the photocell over time. The advantage of this protocol is that it is a simple, reliable assay that can be applied to assess antibody activity in thrombotic conditions. Light transmission aggregometry does not require the use of radioactive reagents and is technically less demanding compared with 14C-serotonin release assay, another common assay for detecting antibody activity.
Key features
• This protocol can be used to assess platelet function and to detect platelet activating antibodies in diseases such as HIT and VITT.
• Does not require radioactive reagents, requires an aggregometer; based on the light transmission aggregometry protocol, adapted for detection of VITT and other platelet-activating antibodies.
• Two positive controls are required for reliable detection of antibodies in diseases such as HIT/VITT, namely a weak HIT/VITT antibody and a physiological agonist.
• For detection of HIT/VITT antibodies, it is essential to use donors known to have platelets reactive to these antibodies to avoid false negative results.
Keywords: Platelet activationBackground
Platelets play an essential role in hemostasis, bleeding, and thrombosis. Upon activation, platelets undergo shape changes and aggregate, leading to platelet clumping. The rate and extent of aggregation can be measured by light transmission via a platelet aggregometer. Platelet-rich plasma (PRP), which is turbid, is stirred in a test cuvette. When the agonist is added, the platelets will form increasingly larger aggregates and the PRP will begin to clear, allowing more light to pass through. This increase in light transmittance is directly proportional to the amount of aggregation.
First described by Born in the 1960s (Born, 1962), light transmission aggregometry is considered a gold standard assay for platelet function, often assigned as the first step for analyzing platelet dysfunction in hemorrhagic patients (Kottke-Marchant and Corcoran, 2002; Hayward, 2008; Gadisseur et al., 2009; Hayward et al., 2009; Podda et al., 2012). To detect the presence and activity of pathogenic antibodies, patient sera or purified patient IgG can be used. Purified IgG confirms that the IgG is the component that induces platelet activation, thus excluding other potential platelet activating agents in patient sera. Standardization of the use of light transmission aggregometry to assess platelet function has been published by the International Society of Thrombosis and Haemostasis (Cattaneo et al., 2013).
Another method of analyzing platelet activation is whole-blood aggregometry (Park et al., 2012; Mencarini et al., 2021). This method measures the change in electrical impedance between two electrodes to indicate platelet aggregation. Although this process accounts for the effect of all blood cells on platelet function, it cannot determine with accuracy the direct or indirect contributions of other blood cells to the platelet activation observed. To avoid confounding factors in whole blood, light transmission aggregometry using PRP is an ideal, relatively simple, and reliable method to specifically determine platelet activity.
The 14C-serotonin release assay (SRA) and the heparin-induced platelet activation (HIPA) test are also common assays used to test antibody activity (Arepally et al., 1995; Greinacher et al., 2012 and 2022). They are based on the principle that incubation of patient serum (containing the pathogenic antibody) will activate donor platelets resulting in release of intraplatelet serotonin (SRA) and platelet aggregation (HIPA). SRA is a sensitive method used to detect pathogenic antibody in heparin-induced thrombocytopenia (HIT) (Warkentin et al., 2015) and more recently, vaccine-induced thrombotic thrombocytopenia (VITT) (Leung et al., 2022). For SRA, washed platelets are incubated with radiolabeled serotonin. Upon activation, platelets release radiolabeled serotonin, and this is measured by a beta counter. Despite the high sensitivity of SRA, this method is labor intensive and technically demanding, requiring the user to have radiation training and certification, access to radiation laboratory, and waste disposal streams for the radioactive waste. Due to this, platelet aggregometry is a simpler, more convenient method of assessing platelet activation.
The HIPA test, developed in 1991, is based on the visual assessment of platelet aggregates (Greinacher et al., 1991). It is performed in microtiter plates, allowing multiple samples and platelet donors to be tested simultaneously, uses washed platelets, and is generally considered more sensitive than platelet aggregation tests (Greinacher et al., 1994; Tardy et al., 2020). Experienced personnel are required to conduct this assay, as platelet washing can over-activate platelets, giving false positive results, and estimation of platelet aggregation by eye can be imprecise. In comparison, platelet aggregometry is technically less demanding, does not rely on personnel to be experienced in identifying visual changes in platelet morphology, and the real-time trace clearly shows when platelet activation has taken place. By using platelets from donors pre-determined to be reactive to HIT antibodies, the sensitivity of the assay should approach that of HIPA (Chong et al., 1993). Platelet aggregometry has certain limitations as it cannot be used if donors have low platelet counts and, unlike whole-blood aggregometry, some sample preparation is required.
Here, we provide details on an updated version of Born’s method for determining antibody activity by platelet aggregation (Figure 1). This protocol is improved by standardization, with added appropriate controls and use of reactive donor platelets to increase accuracy, and adapted to test IgG, along with serum and plasma. This protocol can be used for research purposes, as well as in clinical laboratories for testing of HIT/VITT antibodies.
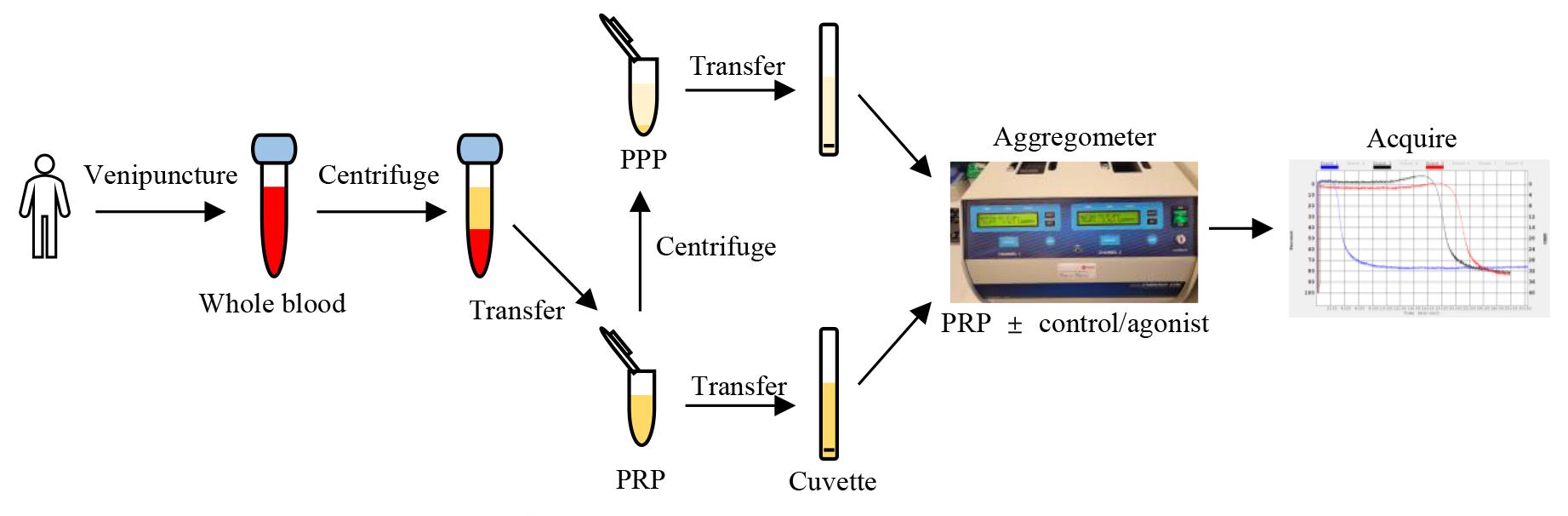
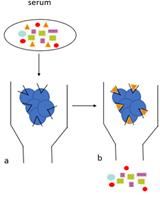
Figure 1. Schematic diagram of the platelet aggregometry assay. Centrifuged tubes show the different layers of blood cells and plasma. PPP: platelet-poor plasma; PRP: platelet-rich plasma.
Materials and reagents
15 mL centrifuge tubes (Greiner, catalog number: 188271)
1.5 mL microcentrifuge tube (Greiner Bio-One, catalog number: 616201)
Pasteur pipette (Transfer) (Labtek, catalog number: 650.050.523)
Direct detect assay-free cards (Merck, catalog number: DDAC00010-GR)
Stir bar, disposable siliconized for P/N 312 cuvettes (DKSH, Part No 311)
Cuvettes, 450 μL (DKSH, Part No 312)
100 kDa Amicon Ultra-15 centrifugal filter unit (Merck, catalog number: UFC910024)
Econo-Column® chromatography column, 1.5 cm × 10 cm (Bio-Rad, catalog number: 7374151)
Fresh or frozen plasma (stored at -80 °C) collected in ACD (BD, catalog number: 366645), trisodium citrate (BD, catalog number: 363095), or EDTA (BD, catalog number: 367839), or sera collected in clot activator/gel vacutainer (BD, catalog number: 367954) from patient for IgG purification
Fresh or frozen plasma (stored at -80 °C) collected in trisodium citrate or sera collected in clot activator/gel vacutainer from patient for direct testing in platelet aggregation
Whole blood from healthy donor, collected in trisodium citrate vacutainer (BD, catalog number: 363095)
Protein G agarose beads (SeraCare, catalog number: 5720-0002)
Dulbecco’s phosphate buffered saline (Sigma-Aldrich, catalog number: D8537)
Control agonists such as collagen (1 μg/mL), thrombin (1 U/mL), plasma sample from positive HIT patients
Sodium phosphate (Merck, catalog number: S8282)
Glycine (Astral Scientific, catalog number: BIOGB0235)
Tris (Amresco, catalog number: 0497)
HCl (Merck, catalog number: 320331)
NaOH (Sigma, catalog number: S-0899)
NaCl (Merck, catalog number: S9625)
EDTA (Invitrogen, catalog number: 15576028)
Starting buffer, pH 7.0 (see Recipes)
Elution buffer, pH 2.7 (see Recipes)
Neutralization buffer, pH 9.0 (see Recipes)
Washing buffer, pH 7.0 (see Recipes)
Equipment
Benchtop centrifuge (Spintron, model: GT-10 SV3)
Tabletop microfuge (Beckman Coulter, Microfuge 20 Centrifuge, model: B31603)
Centrifuge (Eppendorf, model: 5810 R)
Aggregometer (Chrono-Log, model: 700)
Chromatography system (GE Healthcare, model: AKTA Pure. catalog number: 29-0182-24)
Direct detect infrared spectrometer (Millipore, model: DDHW00010)
Rotatory tube mixer (Ratek, model: RSM7DC)
Magnetic stirrer (Bacto Laboratories, model: MS7-H550-Pro)
Calibrated pipettes
-80 °C freezer
4 °C fridge
Software
Aggro/Link8 software (Chronolog, USA)
GraphPad Prism 9 (GraphPad Software, USA)
Microsoft Excel (Microsoft, USA)
Procedure
Preparation of platelet-rich plasma (PRP)
Draw human whole blood into a vacuum tube with trisodium citrate.
Note: PRP is approximately 30%–40% of whole blood volume. Minimum volume of whole blood needed depends on number of assays to be performed [300 μL of PRP per assay is required, plus 500 μL to prepare platelet poor plasma (PPP) for background].
Centrifuge tube at 200× g with no brake for 10 min at room temperature.
Transfer PRP layer using a 3 mL Pasteur pipette to a 15 mL centrifuge tube.
Store the capped PRP sample at room temperature until ready for use, for no longer than 3 h.
Note: Platelets can be activated if stored at 4 °C, affecting subsequent platelet function assays.
Preparation of platelet-poor plasma
Transfer 0.5 mL of PRP to a 1.5 mL microcentrifuge tube.
Centrifuge tube at 1,000× g for 10 min at room temperature.
Transfer the supernatant to another 1.5 mL microcentrifuge tube and label the tube PPP.
Preparation of patient antibody from serum or plasma
Centrifuge patient serum or plasma at 10,000× g for 10 min at 4 °C to remove any cells or debris. Fresh or frozen patient serum or plasma collected in ACD, EDTA, or sodium citrate can be used.
Transfer a minimum of 0.5 mL of supernatant to a clean microcentrifuge tube.
Add Protein G agarose beads to patient serum or plasma at a 1:1 ratio; then, transfer the mixture to a clean 1.5 cm × 10 cm Econo-Column® chromatography column.
Incubate the column on a rotatory tube mixer at 4 °C for 2 h.
Place the column into the AKTA purifier chromatography system.
Wash the column with at least 10× the sample volume of washing buffer. Check the chromatography trace remains at baseline to confirm that the column has been washed sufficiently.
Add 100 μL of neutralization buffer to 1.5 mL microcentrifuge collection tubes and place them on ice.
Add 1× the sample volume of elution buffer to the column to elute bound immunoglobulins. Collect the fraction containing the protein peak in aliquots of 900 μL into the 1.5 mL microcentrifuge tubes containing 100 μL of neutralization buffer. Invert the tubes to mix.
Pool eluted antibody and transfer to a 100 kDa Amicon Ultra-15 centrifugal filter unit.
Centrifuge the Amicon Ultra-15 unit at 4,000× g for 15–20 min. Add 14 mL of PBS and repeat the centrifugation step twice to significantly reduce the concentration of the original buffer. Once buffer exchange to PBS is completed, transfer mixture and aliquot into 2 mL Eppendorf tubes.
Pipette 2 μL of sample into a sample membrane and 2 μL of buffer onto another membrane on a direct detect assay-free card.
Place card into the direct detect infrared spectrometer to dry and measure the sample concentration, setting the buffer as the blank. Expected sample concentration is approximately 7–10 mg/mL.
Note: Other methods such as Bradford assay can be used to determine antibody concentration. The quality of the purified IgG can be tested using assays such as enzyme-linked immunosorbent assay (ELISA) to confirm binding of purified IgG to antigen. Purity can be confirmed by SDS PAGE gel. The presence of multimeric IgG can be analyzed using size exclusion chromatography.
Keep antibody in -80 °C freezer for long-term storage until required or at 4 °C for use the following day.
Measuring antibody activity by platelet aggregation
Transfer 300 μL of PPP into a cuvette. Place cuvette into the reference slot of the aggregometer (100% light transmission, used as a blank) (Figure 2).

Figure 2. Aggregometer setup. A. Image of cuvette with stir bar. B. Location of reference slot for platelet-poor plasma (PPP), heating slots, and measuring slot for platelet-rich plasma (PRP). C. Aggregometer setup for running four samples concurrently.Set temperature to 37 °C and stirring speed to 1,200 revolutions per minute (rpm) and select optical mode on the aggregometer.
For each agonist to be tested, transfer 300 μL of PRP into each cuvette containing a stir bar.
Place PRP cuvettes into heating slots for 2 min to warm up to 37 °C; then, place cuvettes into the measuring slot and ensure stir bars are stirring evenly.
Set baseline on the aggregometer.
Pipette reagent to be assessed into a cuvette containing 300 μL of PRP. Reagents include patient sera (5–50 μL), purified patient IgG (0.1–2 mg/mL), negative controls (equivalent amounts of sera or purified IgG from a healthy donor or buffer), or positive controls (0.5–1 U/mL thrombin, 1 μg/mL collagen, or 50 μL of weak positive HIT patient serum with 0.1 U/mL heparin to allow for detection of weak pathogenic antibodies).
Note: Agonist or control volumes should not exceed 150 μL.
Select Aggregometer, Run a new patient, or click Run new icon on the Aggro/Link software.
Acquire data for 15–30 min (or until platelet aggregation has completed).
Exporting and analyzing data
Export image and raw data in del file format from the Aggro/Link software.
Open raw data file in Excel.
Replot datapoints into GraphPad Prism.
Obtain AUC, maximum aggregation %, velocity, and latency time from Aggro/Link software.
Data analysis
Various parameters can be extrapolated from the platelet aggregometry data. The most essential parameters are amplitude of the platelet aggregation curve, indicating percentage of maximum aggregation, and latency time, indicating time of aggregation onset. Velocity of aggregation can also be analyzed by calculating percentage aggregation per unit of time (Figure 3). Area under the curve (AUC) and maximum aggregation (%) are calculated directly by the Aggro-Link software. These can also be recalculated following export of raw data and replotting using other programs such as Excel or Prism. Velocity and latency time can be extrapolated by selecting a particular aggregation % to compare between samples. It is not recommended to compare data from different days and donors. This assay is semi-quantitative.
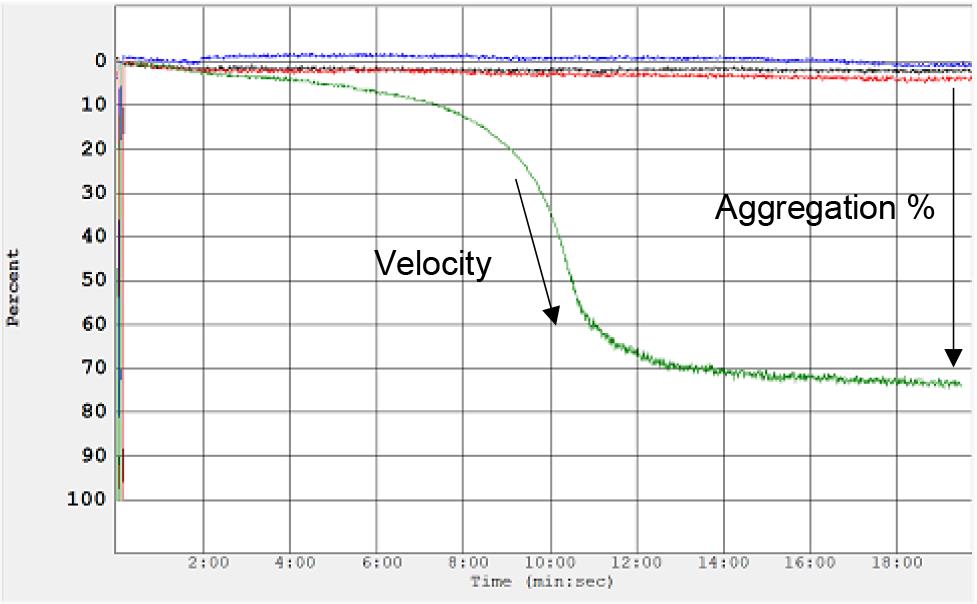
Figure 3. Analysis of platelet aggregation data. Aggregometer traces indicating velocity of aggregation and maximum aggregation percentage following treatment with purified heparin-induced thrombocytopenia (HIT) patient antibody (shown in green). No aggregation is observed following treatment with negative controls (buffer shown in blue; normal IgGs shown in black and red).
To ensure the healthy donor’s platelets are functioning normally, at least one positive control is required. For the detection of platelet activating antibodies in diseases such as HIT and VITT, a weak positive patient serum or IgG is needed as the positive control. A positive result should be attained when using a weak control HIT/VITT antibody to ensure that the donor platelets are reactive enough to detect weak antibodies. If the donor platelets show a negative result for a known weak control HIT/VITT antibody, the donor platelets used are unlikely to detect a weak antibody, leading to false negative results. On the other hand, if the weak positive control gives a positive result, the donor platelets are suitable to detect even weak pathogenic antibodies.
Since only a proportion of healthy individuals are responsive to HIT antibodies, laboratories that regularly conduct HIT antibody testing should have several donors with reactive platelets to HIT antibodies to call upon to donate. These reactive donors are selected by prior testing, identified from a larger group of donors. Alternatively, two to four random donors of unknown platelet reactivity can be used together in the assay as at least one of the donors will have reactive platelets.
As with other platelet function assays, the platelet donor must not have taken drugs (e.g., aspirin) or foods (e.g., garlic), as these drugs/foods could suppress platelet reactivity. To detect this, a physiological agonist, often collagen 1 μg/mL, is used as an additional positive control (see Notes below for more detail). Abnormally shaped curves should be interpreted with caution and assay repeated to ensure samples (suitable donor, assay conducted within 3 h of blood collection) and/or reagents (correct pH and concentration) were prepared and functioning correctly.
Notes
Healthy platelet donors should not be taking any medications known to affect platelet function during the 10 days prior to the experiment. These may include cyclooxygenase (COX)-1 and COX-2 inhibitors, non-steroidal anti-inflammatory drugs (NSAID), ibuprofen, platelet receptor inhibitors [Abciximab (αIIbβ3), Clopidogrel (P2Y12), Prasugrel (P2Y12)], and anticoagulants. Aggregation times and extent of aggregation may vary depending on donor blood platelet concentration; therefore, positive controls are essential. Platelet aggregation assay should be conducted within 3 h of blood collection. The amount of patient sera or IgG needed varies from patient to patient depending on antibody titer, avidity, time of collection, and so on. To study the contribution of various platelet membrane receptors and/or signaling pathways to platelet aggregation, a range of physiological agonists such as arachidonic acid (thromboxane A2), adenosine diphosphate (ADP) (P2Y1 and P2Y12), collagen (GPVI and α2β1), epinephrine (α2A), or thrombin receptor activator peptide (TRAP)-6 [protease-activated receptor (PAR)-1] can be used.
The platelet aggregation assay described in this protocol is used for the detection of HIT and VITT antibodies and for assessment of platelet function in general, e.g., patients suspected of having a platelet function disorder. When this assay is used for the former purpose, specific controls and platelet donor requirements are needed, as described in the protocol. Platelet count of 250,000/μL is recommended. Platelet counts lower than 50,000/μL may result in inconsistent results and cause problems with setting the baseline.
The factors that make certain donor platelets more reactive to HIT/VITT antibodies are unknown. There have been conflicting data on whether reactivity is related to the FcγIIa R131 isoform compared to those with the H131 polymorphism. No significant correlation has been found between Fc receptor number and platelet response. Platelet aggregation induced by other agonists (ADP, collagen) is affected by factors such as age, body mass index, triglyceride levels, and certain foods and drugs. These, together with unknown factors, are also likely to influence reactivity to HIT/VITT antibodies.
Recipes
Starting buffer, pH 7.0
20 mM sodium phosphate
Prepare 800 mL of distilled water in a suitable container.
Add 3.28 g of sodium phosphate and stir.
Adjust solution to final desired pH using HCl or NaOH.
Add distilled water until the volume is 1 L.
Store at room temperature. Stable for more than one year.
Elution buffer, pH 2.7
100 mM glycine
Prepare 800 mL of distilled water in a suitable container.
Add 7.5 g of glycine to the solution and stir.
Adjust the pH to 2.7 with HCl.
Add distilled water until the volume is 1 L.
Store at 4 °C. Stable for one year.
Neutralization buffer, pH 9.0
1 M Tris·HCl
Prepare 800 mL of distilled water in a suitable container.
Add 121.14 g of Tris base to the solution.
Adjust solution to desired pH using HCl.
Add distilled water until the volume is 1 L.
Store at room temperature or at 4 °C. Stable for six months.
Washing buffer, pH 7.0
20 mM sodium phosphate
150 mM NaCl
2 mM EDTA
Prepare 800 mL of distilled water in a suitable container.
Add 3.28 g of sodium phosphate and 8.76 g of NaCl and stir.
Add 4 μL of 0.5M EDTA.
Adjust solution to final desired pH using HCl or NaOH.
Add distilled water until the volume is 1 L.
Store at room temperature. Stable for more than one year.
Dissolve salts in ddH2O and stir with magnetic stirrer. Adjust solution to desired pH by adding HCl or NaOH. All recipes given are for 1 L of solution. Different volumes may be prepared as required.
Acknowledgments
This work was supported by grants from National Health and Medical Research Council, Australia, Program Grant APP1052616 and New South Wales Capacity Program Senior Researcher Grant RG201677 to B.H.C. This protocol was adapted from a previous publication from our group (Leung et al., 2022).
Competing interests
The authors of this manuscript declare that there are no conflicts of interest or competing interests.
Ethics
This protocol was approved by the South Eastern Sydney Human Research Ethics Committee (17/211 LNR/17/POWH/501). Informed consent was obtained from all study participants.
References
- Arepally, G., Reynolds, C., Tomaski, A., Amiral, J., Jawad, A., Poncz, M. and Cines, D. B. (1995). Comparison of PF4/Heparin ELISA Assay With the 14C-Serotonin Release Assay in the Diagnosis of Heparin-induced Thrombocytopenia. Am. J. Clin. Pathol. 104(6): 648–654.
- Born, G. V. (1962). Aggregation of Blood Platelets by Adenosine Diphosphate and its Reversal. Nature 194: 927–929.
- Cattaneo, M., Cerletti, C., Harrison, P., Hayward, C. P., Kenny, D., Nugent, D., Nurden, P., Rao, A. K., Schmaier, A. H., Watson, S. P., et al. (2013). Recommendations for the standardization of light transmission aggregometry: a consensus of the working party from the platelet physiology subcommittee of SSC/ISTH. J. Thromb. Haemost. 11: 1183–1189.
- Chong, B., Burgess, J. and Ismail, F. (1993). The Clinical Usefulness of the Platelet Aggregation Test for the Diagnosis of Heparin-Induced Thrombocytopenia. Thromb. Haemost. 69(4): 344–350.
- Gadisseur, A., Hermans, C., Berneman, Z., Schroyens, W., Deckmyn, H. and Michiels, J. (2009). Laboratory Diagnosis and Molecular Classification of von Willebrand Disease. Acta Haematol. 121: 71–84.
- Greinacher, A., Michels, I., Kiefel, V. and Mueller-Eckhardt, C. (1991). A Rapid and Sensitive Test for Diagnosing Heparin-Associated Thrombocytopenia. Thromb. Haemost. 66(6): 734–736.
- Greinacher, A., Amiral, J., Dummel, V., Vissac, A., Kiefel, V. and Mueller-Eckhardt, C. (1994). Laboratory diagnosis of heparin-associated thrombocytopenia and comparison of platelet aggregation test, heparin-induced platelet activation test, and platelet factor 4/heparin enzyme-linked immunosorbent assay. Transfusion 34(5): 381–385.
- Greinacher, A., Warkentin, T. E. and Chong, B. H. (2012). Heparin-induced thrombocytopenia. In: Michelson, A.D. (Ed.). Platelets (pp. 851–882). Oxford, United Kingdom: Elsevier’s Science and Technology.
- Greinacher, A., Langer, F., Makris, M., Pai, M., Pavord, S., Tran, H. and Warkentin, T. E. (2022). Vaccine‐induced immune thrombotic thrombocytopenia (VITT): Update on diagnosis and management considering different resources. J. Thromb. Haemost. 20(1): 149–156.
- Hayward, C. P. (2008). Diagnostic approach to platelet function disorders. Transfus. Apher Sci. 38(1): 65–76.
- Hayward, C. P., Pai, M., Liu, Y., Moffat, K. A., Seecharan, J., Webert, K. E., Cook, R. J. and Heddle, N. M. (2009). Diagnostic utility of light transmission platelet aggregometry: results from a prospective study of individuals referred for bleeding disorder assessments. J. Thromb. Haemost. 7(4): 676–684.
- Kottke-Marchant, K. and Corcoran, G. (2002). The Laboratory Diagnosis of Platelet Disorders. Arch. Pathol. Lab. Med. 126(2): 133–146.
- Leung, H. H. L., Perdomo, J., Ahmadi, Z., Zheng, S., Rashid, F., Enjeti, A., Ting, S. B., Chong, J. J. H. and Chong, B. H. (2022). NETosis and thrombosis in vaccine-induced immune thrombotic thrombocytopenia. Nat. Commun. 13(1): e1038/s41467-022-32946-1.
- Mencarini, T., Roka-Moiia, Y., Bozzi, S., Redaelli, A. and Slepian, M. (2021). Electrical impedance vs. light transmission aggregometry: Testing platelet reactivity to antiplatelet drugs using the MICELI POC impedance aggregometer as compared to a commercial predecessor. Thromb. Res. 204: 66–75.
- Park, Y., Jeong, Y. H., Kim, I. S., Yun, S. E., Kwon, T. J., Hwang, S. J., Kwak, C. H. and Hwang, J. Y. (2012). The concordance and correlation of measurements by multiple electrode and light transmittance aggregometries based on the pre-defined cutoffs of high and low on-treatment platelet reactivity. Platelets 23(4): 290–298.
- Podda, G., Femia, E. A., Pugliano, M. and Cattaneo, M. (2012). Congenital defects of platelet function. Platelets 23(7): 552–563.
- Tardy, B., Lecompte, T., Mullier, F., Vayne, C. and Pouplard, C. (2020). Detection of Platelet-Activating Antibodies Associated with Heparin-Induced Thrombocytopenia. J. Clin. Med. 9(4): 1226.
- Warkentin, T. E., Arnold, D. M., Nazi, I. and Kelton, J. G. (2015). The platelet serotonin-release assay. Am. J. Hematol. 90(6): 564–572.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Leung, H., Perdomo, J., Ahmadi, Z. and Chong, B. H. (2023). Determination of Antibody Activity by Platelet Aggregation. Bio-protocol 13(17): e4804. DOI: 10.21769/BioProtoc.4804.
Category
Immunology > Immune mechanisms > In vitro model
Immunology > Antibody analysis > Antibody detection
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








