- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Binging from Food to Alcohol: A Sequential Interaction Between Binging Behaviors in Male Wistar Rats
(*contributed equally to this work) Published: Vol 13, Iss 15, Aug 5, 2023 DOI: 10.21769/BioProtoc.4781 Views: 1345
Reviewed by: Alejandro GrauMohammed Mostafizur RahmanAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Construction of Activity-based Anorexia Mouse Models
Maria Consolata Miletta and Tamas L. Horvath
Aug 5, 2023 1772 Views

The Mouse Social Frailty Index (mSFI): A Standardized Protocol
Charles W. Collinge [...] Alessandro Bartolomucci
Apr 20, 2025 1820 Views

A Low-Stress, Long-Duration Stable Tail Vein Catheterization and Precise Drug Delivery Protocol for Awake, Freely Moving Mice
Yunshuang Ye [...] Jun Fang
Feb 5, 2026 105 Views
Abstract
The development of excessive alcohol (ethanol) and/or highly palatable food self-administration is an essential task to elucidate the neurobiological mechanisms that underlie these behaviors. Previous work has highlighted that ethanol self-administration is modulated by both the induction of aversive states (i.e., stress or frustration) and by the concurrent availability of appetitive stimuli (e.g., food). In our protocol, rats are food deprived for three days until they reach 82%–85% of their ad libitum weight. After that, rats are exposed daily for 10 days to a brief binge or control eating experience with highly sugary and palatable food (i.e., the ingestion of 11.66 and 0.97 kcal/3 min, respectively), which is followed by a two-bottle-choice test (ethanol vs. water) in their home cages for 90 min. This model induces robust binge eating, which is followed by a selective increase in ethanol self-administration. Therefore, this protocol allows to study: a) behavioral and neurobiological factors related to binge eating, b) different stages of alcohol use, and c) interactions between the latter and other addictive-like behaviors, like binge eating.
Keywords: AlcoholBackground
Animal models of binge drinking (BD) are crucial for clarifying the neurobiological and psychobiological mechanisms underlying this harmful behavior [see Jeanblanc et al. (2019) for a review]. Considerable advances have been made by forcibly administering ethanol (Becker and Lopez, 2016), employing operant paradigms (Simms et al., 2010), developing strains with a genetic predisposition to ethanol (Colombo et al., 2014), or using self-administration models in Wistar rats where ethanol is presented in an intermittent and temporally restricted fashion (Salguero et al, 2020; Ruiz-Leyva et al, 2020). These animal models, however, do not always mimic the contextual variables surrounding human drinking behaviors or fail to achieve pharmacologically relevant blood ethanol concentrations within the proposed time frame for this type of consumption. Moreover, alcohol use is driven by several factors. For instance, it has been observed that the induction of aversive states in conjunction with ethanol availability generally leads to an increase in ethanol consumption and favors the development of psychiatric diseases—including alcohol use disorders (AUD)—via epigenetic changes [for a review, see Pucci et al. (2019)]. In addition, the induction of frustration and anxiety (Díaz-Morán et al., 2013a and 2013b; Sabariego et al, 2013; Kawasaki et al., 2017; Jiménez-García et al., 2019) is associated with increases in ethanol consumption as a coping strategy (Ramirez-Castillo et al., 2019). Paradoxically, exposure to appetitive stimuli (i.e., food) also enhances ethanol consumption. Seminal studies showed that concurrent access to food and ethanol enhanced drinking behaviors in animals (Mello and Mendelson, 1971; Meisch and Thompson, 1972). In this respect, both substance use disorders (including AUD) and BD appear to be comorbid (or at least closely related) with binge eating (BE) (Ferriter and Ray; 2011; Munn-Chernoff et al., 2020). Conceptually, BE involves ingestion of large amounts of palatable (sugary and/or fatty) food in a short period of time, associated with a feeling of loss of control [see Dingemans et al. (2017) for a review].
The present protocol aims to serve as a guide for researchers interested in the study of the interaction of binge-like behaviors and should help replicability efforts. It is also an improvement over the models described above. An exhaustive description of the novel animal model of BE-BD interaction originally developed by Ruiz-Leyva et al. (2022) is presented. Here, the self-administration model induced remarkably high levels of absolute ethanol consumption (approximately 5 g/kg/90 min) and ethanol preference (80%–90%), much greater than those reported by other preclinical models. For instance, rats exposed to intermittent access to 20% ethanol in a two-bottle-choice procedure achieved 9–10 g/kg, yet they did that in a 24 h period, whereas those tested in the drinking-in-the-dark-multiple-scheduled access rarely exceed 5–6.5 g/kg/day. In this model, food-deprived rats are briefly exposed to a measurable amount of highly palatable sugary pellets immediately followed by a two-bottle-choice test (ethanol vs. water). Here, the authors reported dramatic and specific increases in ethanol intake as the BE behavior develops, leading to pharmacologically relevant blood ethanol concentrations. In addition, ethanol consumption turned compulsive by the last sessions and sensitive to naltrexone administration.
Materials and reagents
Anti-drip bottles with capacity of 150 mL (Classic Drinker de Luxe; Zooplus, Munich, Germany)
Filtered and autoclaved tap water
Rodent Dustless precision pellets (DPPs) (45 mg each, nutritional profile: 59.1% carbohydrate, 18.7% protein, 5.6% fat, 3.6 kcal/g) (Bio-serv, catalog number: F0021). Shelf life: 12 months. Store in a dry and ventilated place. The container should be tightly closed and protected from moisture
Ethyl alcohol (ethanol 96%, v/v; CH3CH2OH; 46.07 g/mol) (PanReac AppliChem, catalog number: 141085). This substance is highly flammable (liquid or vapor) and causes severe eye irritation. Store in a cool, ventilated place away from heat sources such as hot surfaces
Animals: adult male Wistar HAN rats (Envigo Laboratories, Barcelona, Spain), aged 70–80 days and weighing 240 g (± 37) at the beginning of the procedure
Equipment
Rectangular polycarbonate cages (42.5 cm × 26.5 cm × 15 cm) (Figure 1A)
Timer and chronometer (Digital Onstart 100) (Figure 1B)
Small plastic cups for depositing the DPPs
Weighing scale (WLC 1/A2 Precision Balance; Radwag©) (Figure 1C)
Plastic syringes (15 mL) and crystal flasks (600 mL) for diluting ethanol solutions (Figure 1D)
Personal protective equipment (laboratory coat, gloves, masks, etc.)
Figure 1. Equipment. A. Rectangular polycarbonate cage with grid. B. Timer and chronometer. C. Weighing. D. Syringe and flasks.
The alcohol concentrations of 6% or 10% were achieved by pouring 470 (or 450) mL of filtered and autoclaved tap water into 500 mL flasks and then adding 30 (or 50) mL of alcohol with the plastic syringe. Next, the solution was gently stirred to ensure proper dissolution of the alcohol.
Procedure
Stabling conditions, habituation, and general considerations
Stabling
Individualize each animal in polycarbonate standard cages with a bed of sawdust and free access to water and standard chow (i.e., ad libitum conditions). Use anti-drip bottles filled with water from the beginning in order to habituate the animals to them. Moreover, put the bottles in the middle of the cage’s grid to prevent side preferences. Finally, make sure that the animals do not have environmental enrichment in their home cages such as cardboard shavings, cotton strips, or plastic tubes.
Habituation
Once in the stabling module or room, habituate the animals to the new environment for at least five days before starting the procedures. During this period, handle each animal gently and briefly for 3–5 days and preferably by the same person and/or regular experimenter(s). Furthermore, carry out each manipulation at approximately the same time every day. In this respect, each procedure in our protocol is always done during the light cycle and, more specifically, between 9:30 a.m. and 14:00 p.m., so that both handling and weighing should begin between 9:30 a.m. and 10:30 a.m.
Environmental conditions
Monitor environmental conditions throughout the experiment. In this particular case, animals were kept under a 12:12 h light/dark cycle (lights on at 6:00 a.m.) in a room with constant temperature (21 °C) and humidity (50%–60%).
General and methodological considerations
When experimenting with animal models, always try to reduce the number of animals used and minimize suffering. To do that, we recommend the following:
Experiments should always be carried out by qualified personnel with experience in this type of paradigm.
All manipulations must be carried out at the same time every day and preferably by the same experimenter(s).
Animals should be matched by weight and randomly assigned to the different experimental conditions.
Design the experiments in batches of animals, especially when it has not been implemented before. This way, experimenters can become familiar with the protocol, and both the data and experimental conditions are gradually completed.
Taking these principles into account, also make sure that you have enough statistical power when reporting effects and making inferences.
Induction of the interaction between BE-BD behaviors
Food deprivation
Once the animals are completely habituated to the stabling room as well as to the experimenter(s), the food deprivation process can begin (see Figure 2, sessions 1–3) by weighing and labeling each rat.
On this day, register the initial weight of each animal and remove all food from the cage. After that, return the animals to the stabling room and leave them there until the day of habituation.
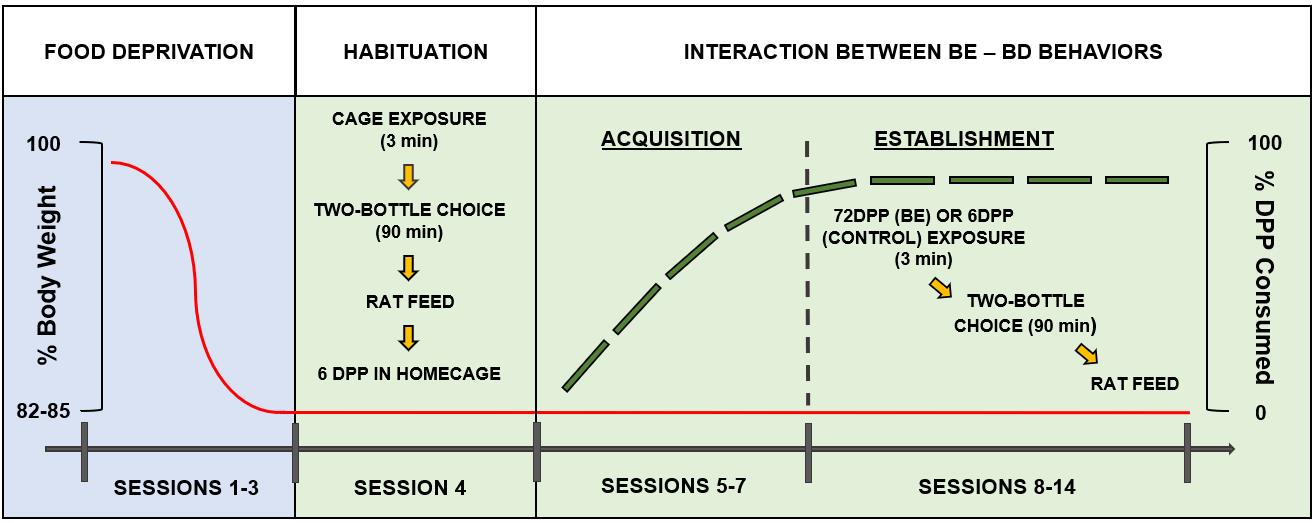
Figure 2. Schematic representation of the experimental procedure. Sessions 1–3 comprise the food deprivation period. Session 4 corresponds to the habituation day. Sessions 5–7 refer to the binge eating (BE)-like behavior acquisition period, while sessions 8–14 cover the BE-binge drinking (BD) interaction establishment period. The solid line represents body weight scores, while the long dash line represents the Dustless precision pellets (DPP) consumption (%) exhibited by the rats all along the experimental timeline. The dotted line marks the beginning of the BE-like DPP consumption (i.e., the ingestion of ≥ 80% of the pellets). Extracted and modified from Ruiz-Leyva et al. (2022) with permission from the authors.
Additional tips for the deprivation procedure
In this section you will find some recommendations that will help to improve the quality of this procedure.
Note that the food deprivation procedure allows rats to acclimatize to the experimental room so that there will be no additional sources of stress on the day of habituation (i.e., session 4).
In adult Wistar rats aged 70–80 days (10–11 weeks), it takes approximately three days of complete deprivation to reach 82%–85% of the ad libitum body weight.
During this time (i.e., sessions 1–3), check the health status of rats daily in order to detect some signs of distress such as piloerection, body dirt, or dehydration. In such case, provide five additional grams of food.
As always, try to carry out this procedure at the same time every day.
Considerations prior to the habituation day
In this section, we provide relevant information to optimize time during the habituation day.
The day before of the habituation day (i.e., session 3), clean and arrange as many empty cages (i.e., without sawdust) with grid (hereinafter referred to as BE cages) as rats that can be assessed simultaneously. These are similar to home cages (see Equipment).
Dispose and arrange the essential material for preparing ethanol solutions (i.e., ethanol 96% v/v, syringes, flasks, weighing scale…). Also, store as many bottles of water as needed to fill every anti-drip bottle that will be used in the subsequent two-bottle-choice test.
Habituation day
On the habituation day (i.e., session 4, see Figure 2), prepare fresh ethanol solutions and fill each pair of anti-drip bottles with a given concentration of ethanol or water, as appropriate. Do not forget to attach a label to each pair of bottles with the corresponding rat identifier.
Once the bottles are prepared, weigh each pair of the anti-drip bottles (do it daily), remove every anti-drip daily water bottle from the home cages, and weigh each rat. At this point, check whether every rat is on the fixed weight interval (82%–85%).
Now, arrange the BE cages in a way that: (1) there is space to place a rat cage in between and (2) each BE cage corresponds to a specific rat or rats if using multiple batches (this must remain constant throughout the experiment). Once so disposed, register the time, prepare the stopwatch, and begin the timer as soon as the first rat touches the floor of their corresponding BE cage. After placing each rat, close the cage’s grid and wait for 3 min. After this, remove the rats from their BE cages, put them back into their home cage, and return them to their corresponding position in the racks. If there is urination or defecation in the box, it should be wiped with a 70% alcohol wipe.
Finally, the two-bottle-choice test will take place. To do that, carefully put each pair of anti-drip bottles on each side of the corresponding home cage grid (i.e., ethanol left and water right, or vice-versa) (see Figure 3A and 3B).

Figure 3. Cage and bottle arrangement in the two-bottle-choice test. A. Upper frontal vision. B. Lateral frontal vision. C. Positioning of Dustless precision pellets (DPPs) and rats during the binge eating (BE) episode.After 90 min, carefully remove each pair of anti-drip bottles. Then, put back the anti-drip daily water bottles in the center of the cage’s grid. Now, weigh again each pair of anti-drip bottles and subtract the loss value of the corresponding ghost bottle (see the section below) from the difference in weights. Finally, feed each rat with the approximate amount of normal chow that it needs to maintain the desired range of body weight and deliver six DPPs to each, in order to avoid neophobia in the following sessions.
Adjustment of the drip loss over the bottle weight difference
The positioning of the bottles during the 90 min two-bottle-choice test (see Figure 3A and 3B) may lead to some very slight losses of contents, which could affect the actual difference in weight (i.e., bottle weight before and after the test). To control this, weigh two extra anti-drip bottles (one filled with the desired ethanol concentration and the other one with water) and place them for 90 min in the grid of an empty rat cage (see Equipment). The positioning of the bottles should be similar to Figure 3.
As a note, these bottles that are meant to control for leakage or spillage are often referred to as ghost bottles.
Desired body weight range considerations and food adjustments
At this point, it is necessary to advise that these considerations depend on the age, sex, strain, and even the supplier, so they must be taken with caution and adjusted for each situation.
It is possible that, at the first weighing after the end of the three days of food deprivation, experimenters may find that not all rats are in the expected range (i.e., 82%–85% of their ad libitum weights). This is perfectly plausible, since the proposed deprivation interval (i.e., three days) for adult Wistar rats of this age and weight is only approximate and is based on previous evidence obtained in our laboratory. Thus, although it is not strictly necessary for all rats to be in this weight range at this moment, it may be relevant when adjusting the amount of food to be administered to each rat at the end of the session (see the section below).
Weight maintenance
Adult Wistar rats of these characteristics (see Materials and reagents) require approximately 15–20 g (±1.5 g) of normal chow to maintain body weight. However, as mentioned above, this may vary depending on two variables:
Current body weight:
• If rats are within the required weight (82%–85%), take the standard feed value (15–20 ± 1.5 g) and adjust the quantity according to the eating condition (binge or control).
• If rats are above the required weight range, reduce* the standard feeding value according to the grams that fall outside the desired range (i.e., upper limit or 85%) and according to the eating condition.
• If rats are below the required weight, increase* the standard value of the feed according to the grams that deviate from the desired range (i.e., lower limit) and according to the intake condition.
An example of weight maintenance is presented below (Table 1).
Table 1. Example of weight maintenance
Initial weight (g) 82% weight (g) Maintenance food (g) control/binge 350 287 16 ± 1.5 g/14 ± 1.5 g 300 246 14 ± 1.5 g/11 ± 1.5 g 280 229 13 ± 1.5 g/10 ± 1.5 g Eating condition:
Take into account (specially from session 5 onward) that the rats have a differential amount of DPPs depending on the intake group to which they belong (i.e., 72 DPPs for binge and 6 DPPs for control) and will therefore be able to ingest a certain maximum amount of calories during the binge episode (i.e., 11.66 kcal/3 min for the binge group and 0.97 kcal/3 min for the control group)*.
*Note: Only six DPPs are available at the end of the session (delivered to their home cages), but the BE cages remain empty. In this sense, this variable has to be considered, particularly during the rest of the sessions, where they are already exposed to the binge context with their corresponding DPPs.
Prevent side preferences and control for potential order effects
When carrying out the two-bottle-choice test, remember to change the position of the anti-drip bottles every day. Thus, if on the habituation day (i.e., session 4) the ethanol bottle is on the right and the water bottle on the left, the next day (i.e., session 5) the position should be reversed and so on until the end of the procedure.
If you use batches of animals in each BE session, keep in mind that you must counterbalance the order of the batches across the sessions. Let us consider an example in which 20 animals are tested in four batches (1, 2, 3, 4) of five animals. On even days, the first batch would be run first, followed by batches 2–4. However, on odd days, batch four would be run first and batch one would close the experimental day.
Acquisition of BE-like behavior as well as the interaction between BE-BD behaviors
This phase begins 24 h after the habituation session and should last 10 consecutive days (see Figure 2, sessions 5–7 and sessions 8–14). It is similar to the habituation session, yet it features some crucial manipulations.
Once all bottles and rats are all weighed, arrange the BE cages as done in the habituation. Next, place the labeled plastic cups containing different amounts of DPPs (i.e., 72 or 6 DPPs for binge or control eating condition, respectively) next to the BE cages in a way that each cage corresponds to one (or more) cups, depending on whether the experiment is being done in batches or all at once. Now, move each rat to its corresponding BE cage and place the DPPs (without the cup) just in front of the wall furthest from where the rat is left and stacked in the middle (see Figure 3C).
Close each cage’s grid as you place each rat. During the 3 min of the BE episode, please ensure that the rats are not interrupted in their ongoing behavior and keep environmental noise to a minimum.
After 3 min, put each rat back in their home cage and put each pair of anti-drip bottles into their corresponding home cage (do not forget to change daily the position of the pairs in order to prevent side preferences) for 90 min.
At the end of the test, sequentially remove each pair of anti-drip bottles and replace them for the anti-drip daily water bottles. Then, weigh again the bottles and apply the correction to the difference of weights. Finally, feed each animal with its corresponding amount of standard chow.
Current body weight status
Be sure to check whether the rats are within the required weight range (and if possible, close to 82%). This is particularly relevant at this point, as it may determine, in part, how quickly the BE behavior develops.
Collect relevant data concerning the progression of the BE behavior
When the rats have gone through the binge episode, collect the remaining DPPs of each particular cage and put them back into their corresponding and labeled plastic cup. Once you have collected them all, weigh the remaining DPPs of each cup to calculate the number of DPPs ingested (i), the % of DPPs ingested over total (ii), and the kcal/3 min for each rat (iii).
DPP ingested: You can calculate the number of DPPs consumed based on the remaining weight. Given that the maximum weight for the binge group is 3.24 g (i.e., 72 DPPs) and for the control group is 0.27 (i.e., 6 DPPs), you only have to subtract the remaining weight in the cup.
For example: if a rat has given up 1.80 g of DPPs during the binge eating episode and the maximum possible is 3.24 g, equal to 72 DPPs, then: (1.80 × 72)/3.24 = 40. This tells us how many DPPs it has given up without consuming, so: 72 - 40 = 32.
*To apply this example to the control intake group, just modify the value of the maximum weight of DPPs to be consumed (0.27 g instead of 3.24 g) and the maximum number of pellets (from 72 DPPs to 6 DPPs)
% DPPs consumed: To get the ratio or percentage of DPPs consumed, you simply divide the number of DPPs ingested by the total (i.e., 72 DPPs for the binge group) and multiply this value by 100.
Based on the previous example, if the animal has consumed 32 DPPs and the total is 72, then: (32/72) × 100 = 44.4%.
Kcal ingested in 3 min: multiply the number of DPPs consumed by the caloric value of each DPP (0.162 kcal). Returning to the initial example, if the animal consumes 32 DPPs, then: 32 × 0.162 = 5.18 kcal/3 min.
Data analysis
Exclusion and inclusion criteria
Binge eating behavior progression:
As mentioned in Ruiz-Leyva et al. (2022), out of a total of 142 adult male Wistar rats used for the experiments, seven were eliminated from the subsequent analyses because they did not acquire the BE behavior (i.e., they completely avoided the DPPs). In this regard (and at least when using males), make sure that rats exhibit the BE behavior from day 4 (i.e., session 8) onwards (i.e., ingestion of ≥ 80% DPPs) or at least show signs that DPP intake is significantly increasing and consistently approaching this percentage.
Preliminary, unpublished data from our laboratory indicate that this is an achievable task for adult male rats, but ingestion must be performed rapidly and without interruption. Furthermore, we have observed that male rats show an average intake of 80% of the available DPP even when adulterated with a bitter flavor (quinine, see Figure 4).
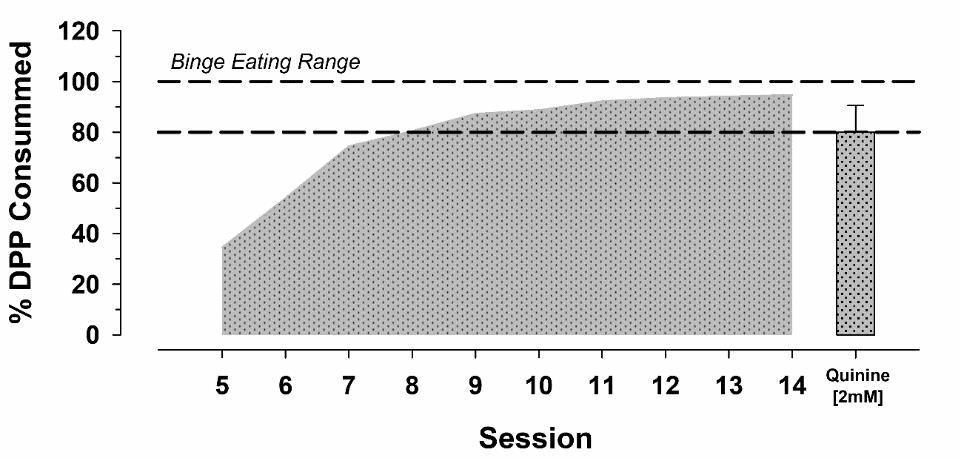
Figure 4. Progression in the consumption of palatable sugar pellets [i.e., Dustless precision pellets (DPPs)] of adult male Wistar rats assigned to the binge eating condition (i.e., ingestion of 72 DPP or 11.66 kcal/3 min). Percent of DPP intake (% DPPs consumed over total) across sessions of the values obtained in 7–9 animals. Dashed lines mark the percentage of DPP consumption in the binge eating range model.Ethanol consumption
Researchers should check the ethanol consumption exhibited by each rat even when they exhibit binge eating behaviors towards sugary pellets. This is due to the existence of rats that completely avoid alcohol consumption and the fact that binge eating is motivated, in part, by calorie restriction. To this end, use statistical criteria for detecting potential outliers. Finally, note that opposite the big amount of ethanol consumed, no taste aversion is development to the pellets (Gallo et al., 1999).
Data calculation
Calculation of the main dependent variable data
There are three dependent variables whose units you can (and should) obtain from the habituation day (i.e., session 4). These are (1) the weight-adjusted water consumption (grams per kilogram of body weight), (2) the weight-adjusted net ethanol consumption (grams of ethanol per kilogram of body weight), and (3) the percentage preference of ethanol over water.
First of all, obtain the difference in weights of the same bottle before (i.e., first weight or PRE) and after the two-bottle-choice test (i.e., second weight or POST) within the same session. To this difference, apply the correction for possible fluid loss.
Water consumption:
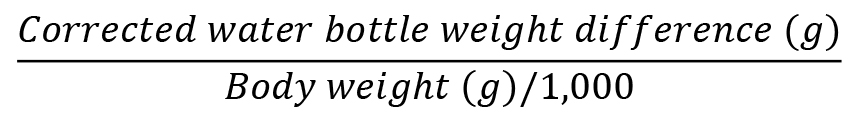
Ethanol intake:
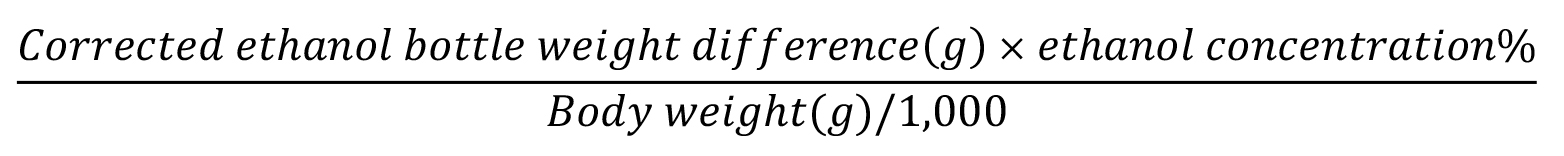
See Figure 5 for a complete overview of the results.
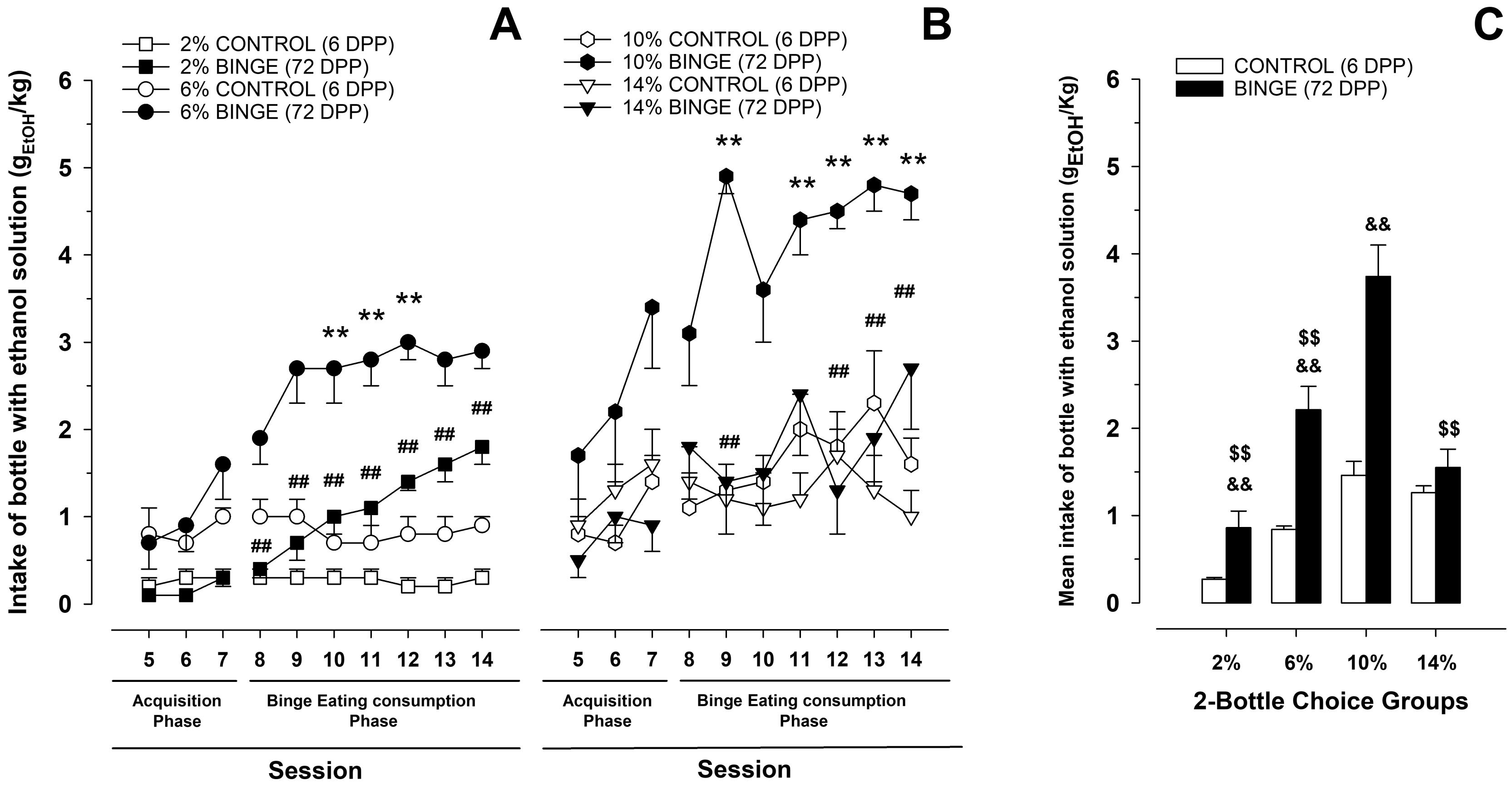
Figure 5. Ethanol intake exhibited by male Wistar rats as a function of ethanol concentration (0%, 2%, 6%, 10%, or 14% w/w), eating condition [binge or control Dustless precision pellets (DPP) exposure], and session. (A) and (B) represent ethanol consumption (net gEtOH/kg) in the acquisition phase (i.e., sessions 5–7) and in the establishment phase (i.e., sessions 8–14), respectively. (C) Depicts the same data as (A) and (B) yet collapsed across sessions. Each point or bar and vertical line represent the mean ± SE obtained in 7–12 animals per group. (A and B) Statistically significant differences between the values obtained in binge and control groups: *p < 0.05, **p < 0.01; and between the values obtained in binge groups compared with 10% ethanol concentration: #p < 0.05, ##p < 0.01. (C) Statistically significant differences between the values obtained in binge and control groups in each ethanol concentration: &&p < 0.01; and between the values obtained in binge groups compared with 10% ethanol concentration: $$p < 0.01. Extracted with permission from Ruiz-Leyva et al. (2022).Preference scores for ethanol over water:
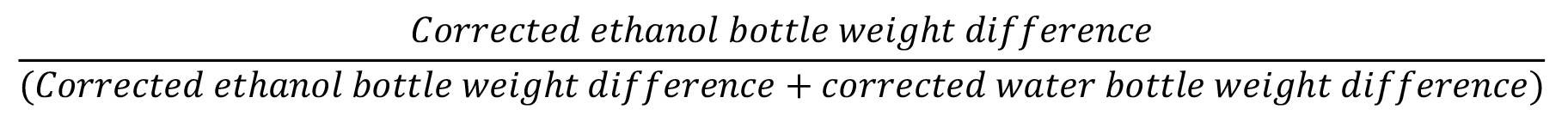
Notes
Up to this point, we have described in detail every aspect of the model and the protocol that has been implemented in our laboratory and that has given us the best convergence of results to date. However, there are certain factors that need to be clarified to improve the reproducibility of the model.
Age and sex
In the published article, all male rats used were young adults (i.e., PD70-80). In the case of using rats in other age range or female rats, it may be necessary to adapt the procedure. Of course, if after day 3 of habituation the animal is below 82%, the amount of food can be increased by 5 g to increase the animal’s weight by 2 g.
Strain differences
To date, we have only used Wistar HAN rats. Despite this, it would be interesting to test the model in other strains or in different intraspecies phenotypes that are more or less vulnerable to relevant variables such as stress, or to psychoactive effects of drugs such as sugar or alcohol.
Ethanol concentration
As shown in Figure 5, ethanol intake exhibited by males is concentration dependent, with 6% and 10% (w/w) being the most consumed.
Food composition
Knowing the macro- and micronutrient composition of the food used to induce binge eating behavior is essential to determine which elements may contribute to it and interact with alcohol and/or its effects. The palatable pellets (i.e., DPPs) used here have a high carbohydrate value (i.e., 59.1%) and sugary taste. However, it would be interesting to try other types of foods (e.g., high fat or mixed) or to try artificial sweeteners (e.g., saccharin) with a much lower caloric value. In this way, the mechanisms underlying the interaction between these addictive behaviors and between the substances involved could be understood in more detail.
Acknowledgments
This paper was partially supported by the Spanish Ministry of Health (Government Delegation for the National Plan on Drugs: PNSD 2020-049), the Junta de Andalucía (grants CTS109 and HUM784), and the University of Granada (PP2022.PP-16). The work was also supported by PICT-2019- 00180 and PICT-2018-00597 of FONCyT-Argentina. This protocol and figures are extracted and/or modified from our previous work (Ruiz-Leyva et al., 2022).
Competing interests
The authors declare no conflicts of interest or competing interests.
Data availability statement
The data from the published paper are available from the corresponding author (see Ruiz-Leyva et al., 2022) upon reasonable request.
Ethics considerations
Animals were maintained according to the EU Directive 2010/63/EU for animal experiments. The experimental protocol was approved by the University of Granada Research Ethics Committee (Protocol Number 09/08/2019/138).
References
- Becker, H. and Lopez, M. (2016). An Animal Model of Alcohol Dependence to Screen Medications for Treating Alcoholism. Int. Rev. Neurobiol. 126: 157–177.
- Colombo, G., Maccioni, P., Acciaro, C., Lobina, C., Loi, B., Zaru, A., Carai, M. A. and Gessa, G. L. (2014). Binge drinking in alcohol-preferring sP rats at the end of the nocturnal period. Alcohol 48(3): 301–311.
- Díaz-Morán, S., Palència, M., Mont-Cardona, C., Cañete, T., Blázquez, G., Martínez-Membrives, E., López-Aumatell, R., Sabariego, M., Donaire, R., Morón, I., et al. (2013a). Gene expression in amygdala as a function of differential trait anxiety levels in genetically heterogeneous NIH-HS rats. Behav. Brain Res. 252: 422–431.
- Díaz-Morán, S., Palència, M., Mont-Cardona, C., Cañete, T., Blázquez, G., Martínez-Membrives, E., López-Aumatell, R., Sabariego, M., Donaire, R., Morón, I., et al. (2013b). Gene expression in hippocampus as a function of differential trait anxiety levels in genetically heterogeneous NIH-HS rats. Behav. Brain Res. 257: 129–139.
- Dingemans, A., Danner, U. and Parks, M. (2017). Emotion Regulation in Binge Eating Disorder: A Review. Nutrients 9(11): 1274.
- Gallo, M., Ballesteros, M., Molero, A. and Morón, I. (1999). Taste Aversion Learning as a Tool for the Study of Hippocampal and Non-Hippocampal Brain Memory Circuits Regulating Diet Selection. Nutr. Neurosci. 2(5): 277–302.
- Ferriter, C. and Ray, L. A. (2011). Binge eating and binge drinking: An integrative review. Eating Behaviors 12(2): 99–107.
- Jeanblanc, J., Rolland, B., Gierski, F., Martinetti, M. P. and Naassila, M. (2019). Animal models of binge drinking, current challenges to improve face validity. Neurosci. Biobehav. Rev. 106: 112–121.
- Jiménez-García, A., Ruiz-Leyva, L., Vázquez-Ágredos, A., Torres, C., Papini, M., Cendán, C. and Morón, I. (2019). Consummatory Successive Negative Contrast in Rats. Bio Protoc 9(7): e3201.
- Kawasaki, K., Annicchiarico, I., Glueck, A. C., Morón, I. and Papini, M. R. (2017). Reward loss and the basolateral amygdala: A function in reward comparisons. Behav. Brain Res. 331: 205–213.
- Meisch, R. A. and Thompson, T. (1972). Ethanol intake during schedule-induced polydipsia. Physiol. Behav. 8(3): 471–475.
- Mello, N. K. and Mendelson, J. H. (1971). Evaluation of a polydipsia technique to induce alcohol consumption in monkeys. Physiol. Behav. 7(6): 827–836.
- Munn-Chernoff, M. A., Johnson, E. C., Chou, Y., Coleman, J. R., Thornton, L. M., Walters, R. K., Yilmaz, Z., Baker, J. H., Hübel, C., Gordon, S., et al. (2020). Shared genetic risk between eating disorder‐ and substance‐use‐related phenotypes: Evidence from genome‐wide association studies. Addict. Biol. 26(1): e12880.
- Pucci, M., Micioni Di Bonaventura, M. V., Wille-Bille, A., Fernández, M. S., Maccarrone, M., Pautassi, R. M., Cifani, C. and D’Addario, C. (2019). Environmental stressors and alcoholism development: Focus on molecular targets and their epigenetic regulation. Neurosci. Biobehav. Rev. 106: 165–181.
- Ramirez-Castillo, D., Garcia-Roda, C., Guell, F., Fernandez-Montalvo, J., Bernacer, J. and Morón, I. (2019). Frustration Tolerance and Personality Traits in Patients with Substance Use Disorders. Frontiers in Psychiatry 10:e00421.
- Ruiz-Leyva, L., Salguero, A., Morón, I., Portillo-Salido, E., Cendán, C. M. and Pautassi, R. M. (2020). Sigma-1 antagonism inhibits binge ethanol drinking at adolescence. Drug Alcohol Depend. 215: 108214.
- Ruiz-Leyva, L., Vázquez‐Ágredos, A., Jiménez‐García, A. M., López‐Guarnido, O., Pla, A., Pautassi, R. M., Morón Henche, I. and Cendán, C. M. (2022). From binge eating to binge drinking: A new and robust paradigm for assessing binge ethanol self‐administration in male rats. Addict. Biol. 27(2): e13153.
- Sabariego, M., Morón, I., Gómez, M. J., Donaire, R., Tobeña, A., Fernández-Teruel, A., Martínez-Conejero, J. A., Esteban, F. J. and Torres, C. (2013). Incentive loss and hippocampal gene expression in inbred Roman high- (RHA-I) and Roman low- (RLA-I) avoidance rats. Behav. Brain Res. 257: 62–70.
- Salguero, A., Suarez, A., Luque, M., Ruiz-Leyva, L., Cendán, C. M., Morón, I. and Pautassi, R. M. (2020). Binge-Like, Naloxone-Sensitive, Voluntary Ethanol Intake at Adolescence Is Greater Than at Adulthood, but Does Not Exacerbate Subsequent Two-Bottle Choice Drinking. Front. Behav. Neurosci. 14: e00050.
- Simms, J. A., Bito-Onon, J. J., Chatterjee, S. and Bartlett, S. E. (2010). Long-Evans Rats Acquire Operant Self-Administration of 20% Ethanol Without Sucrose Fading. Neuropsychopharmacology 35(7): 1453–1463.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Cuesta-Martínez, S., Ruiz-Leyva, L., Jiménez-García, A. M., Aparicio-Mescua, T., López-Guarnido, O., Pautassi, R. M., Morón, I. and Cendán, C. M. (2023). Binging from Food to Alcohol: A Sequential Interaction Between Binging Behaviors in Male Wistar Rats. Bio-protocol 13(15): e4781. DOI: 10.21769/BioProtoc.4781.
Category
Neuroscience > Behavioral neuroscience > Animal model
Neuroscience > Behavioral neuroscience > Addiction
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








