- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Determination of Poly(3-hydroxybutyrate) Content in Cyanobacterium Synechocystis sp. PCC 6803 Using Acid Hydrolysis Followed by High-performance Liquid Chromatography
Published: Vol 13, Iss 16, Aug 20, 2023 DOI: 10.21769/BioProtoc.4790 Views: 1785
Reviewed by: Dennis J NürnbergAmberley D. StephensAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Separating Inner and Outer Membranes of Escherichia coli by EDTA-free Sucrose Gradient Centrifugation
Sheng Shu and Wei Mi
Mar 20, 2023 2858 Views
β-lactamase (Bla) Reporter-based System to Study Flagellar Type 3 Secretion in Salmonella
Fabienne F. V. Chevance and Kelly T. Hughes
Jun 20, 2023 1750 Views
An HPLC-based Assay to Study the Activity of Cyclic Diadenosine Monophosphate (C-di-AMP) Synthase DisA from Mycobacterium smegmatis
Avisek Mahapa [...] Dipankar Chatterji
Dec 20, 2024 1758 Views
Abstract
Various photoautotrophic cyanobacteria accumulate intracellular poly(3-hydroxybutyrate) (PHB) granules. This protocol can be used for determining the PHB contents of the cells as % PHB weight per dry cell weight using acid hydrolysis followed by high-performance liquid chromatography (HPLC). This HPLC analysis is rapid, with a running time of approximately 5 min per sample. The technique can accurately determine PHB concentrations in the range of 2–1,000 μg/mL PHB. However, this technique is not applicable for determining the contents of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in cyanobacteria.
Keywords: CyanobacteriaBackground
Poly(3-hydroxybutyrate) (PHB) is a common biopolymer accumulated in various heterotrophic bacteria and autophototrophic cyanobacteria as a storage for carbon and energy (Amadu et al., 2021; Yashavanth et al., 2021). PHB can be used as biodegradable plastic and scaffold for tissue engineering (Bhati et al., 2010; Monshupanee et al., 2016; Tarawat et al., 2020). In the well-studied cyanobacterium Synechocystis sp. PCC 6803 (hereafter, Synechocystis), cells have low PHB contents 0.4%–5% under a normal growth condition but, under nitrogen or phosphorus deprivation, PHB accumulation is significantly increased, up to 5%–13% w/w dry weight (Wu et al., 2001; Panda and Mallick, 2007; Monshupanee and Incharoensakdi, 2014; Koch et al., 2019). In Synechocystis, PHB biosynthesis is catalyzed by three enzymes (Figure 1). First, two acetyl-CoA are combined into acetoacetyl-CoA by 3-ketothiolase (PhaA). Second, acetoacetyl-CoA is reduced to 3-hydroxybutyryl-CoA by acetoacetyl-CoA reductase (PhaB). Finally, PHA synthase (PhaEC) polymerizes 3-hydroxybutyryl-CoA to form PHB (Taroncher-Oldenburg et al., 2000).
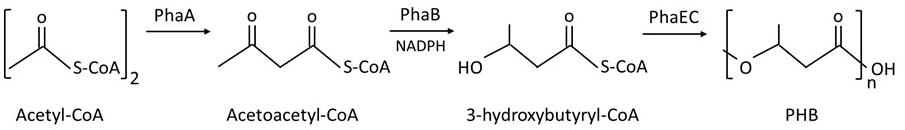
Figure 1. Poly(3-hydroxybutyrate) (PHB) biosynthesis in Synechocystis according to Taroncher-Oldenburg et al. (2000)
Various cyanobacteria accumulate PHB in the absence of essential nutrients including nitrogen or phosphorus (Drosg et al., 2015; Koller and Maršálek, 2015; Kaewbai-ngam et al., 2016). The previous reports on Synechocystis suggested that PHB serves as a carbon and energy reserve for growth recovery under nitrogen repletion (Koch et al., 2019; Kaewbai-ngam et al., 2022).
The common method for quantifying PHB content in microbes is the depolymerization of PHB by methanolysis in acid, with subsequent analysis by gas chromatography (GC) (Juengert et al., 2018). Another alternative method is the alkaline lysis of PHB, followed by the enzymatic assay method using 3-hydroxybutyrate dehydrogenase (Zilliges and Damrow, 2017). However, the GC method is time consuming, and the enzymatic assay requires enzyme preparation, which is costly. Here, we describe a rapid quantification of PHB content in cyanobacterial cells using acid hydrolysis followed by high-performance liquid chromatography (HPLC). This technique takes only 5 min of HPLC operation per sample and can be used to determine PHB contents in 135 evolutionarily diverged cyanobacterial species (Kaewbai-ngam et al., 2016). Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) is the co-polymer, which comprises 3-hydroxybutyrate (HB) and 3-hydroxyvalerate (HV) monomers (Verlinden et al., 2007; Balaji et al., 2013). PHBV changes material properties upon altering mole ratio (mol %) of the two monomers: 3-hydroxybutyrate (HB) and 3-hydroxyvalerate (HV) (Tarawat et al., 2020). We are reporting here a detailed protocol of PHB content determination that summarizes the steps for converting PHB to crotonic acid by acid hydrolysis, followed by the determination of crotonic acid contents using HPLC. We also show that this protocol cannot be used to determine PHBV content.
Materials and reagents
Organism: wildtype Synechocystis sp. PCC 6803 (Pasteur Culture Collection, France)
Pipette tips 5,000 μL (Biohit, catalog number: 780 300)
Pipette tips 1,000 μL (Labcon, catalog number: 1046-800-000-9)
Pipette tips 200 μL (Labcon, catalog number: 1165-800-000)
Pipette tips 10 μL (Labcon, catalog number: 1161-800-000)
Plastic cuvette for spectrophotometer (Brand, catalog number: 7591 50)
50 mL tubes (Axygen Scientific, catalog number: SCT-50ml-25-S)
10 mL glass tubes with screw cap (Pyrex, catalog number: 9825)
2 mL HPLC glass vials with septum cap (Thermo Scientific, catalog number: CHSV9-10P)
1.5 mL reaction tubes (Labcon, catalog number: 3016-870-000)
3 mL disposable syringes (Nipro, catalog number: 20B20/FEB2020)
Sterile syringe polypropylene filter, 0.22 μm (Filtep-bio, catalog number: 20220516002)
Polypropylene membrane, 0.45 μm (Filtrex, catalog number: FTM-PP47045-010)
Poly(3-hydroxybutyrate) (PHB) (Sigma-Aldrich, catalog number: 363502)
Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) (Sigma-Aldrich, catalog number: 403121)
Crotonic acid (Sigma-Aldrich, catalog number: 113018)
Adipic acid (Fluka Analytical, catalog number: 09582)
Distilled water
Acetonitrile, HPLC grade (Honeywell, catalog number: AHO15-4A)
Methanol, HPLC grade (VWR chemicals, catalog number: 67-56-1)
Absolute ethanol (Merck, catalog number: 100983)
Citric acid (BDH, catalog number: 08D150022)
Sodium hydroxide (Merck, catalog number: 1310-73-2)
Potassium dihydrogen phosphate (KH2PO4) (Merck, catalog number: 104873)
Ortho-phosphoric acid (H3PO4) (Sigma-Aldrich, catalog number: 7664-38-2)
Calcium chloride dihydrate (CaCl2·2H2O) (Merck, catalog number: 102382)
Ethylenediamine tetraacetic acid disodium salt dihydrate (Na2EDTA·2H2O) (Merck, catalog number: 108418)
Iron (III) chloride (FeCl3) (Ajax Finechem, catalog number: AF608291)
Magnesium sulfate heptahydrate (MgSO4·7H2O) (Merck, catalog number: 105886)
Sodium carbonate (Na2CO3) (Carlo Erba Reagenti, catalog number: 7783-20-2)
Sodium nitrate (NaNO3) (Ajax Finechem, catalog number: 1502523945)
(4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid)-NaOH (HEPES-NaOH) (OmniPur, catalog number: 7365-45-9)
Boric acid (H3BO3) (Scharlau, catalog number: 10043-35-3)
Manganese (II) chloride tetrahydrate (MnCl2·4H2O) (Kemaus, catalog number: 13446-34-9)
Zinc sulfate heptahydrate (ZnSO4·7H2O) (Ajax Finechem, catalog number: 0707330)
Sodium molybdate dihydrate (Na2MoO4·2H2O) (Kemaus, catalog number: 10102-40-6)
Copper (II) sulfate pentahydrate (CuSO4·5H2O) (BDH, catalog number: 100915R)
Cobalt (II) nitrate hexahydrate (Co(NO3)2·6H2O) (Carlo Erba Reagenti, catalog number: 10026-22-9)
Dipotassium hydrogen phosphate (K2HPO4) (Carlo Erba Reagenti, catalog number: 7758-11-4)
Sulfuric acid (H2SO4) (Merck, catalog number: 100731)
BG11 media (see Recipes)
Standard sulfuric acid solution (see Recipes)
Standard adipic acid solution (see Recipes)
Standard crotonic acid solution (see Recipes)
Premix solution used in Table 1 (see Recipes)
Mobile phase for HPLC analysis (see Recipes)
Equipment
Micro pipettes (Gilson: 20–1,000 μL)
Micro pipettes (Boeco, model: SA series ADJ 500–5,000 μL)
Glass pipettes (Qualicolor: 1–10 mL) and rubber pipette bulbs
Wide-neck 250 mL Erlenmeyer glass flasks (Duran)
Laboratory glass bottles (Duran)
Personal protective equipment (PPE)
Note: This should be worn at all times when working with concentrated acids.
Safety glasses
Lab coat
Gloves, purple nitrile (Kimtech, catalog number: SM0182ZZZ_37AX)
Autoclave (Hirayama manufacturing corporation, model: HA-30)
Laminar flow bench (Boss tech, model: HBV120)
Fume hood (HYSC LAB, model: FH-120)
Spectrophotometer (Jenway, model: UV/VIS 6400)
Culture shaker [Sac Science, model: Sling shaker (120–150 rpm), 3 levels]
Centrifuge (Hettich zentrifugen, model: Mikro 220/220 R)
Microcentrifuge (Eppendorf, model: 5417C)
Analytical balance (Mettler Toledo, model: PJ360)
Water bath (Hangzhou Bioer technology, model: N series)
Oven (Contherm, model: 2050)
Vortex shaker (Scientific industries, model: K-550-GE)
pH meter (Mettler Toledo, model: FiveEasy F20)
Filtration equipment (Pyrex, model: 33980-1L)
HPLC machine (Shimadzu, model: Shimadzu-02-1060)
HPLC UV/Vis detector (Shimadzu, model: SPD-10A)
HPLC pump (Shimadzu, model: LC-10AD)
HPLC auto-injector (Shimadzu, model: SIL-10AD VP)
HPLC column oven (Shimadzu, model: CTO-10A VP)
HPLC column (GL Sciences, model: InertSustain C18, 4.6 × 150 mm, 5 μm)
Conventional guard column (GL Sciences, model: InertSustain C18, 4.0 mm × 10 mm, 5 μm)
Software
Shimadzu HPLC LGE system, Japan
Excel, Microsoft, USA
Procedure
The procedure for determining the PHB content of Synechocystis cells is described in Figure 2. It consists of four steps, including (A) the cultivation of cyanobacteria, (B) acid hydrolysis to convert PHB into crotonic acid, (C) HPLC sample preparation, and (D) determination of PHB content using HPLC.
Two-step Synechocystis cultivation: grow cells under standard growth conditions to increase cell biomass and then transfer cells to nitrogen-deprived media to stimulate PHB accumulation
Inoculate BG11 media (Rippka et al., 1979) (see Recipes) with Synechocystis in Erlenmeyer flasks using the initial cell density of OD730nm = 0.2 and a total volume of 100 mL. Then, cultivate cells at 28 °C under continuous white light at 50 μmol m-2·s-1 with an atmospheric CO2 supply via culture shaking at 160 rpm. Cultivate cells for 4–5 days to achieve the cell density of OD730nm = 0.6–0.8.
Harvest cells by centrifugation at 4,000× g for 10 min at room temperature and wash cells twice with BG110 media (nitrogen-deprived medium) (see Recipes), followed by using the aforementioned centrifugation step. Remove supernatant medium in each washing step.
Perform the second cultivation step by inoculating the harvested cells in BG110 medium, using the initial OD730nm = 0.4 and the total volume of 100 mL. Next, cultivate cells for 7–14 days to induce PHB accumulation at 28 °C under continuous white light at 50 μmol m-2·s-1 with an atmospheric CO2 supply via culture shaking at 160 rpm.
Harvest cells from 40 mL of cell culture by centrifugation at 4,000× g for 10 min at room temperature. Next, resuspend fresh biomass in BG11 (total volume not exceeding 1.4 mL) and transfer the resuspended cell sample to the 1.5 mL tube. Then, centrifuge the tube, remove the liquid medium, and immediately dry cells at 60 °C in a hot oven until the weight of the sample stabilizes.
Note: The wet cell pellets can be stored at -20 °C or dried at 60 °C immediately before being used.
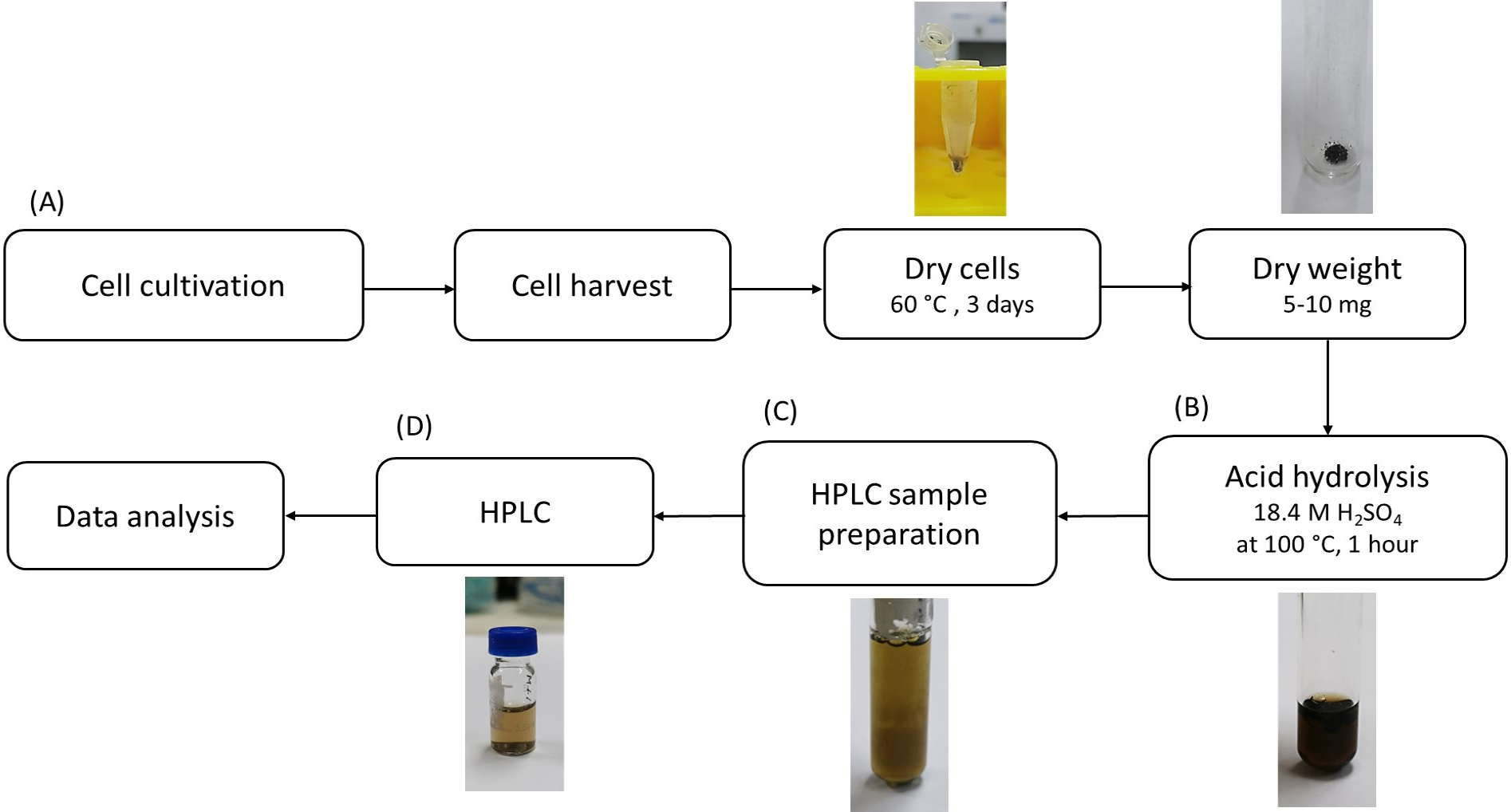
Figure 2. Overview of procedures for determining poly(3-hydroxybutyrate) (PHB) content of Synechocystis. Cells are cultivated, harvested, and dried. Next, dry cells are hydrolyzed by acid to convert PHB into crotonic acid. Then, crotonic acid content is quantified using HPLC. Details of each step are described in the procedure.
Acid hydrolysis to convert PHB into crotonic acid
Note: This step uses a strong acid, thus it must be performed under the fume hood and while wearing personal protective equipment.
Weigh approximately 5–10 mg of dry cells or 5 mg of the standard commercial PHB into 10 mL screw-capped glass tubes.
Add 1 mL of 18.4 M sulfuric acid by using a glass pipette to dry cells; boil at 100 °C for 1 h in a hot water bath to break cells and hydrolyze PHB into crotonic acid (Figure 3). The typical percentage of mass conversion of PHB to crotonic acid is in the range of 83% ± 7% (w/w) (Singhon et al., 2021; Kaewbai-ngam et al., 2022).
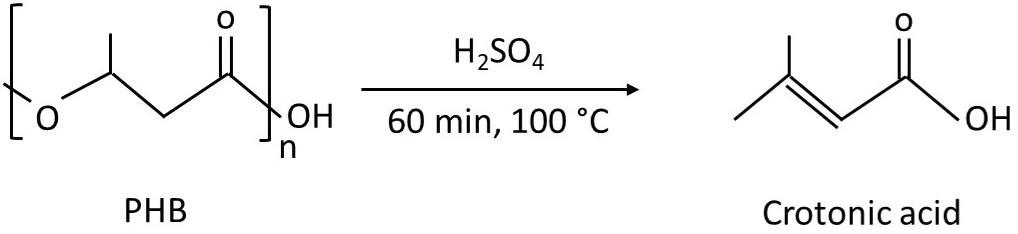
Figure 3. Depolymerization of poly(3-hydroxybutyrate) (PHB) by sulfuric acid to form crotonic acid. The reaction information is according to Koch et al. (2020).Cool sample until reaching room temperature; then, dilute the obtained total hydrolysate (1 mL) by adding 4 mL of distilled water and mix thoroughly by vortexing. The total volume of sample is 5 mL.
Note: For safety reasons, assure that the hot samples after acid hydrolysis are cooled down before proceeding to the next step.
For the commercial PHB sample (5 mg) that has been hydrolyzed and diluted to the total 5 mL in the step B3, make a 1:2, 1:5, 1:10, 1:50, 1:100, and 1:500 dilution in the total 5 mL volume to generate the standard PHB samples corresponding to the total PHB of 2.5, 1, 0.5, 0.1, 0.05, 0.01 mg of PHB in the 5 mL total volume.
HPLC sample preparation
Prepare HPLC samples according to Table 1 and mix thoroughly by vortexing.
Filter HPLC sample using a syringe and the polypropylene filter (0.22 μm); then, transfer the obtained filtrate to HPLC glass vials.
Table 1. HPLC sample preparation. N.A.: no addition
Note: The acid hydrolysis of biomass and HPLC analysis should be completely conducted within two days. The hydrolyzed samples can be stored at 4 °C for maximum of two days.HPLC sample name Component 1 Component 2 Total sample volume 1. Hydrolysate from Synechocystis cells 200 μL of cell hydrolysate from step B3 800 μL of the premix solution (see Recipes) 1,000 μL 2. Hydrolysate from commercial PHB (PHB standard) 200 μL of PHB hydrolysate and its six diluted samples from step B4 800 μL of the premix solution (see Recipes) 3. Standard sulfuric acid (negative control) 1,000 μL of standard sulfuric acid (see Recipe 2) N.A. 4. Standard adipic acid (internal standard) 1,000 μL of standard adipic acid (see Recipe 3) N.A. 5. Standard crotonic acid (positive control) 1,000 μL of standard crotonic acid (see Recipe 4) N.A.
Determination of PHB content using HPLC
Set up the HPLC operation as shown below:
• InertSustain Carbon-18 column
• UV detection at 210 nm
• 40 °C oven temperature
• 10 μL injection volume
• 1 mL/min flow rate
• 5 min running time per one sample
• Mobile phase: [30:70 (% v/v) acetonitrile:10 mM KH2PO4 buffer pH 2.3]
Note: There is no washing step between runs because no gradient of the mobile phase is used.
Data analysis
By using the mobile phase [30:70 (% v/v) acetonitrile:10 mM KH2PO4 buffer pH 2.3], the obtained retention times of the samples are shown in Table 2. Note that these retention times are specific for the HPLC column, the HPLC system, and the mobile phase used in this study.
Table 2. Obtained retention time of the HPLC standards and cell sample using 30% (v/v) acetonitrile in 10 mM KH2PO4 buffer as the mobile phase
Sample Obtained retention times (min) Chromatogram peak 1. Hydrolysate from commercial PHB 3.02 (crotonic acid) and 2.25 (adipic acid internal standard) Figure 5A 2. Hydrolysate from Synechocystis 3.09 (crotonic acid) and 2.29 (adipic acid internal standard) 3. Standard sulfuric acid (negative control) 1.59–1.65 Figure 5B 4. Standard crotonic acid (positive control) 3.00–3.10 5. Standard adipic acid (internal standard) 2.20–2.30 Calculation of PHB content
Generate a standard curve of the six amounts (0–5 mg, see step B4) of the standard PHB sample.
i. Collect HPLC peak areas of crotonic acid and adipic acid from each amount of PHB standard.
ii. Calculate the ratio between the peak area of crotonic and the peak area of adipic acid. All peak areas used in this study were defined by the HPLC software.
iii. Plot the graph: the ratio between the peak area of crotonic and the peak area of adipic acid on the y-axis against the amount of PHB (0–5 mg) on the x-axis.
iv. Determine the linear equation (y = a × x + b, where b = 0). The linear regression coefficient should be in the range of 0.99 ≤ R2 ≤ 1 (Figure 4).
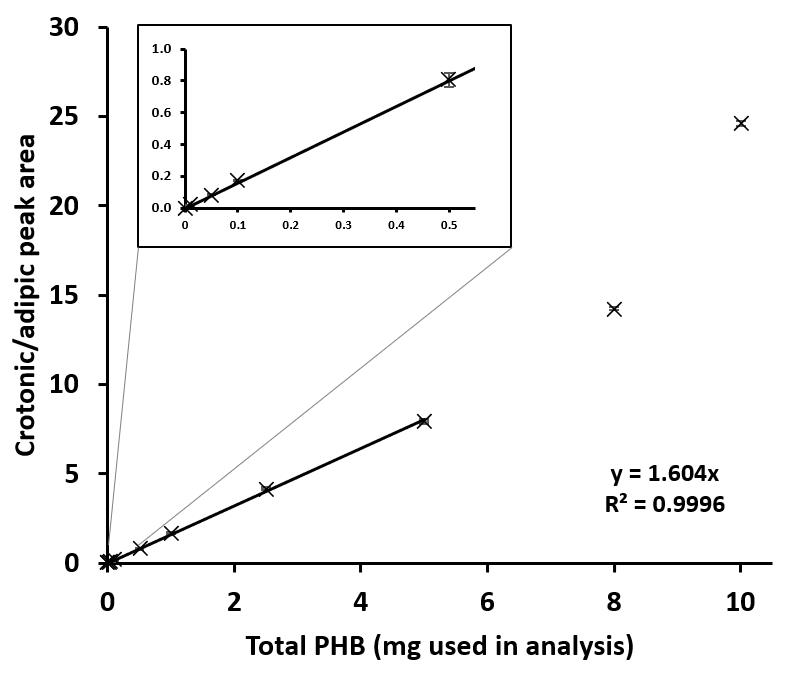
Figure 4. Standard curve for the determination of poly(3-hydroxybutyrate) (PHB) weight. The linear correlation was obtained under standard PHB = 0–5 mg, but not at 8 and 10 mg in the total volume of 5 mL sample. Therefore, the maximum standard PHB used for the analysis was 5 mg in 5 mL.Calculate the weight of PHB in the cell sample using the formula:
x = y/a
x: Weight of PHB (mg) in cell sample
y: The ratio between the peak area of crotonic: the peak area of adipic acid derived from cell sample
a: The value obtained from the formula (y = a × x) in step B1
Calculate PHB content in Synechocystis cells as % w/w dry weight (DW) using the formula:
PHB content (% w/w DW) = [x (mg)/cell weight (mg)] × 100
x: Weight of PHB (mg) in cell sample obtained in step B2
Samples from at least three independent cultures must be used.
Increased acetonitrile concentrations in the mobile phase significantly reduced the retention times of all target samples (adipic acid and crotonic acid) (Figure 5A). For example, the HPLC using the mobile phase without acetonitrile showed the retention time of crotonic acid at 34.7 min, while HPLC using the mobile phase containing 30% (v/v) acetonitrile significantly reduced the retention time of crotonic acid to only 3.0 min (Figure 5A). Therefore, the recommended percentage of acetonitrile in mobile phase is 30% (v/v), which was used throughout this experiment, and the HPLC running time per sample is only 5 min (Figure 5A).
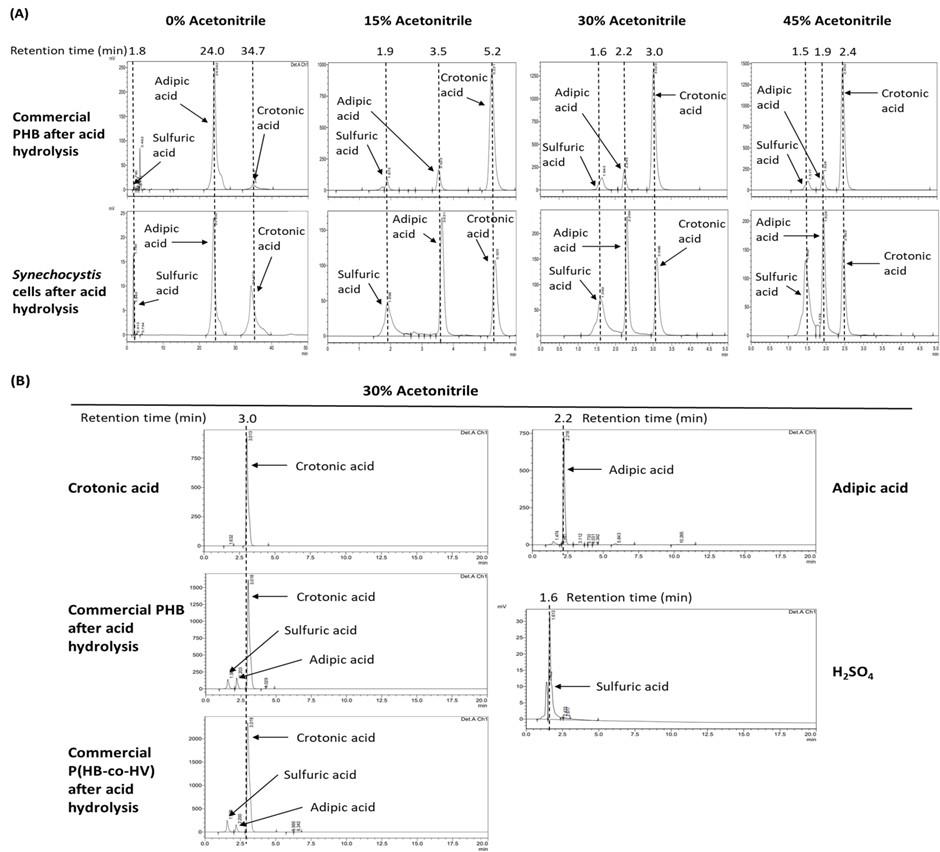
Figure 5. HPLC chromatograms of all samples used in this study. (A) The chromatograms of the commercial poly(3-hydroxybutyrate) (PHB) and the cell sample in the mobile phase containing 0, 15, 30, and 45% (v/v) acetonitrile. (B) The chromatograms of the indicated samples in the mobile phase containing 30% (v/v) acetonitrile. PHBV: commercial poly(3-hydroxybutyrate-co-3-hydroxyvalerate).The lower limit of the sensitivity of this protocol is a PHB content of 0.01 mg per 5 mL of total volume, corresponding to a PHB concentration of 2 μg/mL (Figure 4). The recommended maximal amount of PHB to be analyzed by this technique is 5 mg per 5 mL of total volume, corresponding to a PHB concentration of 1 mg/mL (Figure 4). If the PHB concentration is higher than 1 mg/mL, this sample must be diluted after acid hydrolysis prior to HPLC analysis.
The analysis of the acid hydrolysis followed by HPLC of the commercial poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) showed only the peak of crotonic acid (the hydrolyzed product of 3-hydroxybutyrate), and no peak of the hydrolyzed product of 3-hydroxyvalerate (Figure 5B) as detected by 210 nm UV absorption. Thus, the hydrolyzed product of 3-hydroxyvalerate may not absorb the light at 210 nm. There is no current report on the chemical identity of the hydrolyzed product of 3-hydroxyvalerate. Therefore, the acid hydrolysis followed by HPLC used in this study cannot be used to determine the content of P(HB-co-HV) in cyanobacteria. It is noted that successful analyses of P(HB-co-HV) from cyanobacterial cells using methanolysis in acid followed by gas chromatography have been reported previously (Bhati and Mallick, 2015; Taepucharoen et al., 2017; Tarawat et al., 2020).
Recipes
BG11 media (autoclaved)
The medium composition is according to Rippka et al. (1979); (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid)-NaOH (HEPES-NaOH) was added to the medium.
Number
Trace element component
Final concentration [mM]
BG11 media
BG110 media
1
NaNO3
17.60
0
2
K2HPO4
0.23
0.23
3
MgSO4·7H2O
0.30
0.30
4
CaCl2·2H2O
0.24
0.24
5
FeCl3
0.021
0.021
6
Na2EDTA·2H2O
0.0027
0.0027
7
Na2CO3
0.19
0.19
8
H3BO3
46.00
46.00
9
MnCl2·4H2O
9.00
9.00
10
ZnSO4·7H2O
0.77
0.77
11
Na2MoO4·2H2O
1.60
1.60
12
CuSO4·5H2O
0.30
0.30
13
Co(NO3)2·6H2O
0.17
0.17
14
HEPES-NaOH (pH 7.5)
20.00
20.00
Standard sulfuric acid (negative control) (5 mL)
0.74 M H2SO4
Standard adipic acid (internal standard) (10 mL)
15 mg/mL adipic acid in 0.74 M H2SO4
Standard crotonic acid (10 mL)
10 mg/mL crotonic acid in distilled H2O
Premix solution used in Table 1 (100 mL per 100 samples)
0.375 g of adipic acid in 100 mL of 1.84 M H2SO4
Mobile phase for HPLC analysis (1 L per 100 samples)
1.36 g/L KH2PO4 buffer [adjust to pH 2.3 using H3PO4 and filtrate the solution using the polypropylene membrane (0.45 μm) and 30% (v/v) acetonitrile (HPLC grade)]
Note: Solutions 2–6 must be freshly prepared just before use.
Acknowledgments
This protocol was adapted from the previous protocol described by Schlebusch and Forchhammer (2010). This work was supported by The Royal Golden Jubilee Ph.D. Program (PHD/0011/2560) and National Research Council of Thailand (NRCT): NRCT5-RSA63001-21. The authors declare no conflicts of interest.
Competing interests
We have no competing interests.
References
- Amadu, A. A., Qiu, S., Ge, S., Addico, G. N. D., Ameka, G. K., Yu, Z., Xia, W., Abbew, A. W., Shao, D., Champagne, P., et al. (2021). A review of biopolymer (Poly-β-hydroxybutyrate) synthesis in microbes cultivated on wastewater. Sci. Total Environ. 756: 143729.
- Balaji, S., Gopi, K. and Muthuvelan B. (2013). A review on production of poly β hydroxybutyrates from cyanobacteria for the production of bio plastics. Algal. Res. 2: 278–285.
- Bhati, R. and Mallick, N. (2015). Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer production by the diazotrophic cyanobacterium Nostoc muscorum Agardh: Process optimization and polymer characterization. Algal Res. 7: 78–85.
- Bhati, R., Samantaray, S., Sharma, L. and Mallick, N. (2010). Poly-β-hydroxybutyrate accumulation in cyanobacteria under photoautotrophy. Biotechnol. J. 5(11): 1181–1185.
- Drosg, B., Fritz, I., Gattermayr, F. and Silvestrini, L. (2015). Photoautotrophic production of poly(hydroxyalkanoates) in cyanobacteria.Chem. Biochem. Eng. Q. 29: 145–156.
- Juengert, J., Bresan, S. and Jendrossek, D. (2018). Determination of Polyhydroxybutyrate (PHB) Content in Ralstonia eutropha Using Gas Chromatography and Nile Red Staining. Bio Protoc 8(5): e2748.
- Kaewbai-ngam, A., Incharoensakdi, A. and Monshupanee, T. (2016). Increased accumulation of polyhydroxybutyrate in divergent cyanobacteria under nutrient-deprived photoautotrophy: An efficient conversion of solar energy and carbon dioxide to polyhydroxybutyrate by Calothrix scytonemicola TISTR 8095. Bioresour. Technol. 212: 342–347.
- Kaewbai-ngam, J., Sukkasam, N., Phoraksa, O., Incharoensakdi, A. and Monshupanee, T. (2022). Production of glycogen, PHB, biohydrogen, NAD(P)H, and proteins in Synechocystis sp. PCC 6803 disrupted in metabolically linked biosynthetic pathway(s). J. Appl. Phycol. 34(4): 1983–1995.
- Koch, M., Bruckmoser, J., Scholl, J., Hauf, W., Rieger, B. and Forchhammer, K. (2020). Maximizing PHB content in Synechocystis sp. PCC 6803: a new metabolic engineering strategy based on the regulator PirC. Microb. Cell Fact. 19(1): e1186/s12934-020-01491-1.
- Koch, M., Doello, S., Gutekunst, K. and Forchhammer, K. (2019). PHB is Produced from Glycogen Turn-over during Nitrogen Starvation in Synechocystis sp. PCC 6803. Int. J. Mol. Sci. 20(8): 1942.
- Koller, M. and Maršálek, L. (2015). Cyanobacterial polyhydroxyalkanoate production: status quo and quo vadis? Curr. Biol. 4(3): 464–480.
- Monshupanee, T. and Incharoensakdi, A. (2014). Enhanced accumulation of glycogen, lipids and polyhydroxybutyrate under optimal nutrients and light intensities in the cyanobacterium Synechocystis sp. PCC 6803. J. Appl. Microbiol. 116(4): 830–838.
- Monshupanee, T., Nimdach, P. and Incharoensakdi, A. (2016). Two-stage (photoautotrophy and heterotrophy) cultivation enables efficient production of bioplastic poly-3-hydroxybutyrate in auto-sedimenting cyanobacterium. Sci. Rep. 6(1): e1038/srep37121.
- Panda, B. and Mallick, N. (2007). Enhanced poly-?-hydroxybutyrate accumulation in a unicellular cyanobacterium, Synechocystis sp. PCC 6803. Lett. Appl. Microbiol. 44(2): 194–198.
- Rippka, R., Stanier, R. Y., Deruelles, J., Herdman, M. and Waterbury, J. B. (1979). Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria. Microbiology 111(1): 1–61.
- Schlebusch, M. and Forchhammer, K. (2010). Requirement of the Nitrogen Starvation-Induced Protein Sll0783 for Polyhydroxybutyrate Accumulation in Synechocystis sp. Strain PCC 6803. Appl. Environ. Microbiol. 76(18): 6101–6107.
- Singhon, P., Phoraksa, O., Incharoensakdi, A. and Monshupanee, T. (2021). Increased bioproduction of glycogen, lipids, and poly(3-hydroxybutyrate) under partial supply of nitrogen and phosphorus by photoautotrophic cyanobacterium Synechocystis sp. PCC 6803. J. Appl. Phycol. 33(5): 2833–2843.
- Taepucharoen, K., Tarawat, S., Puangcharoen, M., Incharoensakdi, A. and Monshupanee, T. (2017). Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) under photoautotrophy and heterotrophy by non-heterocystous N 2 -fixing cyanobacterium. Bioresour. Technol. 239: 523–527.
- Tarawat, S., Incharoensakdi, A. and Monshupanee, T. (2020). Cyanobacterial production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from carbon dioxide or a single organic substrate: improved polymer elongation with an extremely high 3-hydroxyvalerate mole proportion. J. Appl. Phycol. 32(2): 1095–1102.
- Taroncher-Oldenburg, G., Nishina, K. and Stephanopoulos, G. (2000). Identification and Analysis of the Polyhydroxyalkanoate-Specific β-Ketothiolase and Acetoacetyl Coenzyme A Reductase Genes in the Cyanobacterium Synechocystis sp. Strain PCC6803. Appl. Environ. Microbiol. 66(10): 4440–4448.
- Verlinden, R. A., Hill, D. J., Kenward, M. A., Williams, C. D. and Radecka, I. (2007). Bacterial synthesis of biodegradable polyhydroxyalkanoates. J. Appl. Microbiol. 102(6): 1437–49.
- Wu, G. F., Wu, Q. Y. and Shen, Z. Y. (2001). Accumulation of poly-β-hydroxybutyrate in cyanobacterium Synechocystis sp. PCC6803. Bioresour. Technol. 76(2): 85–90.
- Yashavanth, P. R., Das, M. and Maiti, S. K. (2021). Recent progress and challenges in cyanobacterial autotrophic production of polyhydroxybutyrate (PHB), a bioplastic. J. Environ. Chem. Eng. 9(4): 105379.
- Zilliges, Y. and Damrow, R. (2017). Quantitative Determination of Poly-β-hydroxybutyrate in Synechocystis sp. PCC 6803. Bio Protoc 7(14): e2402.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Kaewbai-ngam, J., Incharoensakdi, A. and Monshupanee, T. (2023). Determination of Poly(3-hydroxybutyrate) Content in Cyanobacterium Synechocystis sp. PCC 6803 Using Acid Hydrolysis Followed by High-performance Liquid Chromatography. Bio-protocol 13(16): e4790. DOI: 10.21769/BioProtoc.4790.
Category
Microbiology > Microbial biochemistry > Other compound
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










