- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
SIMBA Method—Simultaneous Detection of Antimicrobial and Anti-biofilm Activity of New Compounds Using Salmonella Infantis
Published: Vol 13, Iss 15, Aug 5, 2023 DOI: 10.21769/BioProtoc.4783 Views: 1988
Reviewed by: Shailesh KumarAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Mobilization of Plasmids from Bacteria into Diatoms by Conjugation Technique
Federico Berdun [...] Eduardo Zabaleta
Mar 5, 2024 1908 Views
Identification of Mycobacterium tuberculosis and its Drug Resistance by Targeted Nanopore Sequencing Technology
Chen Tang [...] Guangxin Xiang
Feb 5, 2025 1964 Views

Artificial Metalloenzymes in Artificial Sanctuaries Through Liquid–Liquid Phase Separation
Kaixin Wang [...] Tong Wu
Oct 5, 2025 1536 Views
Abstract
The development of antimicrobial resistance and the formation of Salmonella biofilms are serious public health problems. For this reason, new natural compounds with antimicrobial and anti-biofilm activity are being sought, and wild fungi represent an untapped potential. Various extraction agents, including organic solvents and aqueous buffers, can be used to obtain bioactive compounds from natural sources. To evaluate their bioactivity, extensive screening studies are required to determine antimicrobial and anti-biofilm activity using methods such as broth microdilution or crystal violet assay, respectively, but none of these methods allow simultaneous evaluation of both activities against bacteria. Cold water extraction from wild fungi offers the advantage of extracting water-soluble compounds. The SIMultaneous detection of antiMicrobial and anti-Biofilm Activity (SIMBA) method combines the testing of both types of activity against bacteria with the evaluation of the 20 h growth curve of the Salmonella Infantis ŽM9 strain determined with absorbance measurements at 600 nm in a 96-well plate. SIMBA method thus shortens the time to determine the bioactivity of extracts, reduces material consumption, and eliminates the need for additional reagents. SIMBA enables rapid selection of bioactive extracts for their fractionation and shortens the time to determine new natural products with antimicrobial and anti-biofilm activity.
Graphical overview

Background
Biofilm formation is a persistence strategy for bacteria that plays a role both for biotic surfaces causing chronic infections and for colonization and survival on abiotic surfaces. After attachment to different surfaces, Salmonella spp. can form a protective matrix of extracellular polymeric substances (EPS) that protects bacteria from environmental factors, facilitates gene transfer, and increases nutrient availability and bacterial resistance to antimicrobial agents. Therefore, Salmonella biofilm cells exhibit higher environment survival and increased antimicrobial resistance, both of which are associated with more human infections (Steenackers et al., 2012; MacKenzie et al., 2017; González et al., 2018; Tassinari et al., 2019). In addition, antimicrobial resistant Salmonella are widespread in animals and on various surfaces associated with animal production and food processing (Vestby et al., 2009; Pate et al., 2019; Tassinari et al., 2019). Alternative control strategies for these Salmonella are being sought due to their resistance to environmental stress and to commercial antibacterial agents.
Higher fungi represent an underexplored source of antimicrobial compounds; fruiting bodies of cultivated and wild mushrooms have been shown to contain various antimicrobial compounds, ranging from secondary metabolites such as steroids, terpenes, and quinolines, to peptides and proteins (Alves et al., 2012; Erjavec et al., 2012). Different solvents have been used for the extraction of new potential antimicrobial compounds. Organic solvents such as methanol, ethanol, ethyl acetate, chloroform, acetone, ether, xylene, cyclohexane, or dichloromethane are the most commonly used, while aqueous extraction with hot water or cold aqueous buffers is rarely used (Alves et al., 2012; Klančnik et al., 2017; Gómez Román et al., 2020). The advantage of using water for extraction is that water-soluble compounds can be extracted, which is beneficial for their potential use as antimicrobials. Water solubility is a very important parameter for drug formulations, as poorly soluble drugs are associated with poor absorption and low bioavailability and often require higher doses (Savjani et al., 2012).
Antimicrobials inhibit bacterial growth or kill bacteria, while specific anti-biofilm agents can interact with biofilm-related metabolic processes without affecting bacterial growth (Steenackers et al., 2012). These two concepts must be considered separately, as compounds that interfere with growth may also contribute to faster development of bacterial resistance. Because of their importance in bacterial persistence, there is a need to control biofilms and their formation without affecting bacterial growth (Dieltjens et al., 2020). Currently, different methods are used to test antimicrobial activity and anti-biofilm activity. Methods performed in microtiter plates are based on similar principles, and determination of minimum inhibitory concentration in the form of broth microdilution is used to evaluate antimicrobial activity. Determination of anti-biofilm activity requires more manipulation because the biofilm is formed on the surface, and therefore steps to wash and detach the cells are required, followed by determination of the culturable cells by dilution and plating. In addition, various indicators can be used as colorimetric indicators of microbial metabolic activity of microorganisms to evaluate viability in antimicrobial activity analysis, for example, tetrazolium salts such as 2,3,5-triphenyltetrazolium chloride (TTC), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenlytetrazolium bromide (MTT), or 2-(4-iodophenil)-3-(4-dinitrophenyl)-5-phenyltetrazolium chloride (INT). Resazurin can also be used to determine the viability of microorganisms, measured as a fluorescence signal or with some commercially available kits such as PrestoBlueTM. The bioluminescence signal can also be used to determine the ATP activity of microorganisms using commercially available kits such as BacTiter-GloTM. Similarly, dye assays can also be used to assess anti-biofilm activity, such as crystal violet, safranin, or acridine orange (Klančnik et al., 2010 and 2021; Eloff, 2019).
All of these methods require manipulation after initial incubation; some use reagents that are hazardous to the environment, and two methods must be performed to evaluate both activities. Here, we present a method for SIMultaneous detection of antiMicrobial and anti-Biofilm Activity (SIMBA) published in Sterniša et al. (2022a). It is a 96-well microplate-based method that, compared to other antimicrobial and anti-biofilm assays, does not require additional plate manipulations after plate preparation, does not require reagents, and provides results within 20 h. We additionally provide the workflow to predict antibacterial or anti-biofilm activity using freely available software Orange (Demšar et al., 2013). A learner on predicting the activity of new compounds is trained on a provided dataset consisting of experimentally verified data and then a workflow is provided to predict the activity of the unknown compound. This presents a step forward to analyzing growth curves by advanced algorithms further supporting high-throughput methods like SIMBA. Namely, SIMBA was shown to reduce costs by up to 50% and time to completion in the laboratory by up to 80%. Interpretation of results is based on a computer program, making it objective and straightforward. This protocol can be used for screening extracts for novel antimicrobial and/or anti-biofilm compounds, as well as for bioactivity-guided purification of such compounds. Other areas where this protocol can be useful for faster analysis of antimicrobial and anti-biofilm activities include testing of commercial products such as food or feed additives, pesticides, biocides, or disinfectants and wastewater analysis.
Materials and reagents
Ice
600 mL glass beaker (Brand, catalog number: BR90648)
Disposable viscose cloths (Tosama, catalog number: 23073)
Large potato ricer
HiPrep 26/60 Sephacryl® S-200 HR column (Cytiva, catalog number: 17-1195-01)
Liquid chromatography column 3 × 100 cm (custom made)
0.2 μm syringe filters (Minisart NML, Sartorius, catalog number: 16534)
10 mL syringe (BD Emerald, catalog number: 307736)
50 mL centrifuge tube (Sarstedt, catalog number: 62.547.004)
Transparent flat-bottomed 96-well microplates (Thermo Scientific Nunc, catalog number: 266120)
15 mL centrifuge tube (Sarstedt, catalog number: 62.554.502)
1.5 mL microcentrifuge tubes (Eppendorf, catalog number: 0030 120.086)
1–10 mL tips (Eppendorf, catalog number: 0030 000.765)
50–1,000 μL tips (Eppendorf, catalog number: 0030 000.927)
2–200 μL tips (Eppendorf, catalog number: 0030 000.870)
Cuvettes (Brand, catalog number: 7590 15)
Tryptic soy broth (TSB) (Biolife, catalog number: 4021552)
Tryptic soy agar (TSA) (Biolife, catalog number: 4021502)
Phosphate buffered saline (PBS) (Oxoid, catalog number: BR0014G)
Na2HPO4 (Sigma-Aldrich, catalog number: S9763)
NaCl (Sigma-Aldrich, catalog number: S9888)
KH2PO4 (Sigma-Aldrich, catalog number: P0662)
KCl (Sigma-Aldrich, catalog number: P3911)
NaOH (Sigma-Aldrich, catalog number: S5881)
HCl (Sigma-Aldrich, catalog number: 258148)
Tris (Serva, catalog number: 37180)
Salmonella enterica subsp. enterica Infantis ŽM9 (culture collection of the Laboratory for Food Microbiology at the Department of Food Science, Biotechnical Faculty; GenBank accession number JARDYW000000000)
20× PBS (concentrated phosphate buffered saline) (see Recipes)
30 mM Tris-HCl, pH 7.5 with 0.4 M NaCl (see Recipes)
2× TSB (see Recipes)
Equipment
Refrigerated centrifuge (Thermo Fisher Scientific, Sorwall Lynx 4000)
ÄKTA pure protein purification system (Cytiva 29018224)
Microplate reader (Thermo Fisher Scientific, Varioskan Lux)
Incubator (Kambič, I-105CK)
Vortex (Domel, Vibromix 10)
Pipette set (Eppendorf, catalog number: 3120 000.836)
1–10 mL pipette (Eppendorf, catalog number: 3123 000.080)
10–100 μL multichannel pipette (Eppendorf, catalog number: 3125 000.044)
Biosafety cabinet (Iskra Pio, SMBC 122AV)
Ultra-low temperature freezer (Haier, DW-86L728ST)
Freezer (LTH, HG 5.1Z)
Spectrophotometer (Perkin Elmer, Lambda Bio+)
Microplate mixer (Eppendorf, ThermoMixer C)
Software
SkanIt RE 5.0 Plate Reader Software (Thermo Fisher Scientific, https://www.thermofisher.com/si/en/home/life-science/lab-equipment/microplate-instruments/plate-readers/software.html)
Excel (Microsoft Corporation, https://office.microsoft.com/excel)
R: A language and environment for statistical computing (R Core Team, http://www.R-project.org/)
Growthcurver: simple metrics to summarize growth curves (Sprouffske and Wagner, 2016, https://github.com/sprouffske/growthcurver, https://cran.r-project.org/web/packages/growthcurver/vignettes/Growthcurver-vignette.html)
Orange Data Mining (Demšar et al., 2013, University of Ljubljana, Faculty of Computer and Information Science, https://orange.biolab.si)
Procedure
The overall procedure is divided into several parts, with the fungal extracts first prepared (section A) and analyzed by the SIMBA method (sections C–F) and the data analyzed as described in the data analysis sections. For selected extracts showing antimicrobial and/or anti-biofilm activity, the extracts are then fractionated (section B), followed by another round of SIMBA method (sections C–F) and data analysis.
Obtaining cold water fungal extracts (CWFE) and their fractionation
Cold water extraction of fruiting bodies of wild or cultivated fungi
Collect and clean fresh fruiting bodies and store in a freezer at -20 °C (see Note 1).
Thaw frozen fruiting bodies overnight at 4–8 °C.
Place a beaker on ice.
Wrap the thawed fungal sample in the viscose cloth and place inside the potato ricer.
Press the potato ricer firmly over the beaker on ice and rotate the cloth with the sample several times to extract all the liquid that collects in the beaker below.
Measure the volume of crude extract in the beaker.
Add 1/20 of the volume of 20× PBS to obtain a concentration of 1× PBS in the extract and mix well.
Transfer to a suitable centrifuge tube that has been chilled on ice (see Note 2).
Centrifuge at 10,000× g for 10 min at 4 °C to remove insoluble material.
Carefully transfer the supernatant to suitable centrifuge tubes (on ice) (see Note 3).
Filter sterilize the supernatant, which is the cold water fungal extract (CWFE), using a 0.2 μm syringe filter.
Store CWFE in aliquots in the freezer at -20 or -80 °C until analysis with SIMBA method (Note 4).
Fractionation of CWFE with confirmed antimicrobial and/or anti-biofilm activity (see Note 5)
Thaw selected CWFE (with antimicrobial and/or anti-biofilm activity confirmed by SIMBA method) on ice.
Work with refrigeration (4–8 °C) either by using a refrigerated automated liquid chromatography system or in a cold room (see Note 6).
Equilibrate the Sephacryl S-200 column in 30 mM Tris-HCl, pH 7.5 with 0.4 M NaCl.
Load CWFE sample (at up to 2% of the total column volume) and elute in the same buffer at a flow rate of 1 mL/min.
Collect 5 mL fractions and measure the absorbance at 280 nm (Figure 1).
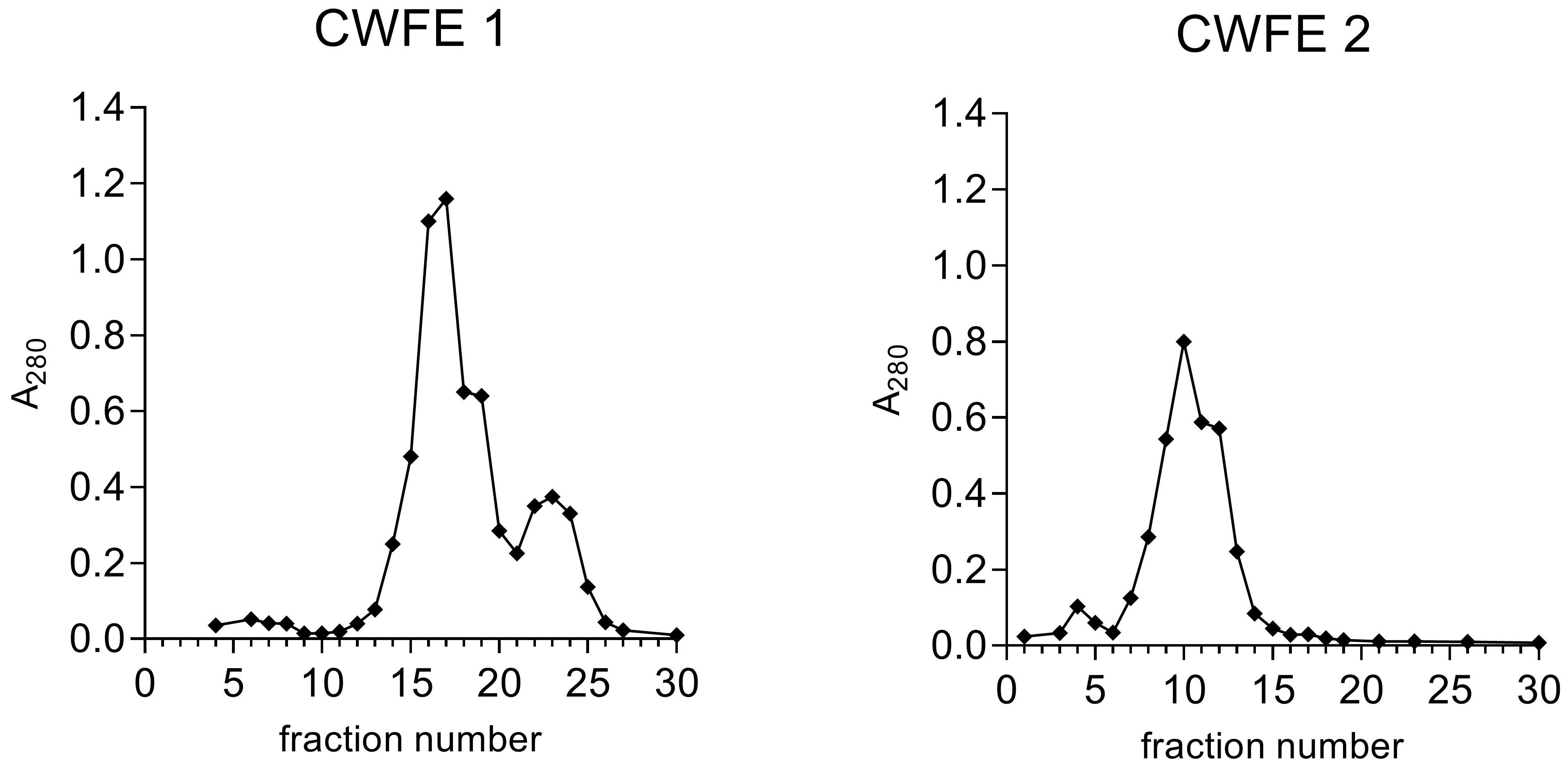
Figure 1. Examples of size-exclusion chromatograms of two different cold water fungal extracts (CWFEs) showing absorbance at 280 nm in fractions.The chromatogram of CWFE 1 shows better resolution than that of CWFE 2, where all proteins elute in a single peak. Fractions are subjected to the SIMBA method, and fractions showing antimicrobial and/or anti-biofilm activity are then selected for further analysis or fractionation.
Filter sterilize fractions using 0.2 μm syringe filters.
Store fractions at 4–8 °C until analysis with SIMBA method (see Note 7).
SIMBA method—Determination of antimicrobial and anti-biofilm activity of CWFE and fractions of CWFE with confirmed antimicrobial and/or anti-biofilm activity
Revival of S. Infantis
Work aseptically.
Remove a vial containing a permanent culture of S. Infantis ŽM9 from the ultra-low temperature freezer and place it on ice.
Scrape off the frozen culture and transfer it to a TSA plate using a sterile loop.
Incubate at 37 °C for 24 h.
Preparation of the inoculum of S. Infantis
Work aseptically.
Prepare 5 mL of PBS in a 15 mL tube.
With a cotton swab, add the biomass (of S. Infantis growth on plate) and vortex for 10 s.
Add 1 mL of the bacterial suspension to a cuvette and measure the absorbance at 600 nm on the spectrophotometer. Use 1 mL of PBS as a blank.
The measured absorbance should be 0.100 ± 0.010, which corresponds to (1–2.5) × 107 CFU/mL. If the measured value is lower than the target value, add more culture. If the measured value is higher than the target value, add PBS.
Transfer 100 μL of the prepared bacterial suspension to 9.9 mL of 2× TSB.
Use the prepared inoculum within 20 min of preparation.
Setup of the microplate
Work aseptically.
Remove the CWFE/CWFE fraction sample from the freezer and place it on ice to thaw.
Vortex the thawed CWFE/CWFE fraction for 10 s.
Pipette the thawed CWFE/CWFE fraction into the wells of the microplate (see also Note 8).
Transfer 100 μL of CWFE/CWFE fraction (row A, columns 1, 2, and 3 of the microplate schematic in Figure 2) if you want to test only a 50% concentration of the extract.
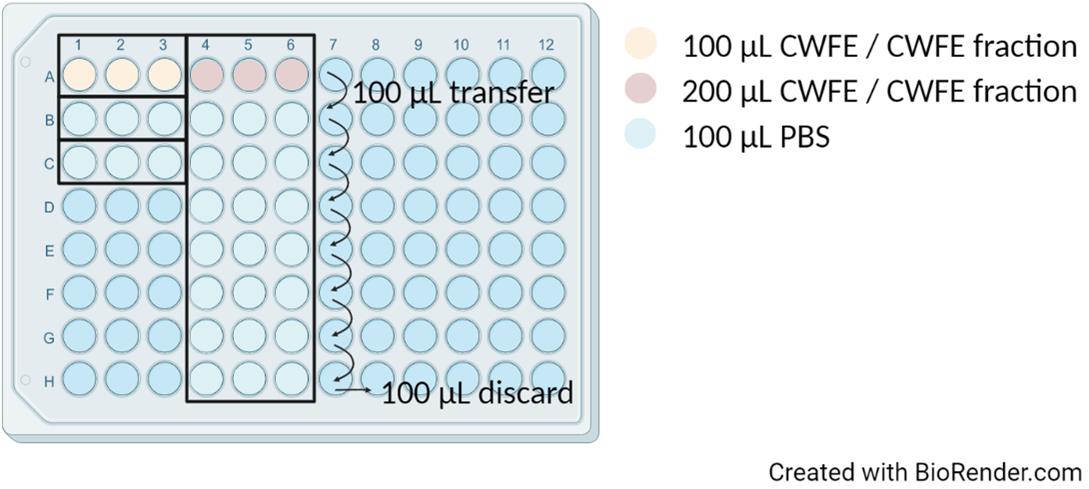
Figure 2. Microplate preparation scheme before adding the prepared bacterial inoculum and 2× TSBTransfer 200 μL of CWFE/CWFE fraction (row A, columns 4, 5, and 6 of the microplate schematic in Figure 2) if you wish to test 50% concentrations and two-fold dilutions of the extract (see Note 9).
i. Pipette 100 μL of PBS (rows B–H, columns 4, 5, and 6 of the microplate schematic in Figure 2).
ii. Transfer 100 μL of CWFE/CWFE fraction from row A to row B and mix by pipetting. Continue these transfers to row H and discard the remaining 100 μL.
Pipette 100 μL of PBS (rows B and C, columns 1, 2, and 3 of the microplate schematic in Figure 2) for positive (row B) and negative (row C) control.
Pipette 100 μL of the prepared inoculum into all wells filled with CWFE/CWFE fraction or its dilutions.
Pipette 100 μL of the prepared inoculum for positive control (row B, columns 1, 2, and 3).
Pipette 100 μL of 2× TSB for the negative control (row C, columns 1, 2, and 3).
Shake the prepared microplate on a microplate shaker at 600 rpm for 2 min.
Absorbance measurement
Set the microplate reader for kinetic measurement.
Set the temperature to 37 °C.
Set 61 kinetic intervals of 20 min, giving a total of 20 h of measurements.
Set 5 s of continuous shaking at 600 rpm before measurement.
Measure the absorbance at 600 nm.
Insert the microplate when the temperature reaches 37 °C and start the kinetic measurements.
Export the absorbance measurements to Excel.
Data analysis
All measurements should be repeated in three biological replicates, each of which is repeated in three technical replicates.
Visualization of obtained data
Open Excel file and mark the samples that were in the individual wells.
Review the initial measured absorbances and discard data where the initial measured absorbance is ≥ 0.500 (see Note 9).
For the repetitions of each extract and controls, calculate the average for each time point (see Note 10).
Plot scatterplot with smooth lines to visualize data. Put time (h) on x-axis and absorbance (600 nm) on y-axis.
Based on obtained curves of S. Infantis growth (Figure 3), visually evaluate the results (see Note 11).
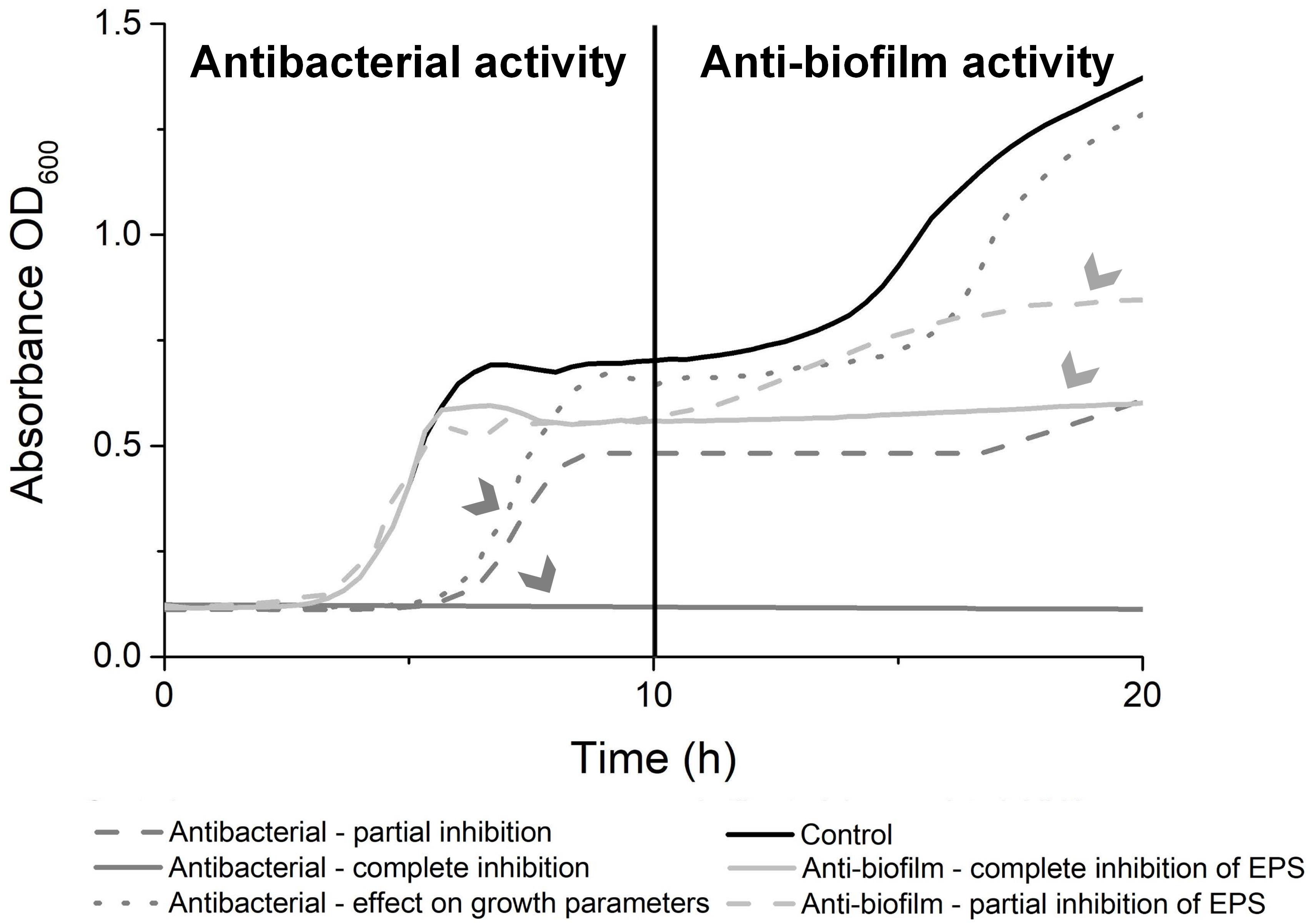
Figure 3. Visual analysis of growth curve of Salmonella Infantis ŽM9. Reprinted from Sterniša et al. (2022a) with permission from Elsevier 5472521010052.
Quantitative evaluation of antimicrobial activity
Use only measurements obtained from 0 to 10 h of incubation.
Prepare data in Excel.
Name the first column as time. The name of other columns should follow the 96-well microtiter plate well marks, i.e. A1, A2, A3, etc.
Insert hours of measurements in the first column and measured absorbances at 600 nm in the corresponding well.
Use a dot to specify a decimal number and save data as tab-delimited .txt file.
Use R package Growthcurver (Sprouffske and Wagner, 2016).
Install Growthcurver package (https://github.com/sprouffske/growthcurver).
Upload data and do analysis as described (https://cran.r-project.org/web/packages/growthcurver/vignettes/Growthcurver-vignette.html).
Compare the obtained parameters of the growth curves of Salmonella exposed to the extracts to the control (growth without the extract). This includes the growth rate, the doubling time, and the carrying capacity.
Quantitative evaluation of anti-biofilm activity
Evaluate difference in endpoint measured absorbances at 600 nm.
Use only endpoint measured absorbances at 600 nm after 20 h of incubation.
Calculate average and standard deviation from all replicas for each extract.
Calculate the percent difference compared to the positive control.
Prediction of antibacterial and anti-biofilm activity from the measurements using Orange
Data formatting:
Export data to Excel.
Calculate differences between neighboring OD600 measurements (first derivative) by subtracting the measurement at time 0 from the measurement at time 1, the measurement at time 1 from the measurement at time 2, and so on until the last measurement for each sample. Please check also the provided template for data formatting.
If necessary, transpose the table in a way that the name of the compound and measurements of OD600 are listed in columns and the time points are listed in the first row, as shown in Table 1.
Table 1. Example of data formatting for prediction of antibacterial or anti-biofilm activity. Data should be formatted in a table where a list of the active compound names and corresponding measurements are presented in columns [first column: name of your sample, second column: value, measurements, and below the calculation of differences (first derivatives), third column: absorbance measurement for each compound for each cycle and calculated first derivatives].
Active compound name Value 0 1 2 3 4 5 my sample 1 technical replicate 1 measurement 0.147 0.153 0.155 0.157 0.170 0.178 my sample 1 first derivative 0.006 0.002 0.002 0.013 0.008
Use provided Orange workflow and model training dataset to predict antibacterial or anti-biofilm activity.
Download and install Orange (https://orangedatamining.com/download/#windows), a free tool for machine learning and data visualization. Data analysis in Orange is done by creating custom workflows; for the purpose of antibacterial or anti-biofilm activity prediction, we provide a workflow and model training data. You will only need to provide your data (measurements) formatted as described above. If you are interested in learning more about the usage of Orange, a wealth of short trainings is provided through Orange Data Mining You Tube channel.
Open the Orange. By clicking open, load the provided workflow named “your_compound_biofilm_activity_prediction_against_Salmonella_revised.ows.” The workflow starts with cross-validation to score learners for prediction of antibacterial or anti-biofilm activity using the provided training set composed of experimentally verified data from Sterniša et al. (2022a). This part is given at the upper part of the workflow as “learner selection.” Each widget is accessed by double-clicking on it. The widgets in the workflow are named as written in this protocol.
Double-click on the first widget on the left called “training_set” and load the provided data in the file “Salmonella_transposed_training_set_Sternisa_et_al_2022.” The type and role of data should be assigned as follows (Table 2):
Table 2. Type and role of data that should be assigned to provided data with known activity
Column name Type Role active compound name text meta value categorical meta antibacterial categorical feature antibiofilm categorical feature time point 1(and all other time points) numeric feature Select features and prediction target (learner selection): target feature is activity, either “antibacterial” or “antibiofilm” (target feature is the feature being predicted); meta-features are “name of the compound,” “value,” and the “activity” that is not target at the moment. You can move features by dragging them into different categories by mouse. The time points should be listed as “features.”
Select curves (learner selection): set a condition “value” “is” “first derivative.” The provided learners (random forrest, kNN, etc.) will then be tested and the widget “score and test” will gather the information on their performance. Based on the presented parameters, we will choose the best-performing learner to be used in the next part of the workflow to predict the antibacterial or anti-biofilm activity of unknown compound. With the current training set, the learner “Random Forest” performs best to predict antibacterial as well as anti-biofilm activity. By clicking on widgets “Test and Score” and “Confusion Matrix,” information on the performance of the model can be accessed.
Now, we will use the best-performing learner (Random Forest) to predict the antibacterial or anti-biofilm activity of the unknown compound (your data or provided dataset for prediction named Salmonella_test_set_for_prediction). Select features and prediction target (train for prediction): target feature is activity “antibacterial” or “antibiofilm,” this target feature has to be the same for “learner selection” and “train for prediction;” meta-features are “name of the compound,” “value,” and the “activity” that is not target at the moment. You can move features by dragging them into different categories by mouse. The measurements for each time should be listed as “features.”
Select curves (train for prediction): set a condition “value” “is” “first derivative.”
Double-click on the widget in the middle at the bottom called “unknown_active_compounds_set.” Load your formatted data as described above. The type and role of data should be assigned as follows (Table 3):
Table 3. Type and role of data that should be assigned to data with unknown activity
Column name Type Role active compound name text meta value categorical meta time point 1(and all other time points) numeric feature Select rows: set a condition “value” “is” “first derivative.” The learner “random forest” will be used to predict the antibacterial or anti-biofilm activity of the unknown compound, depending on which of the activities you chose as the target.
Check the predictions of antibacterial or anti-biofilm activity by double-clicking on the widget “Predictions.” A table will be opened and, on the left side in the column “Random Forest,” you can find the prediction results.
You may also view line plots of growth curves by clicking on the widget line plot. The line plots are associated with data tables and the results of the modeling. When you open these widgets simultaneously, you may view the growth curves selected in the table.
Notes
From approximately 50 g of fresh fruiting bodies, 5–40 mL of extract is obtained according to the protocol in section A, depending on the species.
The centrifuge tube should be compatible for use at 10,000× g, but selection depends on the volume of the extract.
Additional optional steps in extract preparation can include:
Dialysis against PBS in case small molecules should be removed (e.g., with cutoff 1 or 3.5 kDa).
Concentration by ultrafiltration or centrifugal concentrators (with concentration, small molecules are also lost).
Extracts should be filter sterilized (e.g., 0.2 μm filters) if they are to be used in bioassays with live (micro)organisms.
Aliquots of at least 300 μL should be prepared, as this is the volume needed for one Simba experiment.
Fractionation can be performed based on different properties; most often, the first type of fractionation is by size (using size exclusion or gel filtration chromatography) or charge (using ion exchange chromatography), but also hydrophobicity (using hydrophobic interaction chromatography) or affinity (using affinity chromatography) can be exploited. Size exclusion chromatography separates large protein complexes from small proteins and peptides, indicating the nature of molecules with bioactivity. The compounds elute with the peptides but may also remain in complex with larger molecules in the extracts.
When preparing extracts and their fractions, always keep your samples and the buffers you are working with chilled on ice.
If you do not use fractions the same or next day, store them in a freezer at -20 or -80 °C.
According to point E of procedure, fill the microplate as optimally as possible based on your samples. The procedure presented under point E is considered for one biological replicate.
Initial absorbances of ≥ 0.500 proved too high for analysis. In such cases, it is necessary to dilute extract under investigation in order to reduce initial absorbance.
If any of the replicates deviates significantly, exclude it from further analysis.
Define activity:
Antimicrobial effect is defined by effect on the first part of the curve—first 10 h—especially as visible prolongation of lag phase and reduced slope of log phase. Growth inhibition can also affect the second part of the curve, but this does not mean true anti-biofilm activity.
Anti-biofilm effect is defined as no effect on the first part of the curve and visible changes in the second part of the curve—from 10 to 20 h of incubation. This is visible as no secondary increase of curve due to biofilm formation compared to control.
Recipes
20× PBS
20× PBS is prepared as a 20× concentrated solution of PBS.
Dissolve the following in 800 mL of dH2O:
28.8 g of Na2HPO4 (Mw 141.96 g/mol)
160 g of NaCl (Mw 58.44 g/mol)
4.8 g of KH2PO4 (Mw 136.09 g/mol)
4 g of KCl (Mw 74.55 g/mol)
Adjust the pH to 7.4 using 10 M NaOH or HCl.
Add dH2O to 1 L.
Filter sterilize or autoclave.
Store at room temperature.
30 mM Tris-HCl, pH 7.5 with 0.4 M NaCl
Dissolve the following in 0.8 L of dH2O:
3.63 g of Tris (Mw 121.1 g/mol)
23.37 g of NaCl (Mw 58.4 g/mol)
Adjust the pH to 7.5 using HCl.
Add dH2O to 1 L.
Store at 4–8 °C.
2× TSB
2× TSB is prepared by use of double weight of TSB dehydrated culture medium in one volume.
Dissolve 60 g of TSB dehydrated culture medium in 800 mL of dH2O.
Adjust pH to 7.3 ± 0.2.
Fill to 1 L with dH2O.
Store at 4–8 °C.
Acknowledgments
This study was funded by the Slovenian Research Agency (Grant Numbers P2-0209, P4-0432, P4-0127, J3-3079, P4-0116, J4-3088, J4-4548, Z4-4551) and Innovation Fund’s project “Simultaneous detection of antimicrobial and anti-biofilm activity of active substances – development of a prototype application SIMBApp.” This protocol was derived from Sterniša et al. (2022a).
Competing interests
Patent application EP2022058548W·2022-03-31 was published WO2022207781A2·2022-10-06 (Sterniša et al., 2022b).
References
- Alves, M., Ferreira, I., Dias, J., Teixeira, V., Martins, A. and Pintado, M. (2012). A Review on Antimicrobial Activity of Mushroom (Basidiomycetes) Extracts and Isolated Compounds. Planta Med. 78(16): 1707–1718.
- Demšar, J., Curk, T., Erjavec, A., Gorup, C., Hočevar, T., Milutinovič, M., Možina, M., Polajnar, M., Toplak, M., Starič, A., et al. (2013). Orange: Data Mining Toolbox in Python. J. Mach. Learn. Res. 14(1): 2349−2353.
- Dieltjens, L., Appermans, K., Lissens, M., Lories, B., Kim, W., Van der Eycken, E. V., Foster, K. R. and Steenackers, H. P. (2020). Inhibiting bacterial cooperation is an evolutionarily robust anti-biofilm strategy. Nat. Commun. 11(1): e1038/s41467-019-13660-x.
- Eloff, J. N. (2019). Avoiding pitfalls in determining antimicrobial activity of plant extracts and publishing the results. BMC Complement. Altern. Med. 19: 106.
- Erjavec, J., Kos, J., Ravnikar, M., Dreo, T. and Sabotič, J. (2012). Proteins of higher fungi – from forest to application. Trends Biotechnol. 30(5): 259–273.
- Gómez Román, M. P., Badillo Mantilla, N., Andrés Carreño Flórez, S., De Mandal, S., Kumar Passari, A., Ruiz-Villáfan, B., Rodríguez-Sanoja, R. and Sánchez, S. (2020). Antimicrobial and Antioxidant Potential of Wild Edible Mushrooms. In: An Introduction to Mushroom.
- González, J. F., Alberts, H., Lee, J., Doolittle, L. and Gunn, J. S. (2018). Biofilm Formation Protects Salmonella from the Antibiotic Ciprofloxacin In Vitro and In Vivo in the Mouse Model of chronic Carriage. Sci. Rep. 8(1): e1038/s41598-017-18516-2.
- Klančnik, A., Piskernik, S., Jeršek, B. and Možina, S. S. (2010). Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J. Microbiol. Methods 81(2): 121–126.
- Klančnik, A., Megušar, P., Sterniša, M., Jeršek, B., Bucar, F., Smole Možina, S., Kos, J. and Sabotič, J. (2017). Aqueous Extracts of Wild Mushrooms Show Antimicrobial and Antiadhesion Activities against Bacteria and Fungi. Phytother. Res. 31(12): 1971–1976.
- Klančnik, A., Šimunović, K., Sterniša, M., Ramić, D., Smole Možina, S. and Bucar, F. (2021). Anti-adhesion activity of phytochemicals to prevent Campylobacter jejuni biofilm formation on abiotic surfaces. Phytochem. Rev. 20(1): 55–84.
- MacKenzie, K. D., Palmer, M. B., Köster, W. L. and White, A. P. (2017). Examining the Link between Biofilm Formation and the Ability of Pathogenic Salmonella Strains to Colonize Multiple Host Species. Front. Vet. Sci. 4: e00138.
- Pate, M., Mičunovič, J., Golob, M., Vestby, L. K. and Ocepek, M. (2019). Salmonella Infantis in Broiler Flocks in Slovenia: The Prevalence of Multidrug Resistant Strains with High Genetic Homogeneity and Low Biofilm-Forming Ability. Biomed Res. Int. 2019: 1–13.
- Savjani, K. T., Gajjar, A. K. and Savjani, J. K. (2012). Drug Solubility: Importance and Enhancement Techniques. ISRN Pharmaceutics 2012: 1–10.
- Sprouffske, K. and Wagner, A. (2016). Growthcurver: an R package for obtaining interpretable metrics from microbial growth curves. BMC Bioinf. 17(1): e1186/s12859-016-1016-7.
- Steenackers, H., Hermans, K., Vanderleyden, J. and De Keersmaecker, S. C. (2012). Salmonella biofilms: An overview on occurrence, structure, regulation and eradication. Food Res. Int. 45(2): 502–531.
- Sterniša, M., Sabotič, J. and Klančnik, A. (2022a). A novel approach using growth curve analysis to distinguish between antimicrobial and anti-biofilm activities against Salmonella. Int. J. Food Microbiol. 364: 109520.
- Sterniša, M., Sabotič, J. and Klančnik, A. (2022b). International patent application WO2022207781A2. Geneva, Switzerland: World Intellectual Property Organization.
- Tassinari, E., Duffy, G., Bawn, M., Burgess, C. M., McCabe, E. M., Lawlor, P. G., Gardiner, G. and Kingsley, R. A. (2019). Microevolution of antimicrobial resistance and biofilm formation of Salmonella Typhimurium during persistence on pig farms. Sci. Rep. 9(1): e1038/s41598-019-45216-w.
- Vestby, L. K., Møretrø, T., Langsrud, S., Heir, E. and Nesse, L. L. (2009). Biofilm forming abilities of Salmonellaare correlated with persistence in fish meal- and feed factories. BMC Vet. Res. 5(1): e1186/1746-6148-5-20.
Supplementary information
The following supporting information can be downloaded here:
- template_for_data_formatting.xlsx
- Figure workflow Orange
- Salmonella_transposed_training_set_Sternisa_et_al_2022.xlsx
- Salmonella_test_set_for_prediction.xlsx
- your_compound_biofilm_activity_prediction_against_Salmonella_revised.ows
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Sterniša, M., Sabotič, J., Janež, N., Curk, T. and Klančnik, A. (2023). SIMBA Method—Simultaneous Detection of Antimicrobial and Anti-biofilm Activity of New Compounds Using Salmonella Infantis. Bio-protocol 13(15): e4783. DOI: 10.21769/BioProtoc.4783.
Category
Microbiology > Antimicrobial assay > Antibacterial assay
Microbiology > Microbial biofilm
Biological Sciences > Microbiology
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









