- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Cuticular Hydrocarbon Profiling by Fractionation and GC-MS in Socially Parasitic Ants
Published: Vol 13, Iss 13, Jul 5, 2023 DOI: 10.21769/BioProtoc.4772 Views: 1279
Reviewed by: Anonymous reviewer(s)
Abstract
Ants use cuticular hydrocarbon (CHC) as a semiochemical for recognizing their nestmates. For socially parasitic ants, deceiving the CHC is an important survival strategy. Profiling and quantifying CHC is a potent approach to understanding such nestmate discrimination behavior. Thus, a highly efficient, stable, and reproducible extraction method for CHC is essential for this purpose. This paper describes a method for socially parasitic ants to disguise the host species’ CHC profile under laboratory conditions, as well as the extraction and measurement of CHC from ants (from a previous study). First, the artificial isotopic substance is applied to the host worker; then, the socially parasitic ant disguises the host-like CHC profile against the above host worker. Next, the CHC is extracted and fractionated from a socially parasitic ant using hexane and silica gel. After concentrating the fractionated product, this product is then used for measurement by gas chromatographymass spectrometry (GC-MS). The CHC extraction protocol described in this paper may be used for various ant species.
Keywords: Cuticular hydrocarbon (CHC)Background
Cuticular hydrocarbon (CHC) is the generic term for dozens of hydrocarbons on the surface of insect cuticles. CHC protects insects from desiccation and functions as a semiochemical (Howard and Blomquist, 2005; Sturgis and Gordon, 2012). In particular, the role of CHC in the ant social system has been studied in detail. For example, CHC is used to discriminate between nestmates and non-nestmates (Howard and Blomquist, 2005; Sturgis and Gordon, 2012) and inhibit workers’ oviposition (Howard and Blomquist, 2005; Kocher and Grozinger, 2011). Furthermore, some parasitic species take advantage of this CHC-based nestmate discrimination mechanism to invade the nests of host ants (Dettner and Liepert, 1994; Lenoir et al., 2001; Howard and Blomquist, 2005; Akino, 2008; de la Mora et al., 2020). For example, when the newly mated Polyrhachis lamellidens and Polyergus samurai queens invade a host colony, they perform rubbing behavior against the host worker or kill the host queen. These behaviors help the newly mated queen to disguise her CHC profile and match that of the host ants. By disguising the CHC profile of the host ants, the newly mated queen is recognized as a nestmate, allowing her to achieve the early stages of social parasitism (Tsuneoka and Akino, 2012; Iwai et al., 2022).
Behavioral tests and bioassays using ants, as well as profiling and quantifying CHC, are potent approaches to understanding such nestmate discrimination and chemical disguise behavior. A highly efficient, stable, and reproducible extraction method for CHC is essential for this purpose. In addition, reproducing the chemical disguise behavior of socially parasitic ants in the laboratory is necessary. In this paper, we describe a method for CHC extraction and measurement, as well as the induction of chemical disguise by a socially parasitic ant under laboratory conditions, which has been established as an actual experimental system, using P. lamellidens and its host Camponotus japonicus as examples, referring to the practical techniques used in our previous study (on Polyrhachis and Camponotus species) (Iwai et al., 2022; Kurihara et al., 2022). In addition, we describe the method for the tracing assay using artificial isotopic substances to confirm the transition of the CHC profile from the host species to a socially parasitic ant.
First, the artificial isotopic substance is applied to the host worker; then, the socially parasitic ant performs the chemical disguise against the host worker. The crude extract, containing mainly CHC and polar compounds, is obtained from an anesthetized ant with hexane. Then, CHC is separated to remove other substances using silica gel. The fractionated product is concentrated by volatilizing the solvent with N2 gas. A portion of this concentrate is then used for measurements in the gas chromatographymass spectrometry (GC-MS) system. As a result, several ant-derived CHC peaks are detected. This CHC extraction protocol is mainly for large species such as Polyrhachis and Camponotus, although it should be possible to use for small species by adjusting the number of ants used per sample. In addition, since the main targets of CHC profile disguising in socially parasitic ants are adult host workers and host queens, the use of artificial isotopic substances described in this paper should also be applicable to other species.
Materials and reagents
Autosampler vials for Agilent Technologies (Tokyo Garasu Kikai Co., Ltd., catalog number: LH520867-100)
Shimadzu LabTotal vial for GC/GC-MS (Shimadzu GLC Ltd., catalog number: 227-34002-01)
SIL target Polyspring inserts 100/pk (Shimadzu GLC Ltd., catalog number: GLC4010-S630)
Glass capillary tubes, end-to-end tip 100 mm 100 μL, 100 pieces (AS ONE Co., Ltd., catalog number: 3-5998-13)
Pyrex® 10 mm × 75 mm disposable rimless culture tubes, bulk pack (Corning Inc., catalog number: 99445-10)
9" disposable Pasteur pipettes, borosilicate glass/non-sterile, 229 mm, 144 pieces/box × 5 boxes (Fisher Scientific, catalog number: 13-678-20C)
Quartz wool 2–6 μm (10 G) (AS ONE Co., Ltd., catalog number: 6-570-01)
Hexane for chromatography 500 mL (FUJIFILM Wako Pure Chemical Co., Ltd., catalog number: 086-01166)
Wakogel® C-200 for column chromatography, 500 g (FUJIFILM Wako Pure Chemical Co., Ltd., catalog number: 237-00075)
Balance dishes (Ina-optika Co., Ltd., catalog number: AS-DM)
Kimwipe S-200 (Nippon Paper Crecia Co., Ltd., catalog number: 62011)
Parafilm 2" 250 ft (Bemis Co., catalog number: PM992)
Sterilization Petri dish (shallow type AY) (Sansei Medical Co., Ltd., catalog number: 01-003)
C7–C40 saturated alkanes, standard certified reference material, 1,000 μg/mL each component in hexane (Supelco, catalog number: 49452-U)
n-Docosane (C22H46) (FUJIFILM Wako Pure Chemical Co., Ltd., catalog number: 047-08032)
n-Triacontane-d62 (C30D62) (C/D/N Isotopes, CAS: 93952-07-9)
n-Dotriacontane-d66 (C32D66) (C/D/N Isotopes, CAS: 62369-68-0)
Plastic case (5.0 cm × 4.5 cm × 2.5 cm)
Plaster (Katei Kagaku Kogyo Co., Ltd., catalog number: 4905488024103)
Isotope-labeled triacontane and dotriacontane solutions (16 mg/mL in hexane) (see Recipes)
Internal standard material (10 ng/μL docosane in hexane) (see Recipes)
Standards of saturated alkanes (0.5, 1, 2, 5, and 10 ng/μL) (see Recipes)
Equipment
Digital scale (Shimadzu GLC Ltd., catalog number: TWC623N)
Stand (three legs) stainless steel (Yamanaka, catalog number: 1-9789-02) with clamp
Erlenmeyer flask
eVol® XR kit (SGE Analytical Science Pty., Ltd., catalog number: 2910200)
Pasteur pipette bulb, Color Dropper (Tokyo Garasu Kikai Co., Ltd., catalog number: 161-23-03-32)
Agilent 6890N (Agilent Technologies, catalog number: 6890N)
Agilent 5973 MSD (Agilent Technologies, catalog number: 5973 MSD)
Inlet liner, ultra inert, splitless, single taper, glass wool, 5/pk (Agilent Technologies, catalog number: 5190-3163)
HP-5 MS (length 30 m, diameter 0.250 mm, film thickness 0.25 μm) (Agilent Technologies, catalog number: 19091S-433)
N2 gas cylinder (Shonai Gas Co., Ltd.)
Pressure regulator for N2 gas YR-70-1 (GL Sciences, catalog number: 3-703-0010)
Silicon tube 1.5 × 2.5 1 m (AS ONE Co., Ltd., catalog number: 6-586-04)
Dry block bath test tube concentration MC1020 for 20 holes MC-1020 (AS ONE Co., Ltd., catalog number: 1-5144-01)
Mini disk rotor (BIO CRAFT Co., Ltd., catalog number: BC-710)
Software
MSD ChemStation E.02.02.1431 (Agilent Technologies)
Procedure
Application of artificial isotopic substances for the tracing assay
Note: As this protocol uses organic solvents (e.g., hexane), we do not recommend the use of plastic instruments to avoid contamination by impurities from these instruments.
Dissolve n-Triacontane-d62 and n-Dotriacontane-d66 individually in hexane (16 mg/mL). Add 100 μL of both solutions to a SIL target Polyspring inserts in the autosampler vial for Agilent Technologies. To allow the ants to breathe in the later process, drill a hole in the lid of this vial.
Stir the solution with a glass capillary tube; meanwhile, spray nitrogen gas to evenly apply the above artificial isotopic substances over the entire inner wall of the insert, using a dry block bath test tube (already installed on another stand, with silicon tube, pressure regulator for N2 gas, and N2 gas cylinder) (Figure 1).
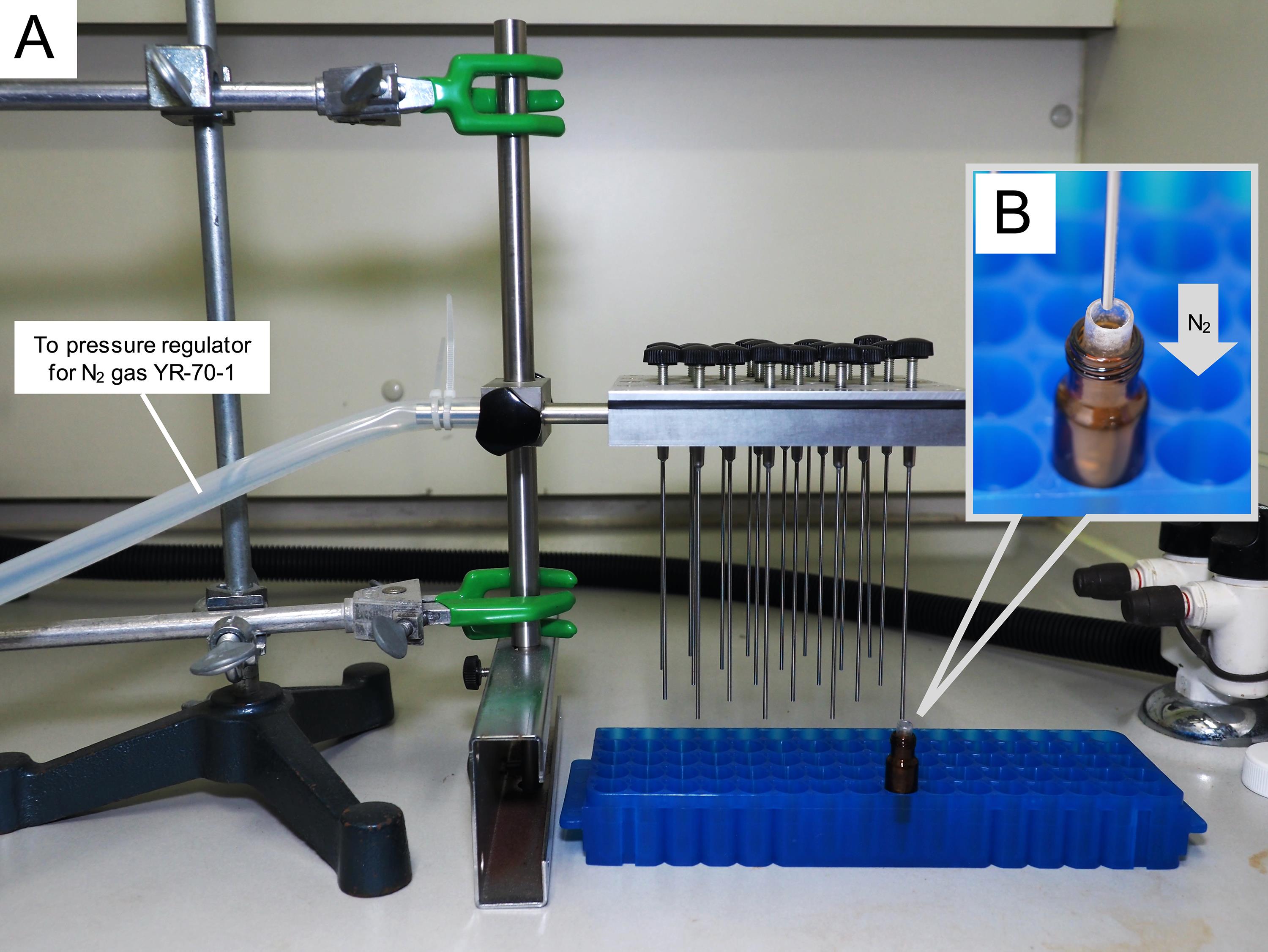
Figure 1. Evaporation system of the solvent. A. The appearance of evaporation. B. The process of spraying N2 gas.Expose a single individual live host worker (e.g., C. japonicus) in a sterilization Petri dish to 4 °C for 2 min and then to -20 °C for 3 min, for anesthetic purposes (Figure 2).
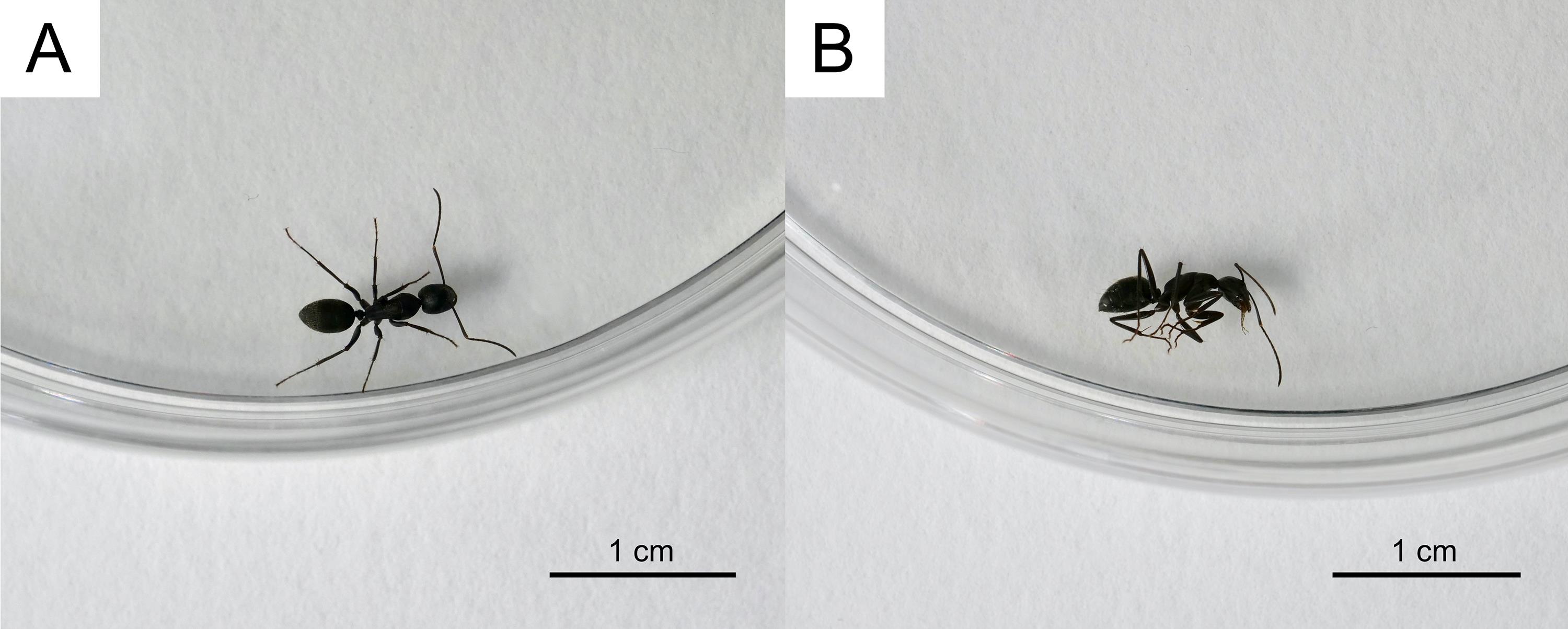
Figure 2. Anesthetizing of an ant. A. Before anesthetizing. B. After anesthetizing.After the solvent is completely volatilized, carefully lift the sterilization Petri dish containing the anesthetized host worker, taking care not to touch it directly with the hands. Move it from the head side into the entrance of the SIL target Polyspring inserts, where the artificial isotopic substances are applied to the inner wall (Figure 3). Gently shake the insert for 1 h using a mini disk rotor. If the anesthetized host worker is alive, it wakes up in this step.
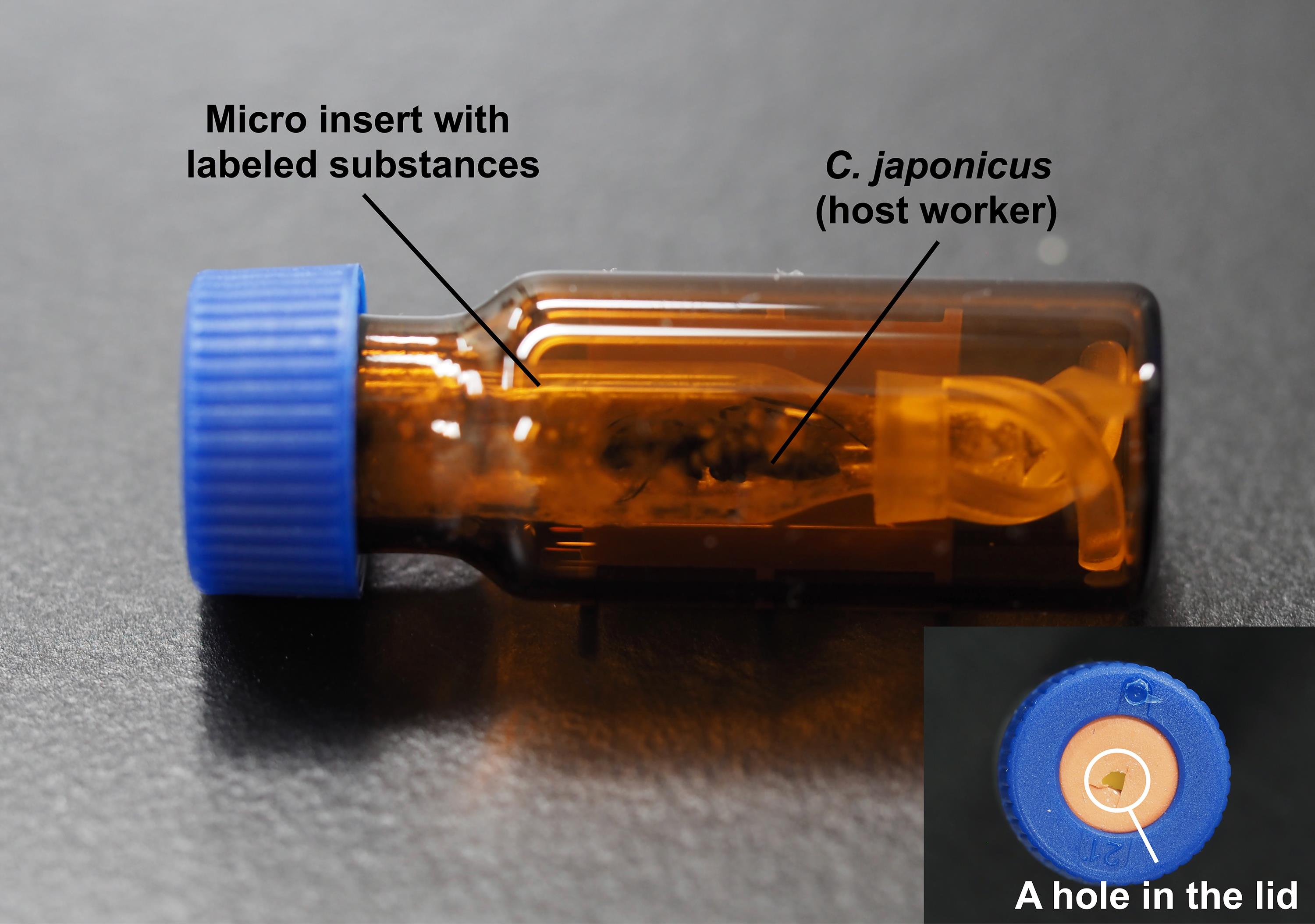
Figure 3. Application of artificial isotopic substances to an ant. An ant is put into the vial with micro inserts coated with artificial isotopic substances.
Induction of chemical disguise behavior under laboratory conditions
To prevent possible attacks from the host worker (C. japonicus) before the newly mated P. lamellidens queen performs the rubbing behavior, anesthetize the host worker with artificial isotopic substances. Use the same method of anesthesia as in step A3; however, to minimize the risk of the artificial isotopic substances coming off from the host worker, the autosampler vial with SIL target Polyspring inserts containing the host worker with the lid removed should be placed in the Petri dish, allowing the host worker to move around in the dish on its own.
Place the newly mated P. lamellidens queen and host workers in plastic cases containing plaster (Figure 4). After dissolving the plaster in water and placing it in a plastic case, make sure the plaster has hardened in the case before using it in the experiment. The plaster acts as both a scaffold for the ants and a moisturizing factor. If a newly mated P. lamellidens queen is able to perform the rubbing behavior continuously, one new host worker with artificial isotopic substances should be added (all from the same colony) every day until the third day. The anesthetized host worker usually wakes up within approximately 10 min of being introduced.
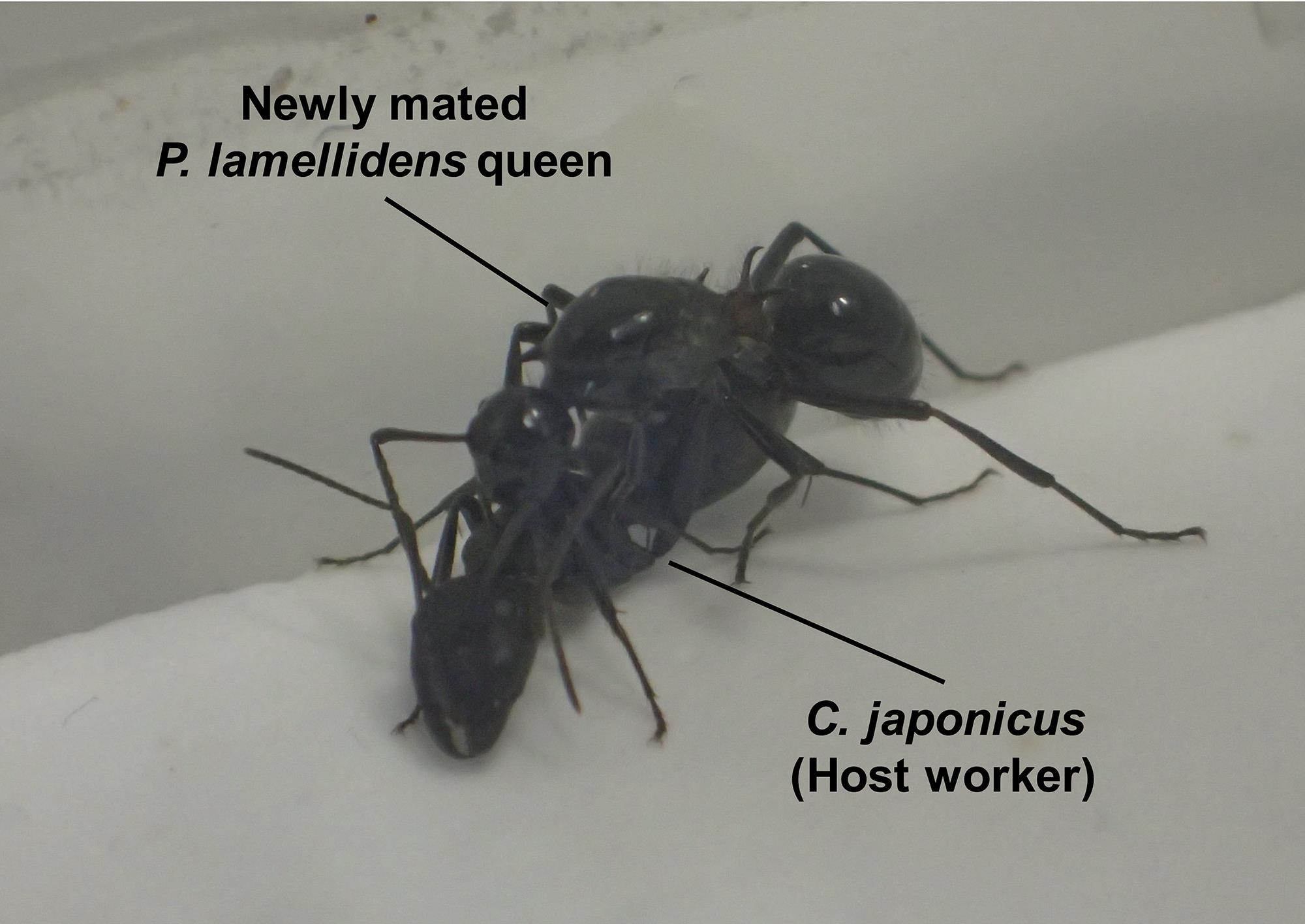
Figure 4. The rubbing behavior of a newly mated P. lamellidens queen. The newly mated P. lamellidens queen exhibits rubbing behavior against a C. japonicus worker under laboratory conditions. Adapted from Iwai et al. (2022).
Extraction and fractionation
Note: Ants of approximately 1–2 cm in size are used as a model case in this protocol. If the ant size is less than 1 cm, the number of ants should be increased per sample (e.g., three ants per sample).
The conditions for selecting the internal standard are: 1) selecting a substance that has similar properties to the CHC of the ants used in the experiment and 2) that is not present naturally in these species. It is necessary to prepare a suitable internal standard (hydrocarbon with a similar chain length to the CHC of the ants) corresponding to that species.
Expose a single individual live ant (e.g., the newly mated P. lamellidens queen or its host worker) in a sterilization Petri dish to 4 °C for 2 min and then to -20 °C for 3 min for anesthetic purposes (similarly to step A3).
Immediately place the anesthetized ant in a disposable rimless culture tube containing 200 μL of hexane mixed with docosane (10 ng/μL) as an internal standard material (Figure 5). The above solvent is accurately measured by the digital syringe eVol® XR. The whole body of an ant should be immersed in hexane.
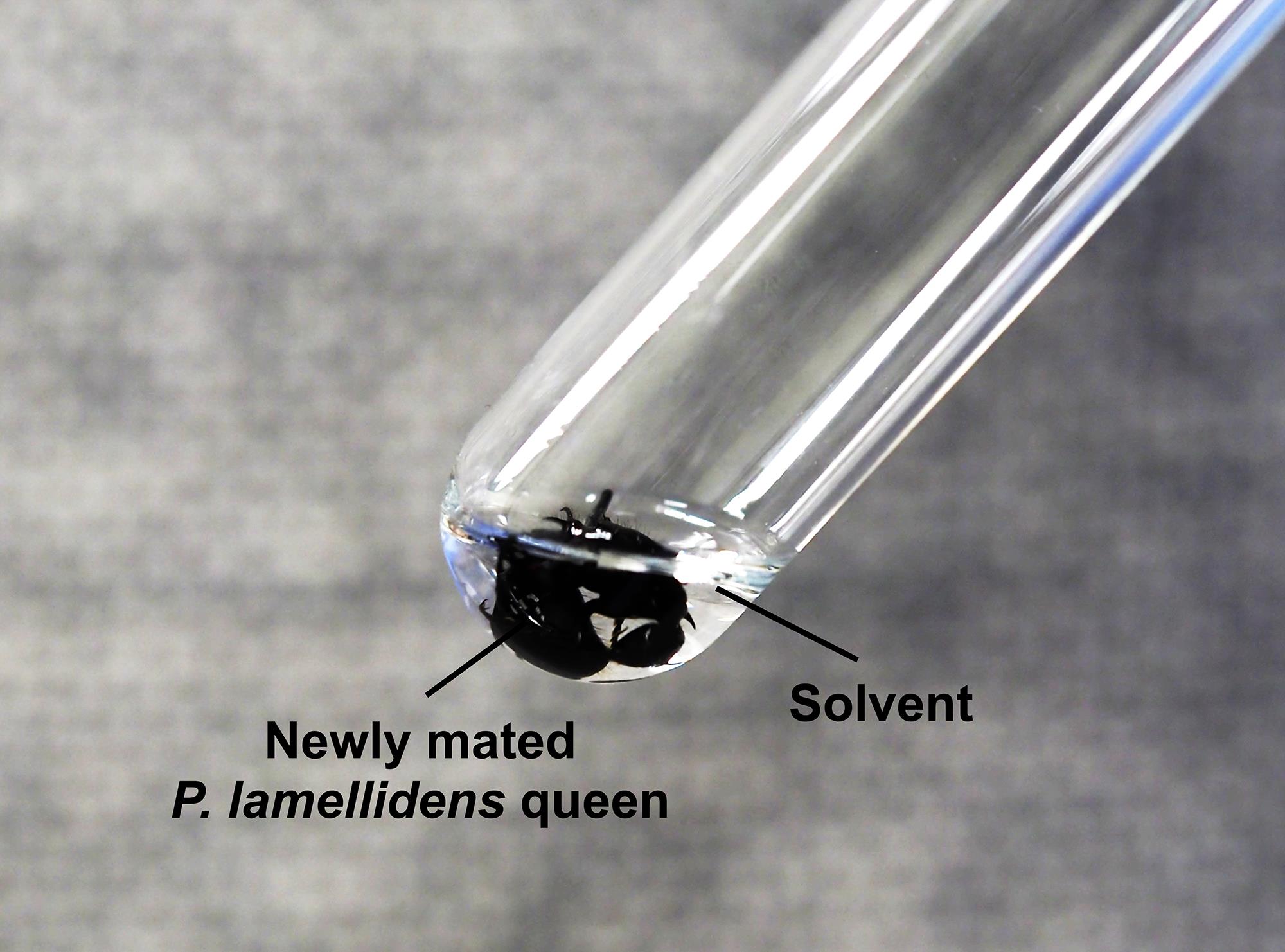
Figure 5. An ant is immersed in the solvent (docosane in hexane). Ensure that the entire ant body is immersed.The chemical materials from the body surfaces of the ant are extracted by dipping the ants in hexane for 5 min. The ant will die during this step.
Add a Pasteur pipette to the stainless-steel stand (three legs) with a clamp and wrap a Kimwipe S-200 around the surface of the Pasteur pipette (Figure 6A). The Kimwipe prevents contamination of the rubber part of the clamp even if a small amount of hexane is accidentally spilled while applying it to the Pasteur pipette.
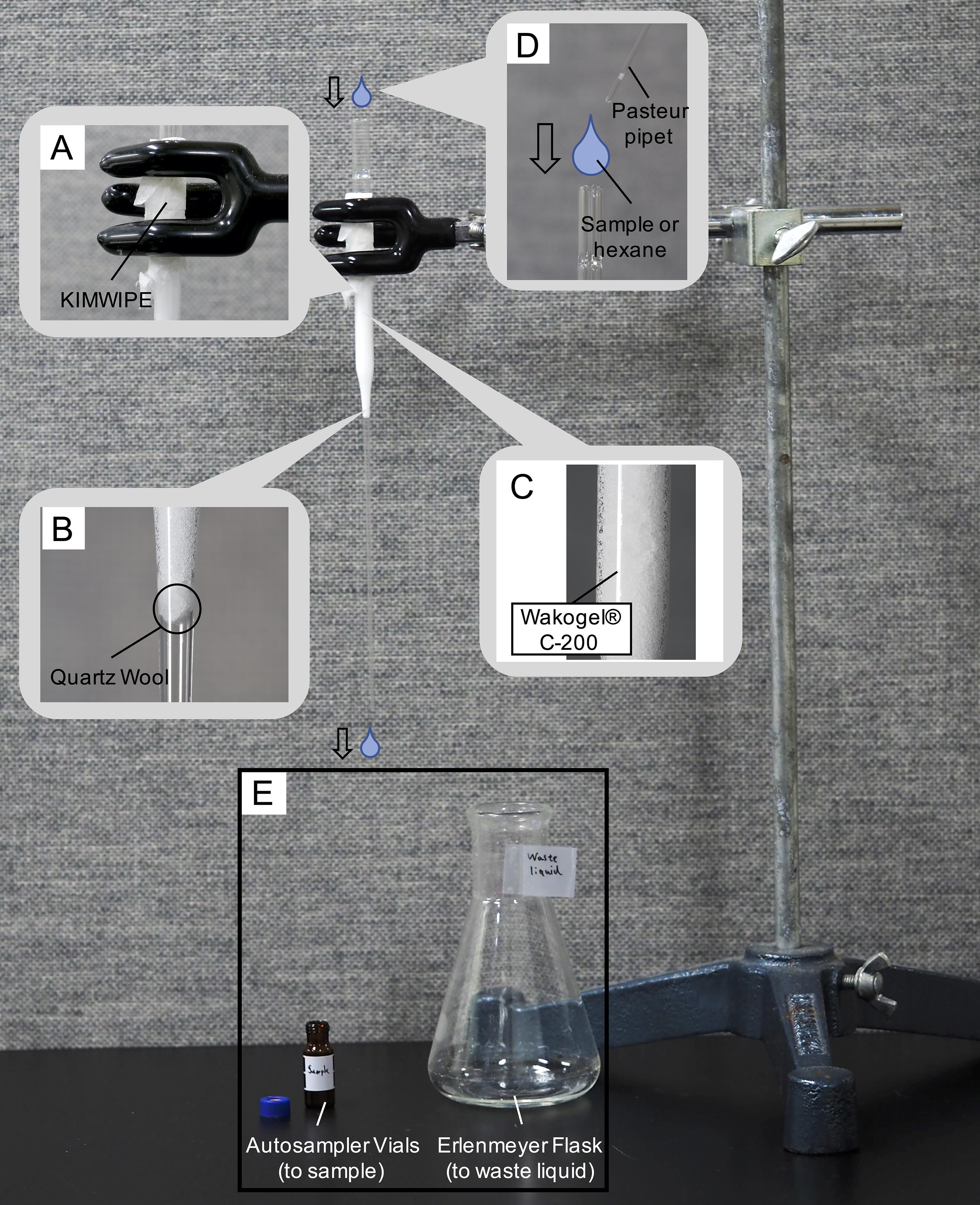
Figure 6. Extraction and fraction of CHC. A. Joint with the clamp. B. Placement of quartz wool. C. Placement of Wakogel® C-200. D and E. Injection of sample or hexane.Add quartz wool to the inside of the Pasteur pipette (Figure 7A). The amount of quartz wool used should be sufficient to fill the bottom hole of the Pasteur pipette. At this point, cram the quartz wool into the bottom using another pipette (Figure 6B and Figure 7A).
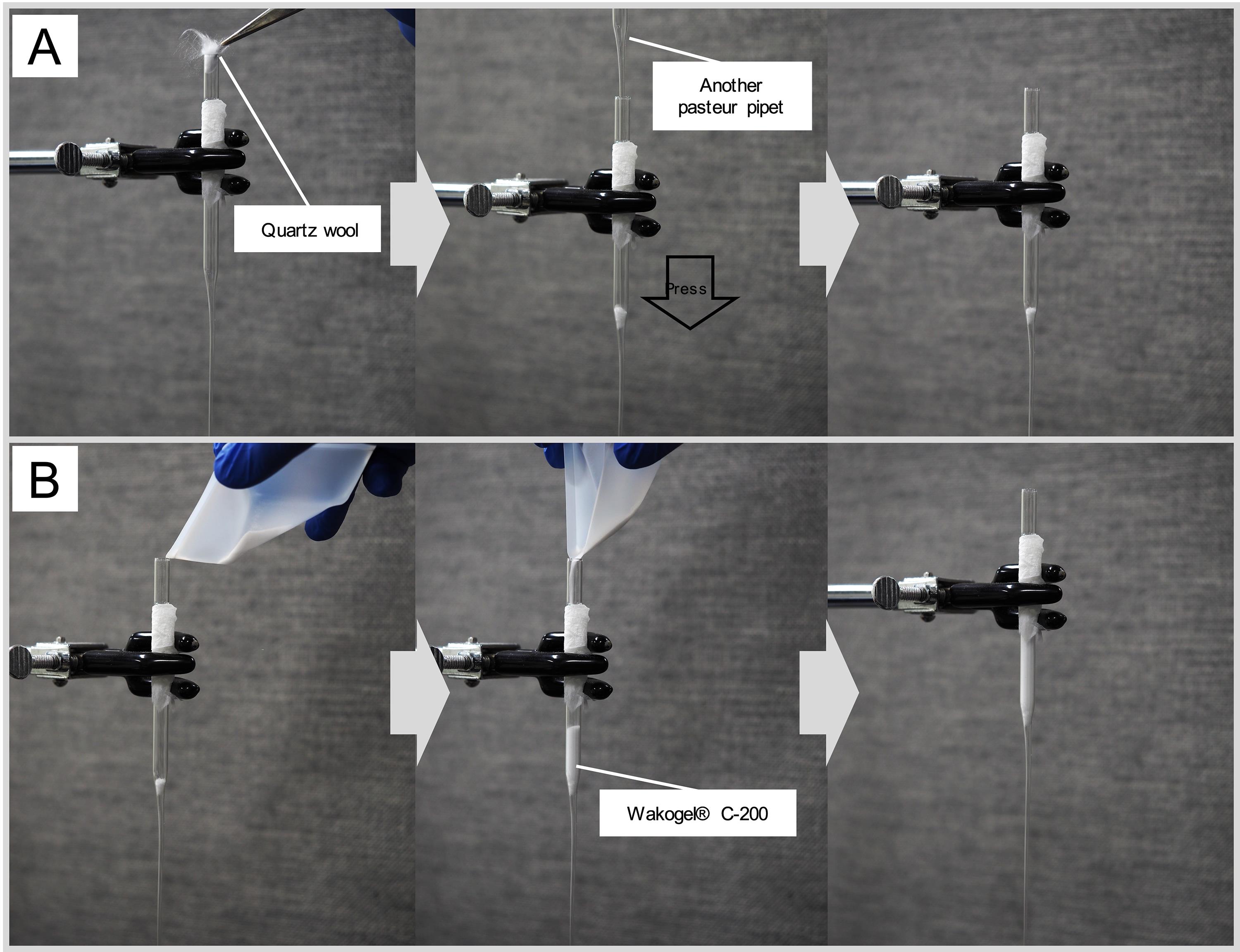
Figure 7. Preparing a column for fractionation. A. Steps for loading Quartz Wool. B. Steps for loading Wakogel® C-200.For column chromatography, add 0.5 g of Wakogel® C-200 to the Pasteur pipette with the quartz wool. Measure the weight of the silica gel using balance dishes and a digital scale (Figure 6C and Figure 7B).
Using another Pasteur pipette, add approximately 1 mL of hexane into the above Pasteur pipette with a Pasteur pipette bulb to wash and deaerate the silica gel. Drip the added hexane through the fractionation column into an empty Erlenmeyer flask using a Pasteur pipette bulb (Figure 6D). As 1 mL of hexane will flow down under its own weight within approximately 10 s of being placed on the Pasteur pipette column, it is necessary to prepare an Erlenmeyer flask before performing this step.
Carry out fractionation by adding the ant extract into the above Pasteur pipette. At this time, dispense the ant extract obtained in steps C1 and C2 using another Pasteur pipette with a Pasteur pipette bulb (Figure 6E).
Add 1 mL of hexane to the column after applying the ant extract using another pre-marked 1 mL scale Pasteur pipette equipped with a Pasteur pipet bulb to elute the hydrocarbons. Drip the fractionated product through the fractionation column into an empty autosampler vial using a Pasteur pipette bulb. This process can only fractionate CHC from ant crude extract. As the fractionated product will flow down under its own weight within approximately 10 s of being placed on the Pasteur pipette column, it is necessary to prepare an empty autosampler vial before performing this step. Approximately 1 mL of the fractionated product is yielded in this step. A new column and an empty vial must be prepared for each sample.
Concentration
By setting the autosampler vial containing the fractional product in a dry block bath test (already installed on another stand with silicon tube, pressure regulator for N2 gas, and N2 gas cylinder), the obtained hydrocarbons are concentrated by applying N2 gas until the solvent hexane is completely evaporated.
To elute the concentrated hydrocarbons, add 50 μL of hexane to the autosampler vial using the digital syringe eVol® XR. After adding 50 μL of hexane, elute hydrocarbons adhering to the inner wall by tilting and turning the autosampler vial.
Set the SIL target Polyspring inserts in the Shimadzu LabTotal Vial for GC/GC-MS and transfer 50 μL of eluted hydrocarbons into the insert using the glass capillary tubes.
Analysis using the GC-MS system
To analyze the samples (concentrated hydrocarbons), set up the GC-MS system under the following conditions. The splitless mode is adopted for the sample injection port and the apparatus is maintained at 300 °C. Helium is used as the carrier gas at a constant flow rate setting of 0.9 mL/min. The oven temperature is set as follows: 40 °C for 3 min, 40–260 °C at 30 °C/min, 260–300 °C at 15 °C/min, and 18 min at the final temperature. See Equipment for the liner and column used.
Inject 2 μL of the sample (concentrated hydrocarbons) into the sample injection port of the GC.
Inject also C7–C40 saturated alkanes of known concentrations (0.5, 1, 2, 5, and 10 ng/μL) to use as standard substances.
Data analysis
To detect and calculate each hydrocarbon peak and its areas, use the MSD ChemStation E.02.02.1431. The equivalent chain length (ECL), the value of fragment and molecular ions, and the mass spectrum pattern are used to estimate the type of each CHC (Figure 8).
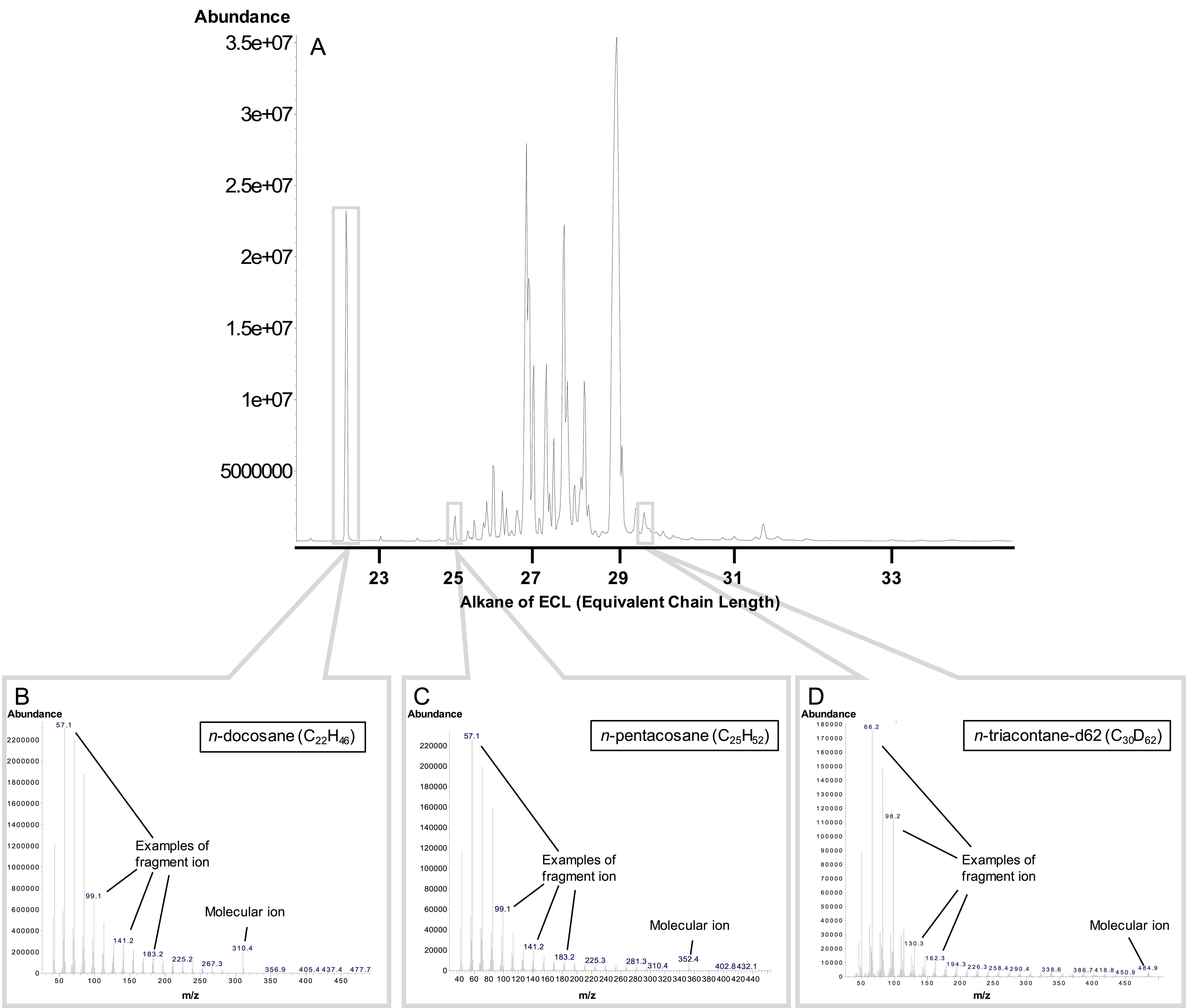
Figure 8. The estimation of each cuticular hydrocarbon (CHC) of a newly mated P. lamellidens queen (before the rubbing behavior). A. Total ion chromatogram of CHC. B. The fragment pattern of n-Docosane (C25H52, internal standard). C. The fragment pattern of n-Pentacosane (C25H52). D. The fragment pattern of n-Triacontane-d62 (C30H62). A list of other CHCs of a newly mated P. lamellidens queen can be found in Iwai et al. (2022).Quantify the concentrations of hydrocarbons using standards (the mixtures of C7–C40 saturated alkanes) of known concentrations (0.5, 1, 2, 5, and 10 ng/μL) diluted at five levels; add the internal standard (docosane) to each sample and to the above standards. After normalizing the peak areas of each standard and sample by using internal standards, create a calibration curve to quantify the concentration of hydrocarbons.
The calculated peak area and concentration of each CHC are used in various statistical analyses (e.g., hierarchical cluster analysis, correlation analysis, and quantitative analysis; refer to Iwai et al., 2022).
Recipes
Isotope-labeled triacontane and dotriacontane solutions (16 mg/mL in hexane)
Add 16 mg of n-Triacontane-d62 and n-Dotriacontane-d66 individually to 1 mL of hexane for chromatography. Store at -20 °C to 4 °C.
Internal standard material (10 ng/μL docosane in hexane)
Add 1 mg of Docosane to 100 mL of hexane for chromatography.
This solution should be stored at -20°C to 4 °C, as it is used regularly in experiments.
Standards of saturated alkanes (0.5, 1, 2, 5, and 10 ng/μL)
Add a total of 20 μg of C7–C40 saturated alkanes (1,000 μg/mL of each component in hexane) to 2 mL of hexane for chromatography.
Additionally, prepare other standards of saturated alkanes (0.5, 1, 2, and 5 ng/μL) containing 10 ng/μL docosane as in Table 1.
Table 1. Recipes for standards of saturated alkanes (0.5, 1, 2, and 5 ng/μL)
Standards of saturated alkanes 10 ng/μL of C7–C40 saturated alkanes 10 ng/μL docosane (internal standard) total 0.5 ng/μL 50 μL 950 μL 1 mL 1 ng/μL 100 μL 900 μL 1 mL 2 ng/μL 200 μL 800 μL 1 mL 5 ng/μL 500 μL 500 μL 1 mL
Acknowledgments
This protocol was adapted from our established publication (Iwai et al., 2022; Kurihara et al., 2022). We thank Toshiharu Akino for advising the extraction method of the CHC and the critical suggestions, and Kazuharu Arakawa, Masaru Tomita, and Masataka Wakayama for their continuous support and help. We also thank Mana Masui and Koh Nakagawa for taking pictures, and Kaoru Ogino for his diligent proofreading of this manuscript. This work was supported by research funds from JSPS Fellows (202021677), Taikichiro Mori Memorial Research Grant, Nakatsuji Foresight Foundation Research Grant, KAKENHI Grant-in-Aid for Scientific Research (B) (21H02210), and Yamagata Prefecture, and Tsuruoka City.
Competing interests
The authors declare no competing interests.
Ethical considerations
This protocol does not use human and animal (e.g., mammal) subjects.
References
- Akino, T. (2008). Chemical strategies to deal with ants: a review of mimicry, camouflage, propaganda, and phytomimesis by ants (Hymenoptera: Formicidae) and other arthropods. Myrmecol. News 11(11): 173–181.
- de la Mora, A., Sankovitz, M. and Purcell, J. (2020). Ants (Hymenoptera: Formicidae) as host and intruder: recent advances and future directions in the study of exploitative strategies.Myrmecol. News 30: 53–71.
- Dettner, K. and Liepert, C. (1994). Chemical mimicry and camouflage. Annu. Rev. Entomol. 39: 129–154.
- Howard, R. W. and Blomquist, G. J. (2005). Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 50: 371–393.
- Iwai, H., Mori, M., Tomita, M., Kono, N. and Arakawa, K. (2022). Molecular evidence of chemical disguise by the socially parasitic spiny ant Polyrhachis lamellidens (Hymenoptera: Formicidae) when invading a host colony. Front. Ecol. Evol. 10: 915517.
- Kocher, S. D. and Grozinger, C. M. (2011). Cooperation, conflict, and the evolution of queen pheromones. J. Chem. Ecol. 37(11): 1263–1275.
- Kurihara, Y., Iwai, H., Kono, N., Tomita, M. and Arakawa, K. (2022). Initial parasitic behaviour of the temporary social parasitic ant Polyrhachis lamellidens can be induced by host-like cuticles in laboratory environment. Biol. Open 11(3): bio058956.
- Lenoir, A., d’Ettorre, P., Errard, C. and Hefetz, A. (2001). Chemical ecology and social parasitism in ants. Annu. Rev. Entomol. 46: 573–599.
- Sturgis, S. J. and Gordon, D. M. (2012). Nestmate recognition in ants (Hymenoptera: Formicidae): a review. Myrmecol. News 16: 101–110.
- Tsuneoka, Y. and Akino, T. (2012). Chemical camouflage of the slave-making ant Polyergus samurai queen in the process of the host colony usurpation (Hymenoptera: Formicidae). Chemoecology 22: 89–99.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Iwai, H. and Kono, N. (2023). Cuticular Hydrocarbon Profiling by Fractionation and GC-MS in Socially Parasitic Ants. Bio-protocol 13(13): e4772. DOI: 10.21769/BioProtoc.4772.
Category
Biochemistry > Other compound > Cuticular hydrocarbon
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










